Abstract
Fifty percent of trauma patients who present sepsis-like syndrome do not have bacterial infections. This condition is known as systemic inflammatory response syndrome (SIRS). A unifying factor of SIRS and sepsis is cardiovascular collapse. Trauma and severe blood loss cause the release of endogenous molecules known as damage-associated molecular patterns. Mitochondrial N-formyl peptides (F-MIT) are damage-associated molecular patterns that share similarities with bacterial N-formylated peptides and are potent immune system activators. The goal of this study was to investigate whether F-MIT trigger SIRS, including hypotension and vascular collapse via formyl peptide receptor (FPR) activation. We evaluated cardiovascular parameters in Wistar rats treated with FPR or histamine receptor antagonists and inhibitors of the nitric oxide pathway before and after F-MIT infusion. F-MIT, but not nonformylated peptides or mitochondrial DNA, induced severe hypotension via FPR activation and nitric oxide and histamine release. Moreover, F-MIT infusion induced hyperthermia, blood clotting, and increased vascular permeability. To evaluate the role of leukocytes in F-MIT-induced hypotension, neutrophil, basophil, or mast cells were depleted. Depletion of basophils, but not neutrophils or mast cells, abolished F-MIT-induced hypotension. Rats that underwent hemorrhagic shock increased plasma levels of mitochondrial formylated proteins associated with lung damage and antagonism of FPR ameliorated hemorrhagic shock-induced lung injury. Finally, F-MIT induced vasodilatation in isolated resistance arteries via FPR activation; however, F-MIT impaired endothelium-dependent relaxation in the presence of blood. These data suggest that F-MIT may be the link among trauma, SIRS, and cardiovascular collapse.
Keywords: mitochondrial N-formyl peptides, cardiovascular collapse, sepsis-like syndrome
the diagnosis of sepsis requires evidence of bacteria in blood cultures, as well as the presence of the following criteria: hypothermia or hyperthermia, hypotension, tachycardia, tachypnea, vascular leakage, and organ damage (13). However, 50% of patients with these symptoms do not have documented infection (1, 13). Accordingly, these patients are diagnosed with sepsis-like syndrome, also called systemic inflammatory response syndrome (SIRS). The diagnosis of SIRS requires the same criteria, minus the presence of infection.
SIRS and sepsis are characterized by increases in inflammatory mediators, and numerous clinical trials have been conducted with agents that block the inflammatory cascade: corticosteroids, anti-endotoxin antibodies, tumor necrosis factor-α (TNF-α) and interleukin-1-receptor antagonists, and various other agents (1, 2, 5, 12, 13). However, these agents have failed to improve morbidity and mortality in these conditions.
The major pathophysiological characteristic of sepsis and SIRS is the loss of control of vascular tone and unresponsiveness to vasoconstrictive drugs (2, 12). Breakdown of the endothelial barrier function results in the loss of fluid into the extravascular space and may lead to edema in the lungs, kidneys, and brain (7). Exacerbated production of nitric oxide (NO) by the inducible form of NO synthase (iNOS) may also contribute to the hypotension and vascular hyporeactivity in these pathologies (19); however, pharmacological interventions using NO synthase inhibitors have not been successful. Although it has been well established that cardiovascular collapse occurs in SIRS and sepsis, the mechanism by which traumatic injury leads to noninfective or “sterile” sepsis-like syndrome is not fully understood.
It has been proposed that in patients without infection, cell components from traumatized tissue are the primary instigators of a systemic inflammatory response (16, 20). These cell components are called damage-associated molecular patterns (DAMPs). DAMPs are endogenous intracellular molecules that are released from cells following injury or death. These factors lead to activation of the innate immune system (16). For evolutionary reasons, mitochondria share several characteristics with bacteria, and when fragments of mitochondria are present in the circulation, they are recognized by the innate immune system as DAMPs. N-formyl peptides, for example, are common molecular signatures of both bacteria and mitochondria and are known to play a role in the initiation of inflammation by activating the formyl peptide receptor (FPR) (9). The FPR has been identified as a subfamily of G protein-coupled receptors (6). It is well known that FPR-1 and FPR-2 are expressed at high levels on leukocytes and that they mediate cell chemotaxis (6). In neutrophils, activation of FPR-1 by binding of N-formyl-methionyl-leucyl-phenylalanine (fMLP), a peptide derived from bacteria, stimulates heterotrimeric G proteins and releases calcium from intracellular stores (6). Other intracellular effectors in the FPR signaling cascade include phospholipase A2 and mitogen-activated protein kinases (6). It has been demonstrated that FPR is also expressed on endothelium, epithelia, microglia, spleen, lung, liver, and skeletal muscle (6, 16). Nevertheless, its biological function in somatic tissues is not fully understood.
We have observed that both mitochondrial N-formyl peptides (F-MIT; N-formyl-Met-Met-Tyr-Ala-Leu-Phe) and formyl peptide from bacteria (fMLP) induce vasodilatation in resistance arteries and that FPR antagonist inhibits this response, suggesting that FPR has an important role in vascular function (15, 16). It is noteworthy that we performed our experiments with formylated peptide corresponding to the NH2-terminus of mitochondria NADPH dehydrogenase subunit 6 (ND6) and structurally distinct from fMLP. In a recent publication, Simmons et al. (14) showed that patients with SIRS had significantly increased circulating levels of mitochondrial DNA, especially in the ND6 gene (14). They suggested that ND6 is associated with the evolution of SIRS and mortality in severely injured human subjects.
To better understand the role of F-MIT in the regulation of hemodynamic responses and vascular biology, we studied the hypothesis that F-MIT (formylated peptide corresponding to the NH2-terminus of mitochondria ND6) lead to cardiovascular collapse and sepsis-like syndrome (SIRS).
METHODS
Animals.
All rats were maintained on a 12-h:12-h light/dark cycle with both rat chow and water ad libitum. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and were reviewed and approved by the Institutional Animal Care and Use Committee of the Georgia Regents University.
Hemodynamic measurements and F-MIT infusion.
Wistar rats (12 wk old) were anesthetized by isoflurane (3%) inhalation. After satisfactory depth of anesthesia, the right femoral artery and vein were cannulated using polyethylene (PE) tubing, PE-10 connected to PE-50, to measure pulsatile blood pressure and administer drug infusion, respectively. F-MIT (0.002, 0.02, or 0.2 mg/rat iv) or vehicle (1% DMSO) were infused 20 min after cannulation or after absence of oscillation in the pulsatile blood pressure values. Some animals received FPR1 [cyclosporine H (CsH), 3 mg/rat iv] or FPR2 [Trp-Arg-Trp-Trp-Trp-Trp-NH2 (WRW4), 2 mg/rat iv] antagonists, cimetidine (histamine H2 receptor antagonist, 50 mg/kg iv), NG-nitro-l-arginine methyl ester (l-NAME; NO synthase inhibitor, 15 mg/rat iv), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one [ODQ; guanylyl cyclase (GC) inhibitor, 0.6 mg/rat iv], or 1400W (iNOS inhibitor 3 mg/rat iv) 30 min before F-MIT or vehicle infusion. To confirm the specificity of F-MIT, some animals were infused with nonformylated peptide (Met-Met-Tyr-Ala-Leu-Phe) in the same dose of F-MIT that were used in other experiments (0.02 mg/rat).
Hemorrhagic shock.
In another group of Wistar rats, after femoral artery and vein catheterization (as described above), treated animals with WRW4 (2 mg/rat iv) or vehicle (1% DMSO) underwent hemorrhage from the femoral artery until a mean arterial pressure of 40–45 mmHg was achieved. This hemorrhage of ∼30% of total blood volume was performed over a 5-min interval. Further hemorrhage or replacement was performed to maintain the mean arterial pressure at 40–45 mmHg. After 1 h of this hemorrhage, reperfusion was initiated using lactated Ringer solution (in equal volume to the blood previously withdrawn), administered via syringe pump (Harvard Apparatus, PHD 2000 infusion, with a 10 ml/14.5 mm diameter glass syringe), for 1 h. Subsequently, the rats were euthanized, and blood samples and lungs were then saved for analysis.
Neutrophil, basophil, and mast cells depletion.
Rabbit anti-rat polymorphonuclear neutrophil antiserum (0.3 ml iv, diluted in 1:5), C48/80 compound (0.75 mg/kg ip) or anti-asialo GM1 antiserum (0.2 ml ip, diluted in 1:10) were injected into the rats 18–24 h before F-MIT infusion to deplete neutrophils, mast cells, and basophils, respectively. Blood samples from cell-depleted rats were withdrawn before injecting antineutrophil and antibasophil antibody or C48/80 compound immediately before F-MIT infusion (0.02 mg/rat). To confirm the absence of basophil, neutrophils, or mast cells, air-dried blood films were stained with Giemsa stain for 2 min. The target cells were counted manually under a light microscope.
F-MIT injections.
Wistar rats (12 wk old) received one intraperitoneal injection of F-MIT (0.02 mg/rat) or vehicle (1% DMSO). After 6 h of the F-MIT injections, the animals were anesthetized during ∼10 min or after satisfactory depth of anesthesia and euthanized to evaluate lung injury.
Lung injury evaluation.
Following hemorrhagic shock or F-MIT treatment for 6 h, the lungs were collected and embedded in tissue medium freeze (OCT, Triangle Biomedical Sciences), cut in cryostat (10 μm), and stained with hematoxylin and eosin. Each slide was evaluated by two or more expert investigators blinded to the experiment groups. Lung injury was evaluated based on three characteristics: edema, neuthrophil infiltration, and alveolar septal thickening. Each item was scored 0–5 (0 = normal, 1 = mild, 3 = moderate, and 5 = severe), and the average of the total score lung injury was then calculated and compared between groups.
Biochemistry assays.
Myeloperoxidase (MPO), TNF-α, and GC activity were measured using ELISA kits as described by the manufacturers (Sigma-Aldrich for MPO, and Cayman Chemical for TNF-α and GC).
Endotoxin detection assay (GenScript) was used to confirm the absence of lipopolysaccharides (LPS) in nonformylated and formylated peptides (8 mg/ml; diluted in saline and 1% DMSO) and in plasma samples from animals treated with F-MIT (0.02 mg/rat) or vehicle.
Evans blue extravasation.
After femoral vein catheterization and F-MIT infusion (as described above), the Evans blue extravasation assay was performed, which is an in vivo permeability assay to test vessel leakage (10). After obtaining a stable value of blood pressure, Evans blue (30 mg/kg) was infused for 30 min. Rats were euthanized, and the third, fourth, and fifth branches of the mesenteric bed and aorta were removed, dissected, and washed three times with PBS for 5 min. Subsequently, the vessels were weighed and incubated with 500 μl formamide to extract extravasated Evans blue. Optical density was measured at 610 nm, and the measurements were converted into mass of dye extravasated (in ng) per mass of tissue (in g) (10).
Vascular function.
In another set of experiments, naive Wistar rats were used to evaluate vascular function. Under deep anesthesia, the mesenteric arcade was carefully removed, and the third-order mesenteric arteries were removed and cleaned of surrounding perivascular tissue in cold Krebs-Henseleit solution containing (in mmol/l) 118 NaCl, 4.7 KCl, 25 NaHCO3, 2.5 CaCl2·2H2O, 1.2 KH2PO4, 1.2 MgSO4·7H2O, 0.01 EDTA, and 11 glucose. Segments (2 mm in length) were mounted in a small vessel myograph chamber (Danish Myo Tech) for isometric tension recordings, as previously described (17). After 15 min, the segments were stretched to their optimal lumen diameter for active tension development (17).
The vessel contractility was tested by exposure to a high-K+ (120 mmol/l) solution. After 15 min, concentration-response curves were constructed to phenylephrine (1 nmol/l–30 μmol/l) or acetylcholine (1 nmol/l–10 μmol/l) in the presence and absence of F-MIT (10 μmol/l). In other segments contracted with phenylephrine (10 μmol/l), F-MIT (300 nmol/l-30 μmol/l) were added to evaluate relaxation. Also, to test whether F-MIT induce vascular dysfunction in the presence of blood, some arterial segments were placed in an organ bath and relaxation responses to acetylcholine were tested. In one experiment, blood from control rats or Krebs-Henseleit solution were “spiked” with F-MIT (10 μmol/l) or vehicle, and the arteries were then incubated in these conditions. In a second experiment, arteries were incubated with blood from a control rat or from a rat treated with F-MIT (0.02 mg/rat ip) for 6 h.
Reactive oxygen species measurement.
The oxidative fluorescent dye hydroethidine was used to evaluate reactive oxygen species (ROS) production in cross-sections of mesenteric resistance arteries incubated with F-MIT (10 μmol/l) or vehicle (1% DMSO) for 15 min (17).
Immunoblotting.
Proteins (10–30 μg) were extracted from the third-order branches of the mesenteric artery, lung, or plasma as previously described (17). The membranes were incubated overnight at 4°C with a primary antibody raised against endothelial NO synthase (eNOS; 1:2,000), cyclooxygenase (COX)-1 (1:2,000), COX-2 (1:1,000), ND6 (1:1,000) phospho-p38 MAPK (1:5,000), p38 MAPK (1:5,000), phospho-p44/42 (1:5,000), ERK-1/2 (p44/42) (1:5,000), FPR (1:1,000), and β-actin (1:40,000).
Reagents and chemicals.
Acetylcholine, phenylephrine, ODQ, l-NAME, protease inhibitor cocktail, Evans blue, formamide, MPO kit assay, C48/80 compounds, DMSO, and antibody to β-actin and anti-ND6 were purchased from Sigma-Aldrich. GC kit assay was purchased from Cayman Chemical. WRW4 was purchased from Tocris Bioscience. F-MIT, nonformylated peptides and endotoxin detection assay were purchased from GenScript. CsH was purchased from Abcam. Antibodies phospho-p38 MAPK (Thr180/Tyr182), p38 MAPK, phospho-p44/42 (ERK-1/2) MAPK (Thr202/Tyr204), and p44/42 MAPK were purchased from Cell Signaling. Antibodies to eNOS, COX-1, and COX-2 were purchase from BD Biosciences (San Jose, CA). Antibody to FPR was purchased from Abcam. Antibody anti-rat polymorphonuclear neutrophil antiserum was purchased from Accurate Chemicals & Scientific. Antibody anti Asialo GM1 (Rabbit) was purchased from Wako Laboratory Chemicals. Dihydroethidium and oligodinucleotide (ODN) were purchased from Invitrogen.
Statistics.
Results are presented as means ± SE. The statistical procedures used included Student's unpaired t-tests, one-way and two-way ANOVA, and nonlinear regression analysis. All analyses were performed using data analysis software GraphPad Prism 5.0. Statistical significance was set at P < 0.05.
RESULTS
F-MIT induce severe hypotension in a dose-dependent manner.
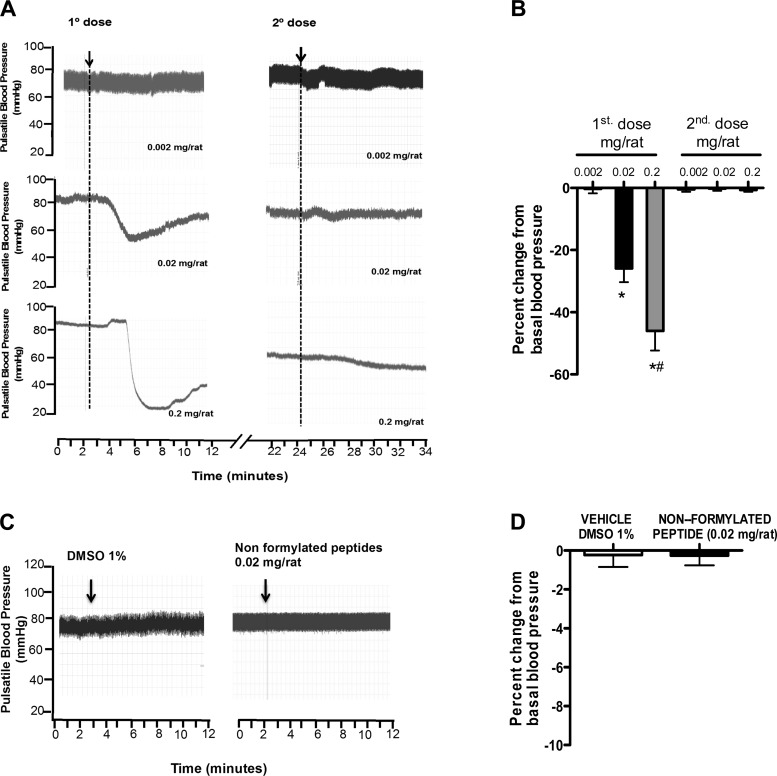
F-MIT infusion (0.002, 0.02, or 0.2 mg/rat iv) decreased blood pressure in a dose-dependent manner in rats (Fig. 1, A and B). The second of two consecutive infusions of the same F-MIT dose, administered 15 min after the first one, did not change blood pressure, suggesting desensitization of the receptor (Fig. 1, A and B). To confirm the necessity of the N-formylated terminus for the observed drop in blood pressure, some animals were infused with peptide of the same amino acid sequence, but nonformylated (Met-Met-Tyr-Ala-Leu-Phe). Nonformylated peptide or vehicle (1% DMSO) did not change blood pressure (Fig. 1, C and D). As described earlier, mitochondria carry hallmarks of their bacterial ancestry (16). One of these hallmarks is that all peptides synthesized by mitochondria begin with N-formyl-Met. Our results suggest that F-MIT, but not nonformylated peptides, as present in other parts of eukaryotic cells, are necessary to decrease blood pressure. Endotoxin detection assay was used to confirm the absence of LPS contamination in nonformylated and formylated peptides and in plasma samples from animals treated with F-MIT (0.02 mg/rat) or vehicle. Endotoxin was absent in all samples evaluated (data not shown). These results confirm that F-MIT induce cardiovascular collapse independently of LPS.
Fig. 1.
Effects of mitochondrial N-formyl peptides (F-MIT; A and B), nonformylated peptide (C and D), and DMSO (C and D) on blood pressure. A and B: subsequent infusion of the same dose of F-MIT (0.002, 0.02, or 0.2 mg/rat), administered 15 min after the first one, does not change blood pressure of Wistar rats. Arrows (A and C) indicate drug infusion. B and D: average values for percent change from basal blood pressure. Means ± SE; n = 4 to 5. One-way ANOVA: *P < 0.05 vs. 0.002 mg/rat; #P < 0.05 vs. 0.02 mg/rat.
To determine whether another compound from mitochondria was able to change hemodynamic parameters, some rats were infused with a mimetic of the biological structure of mitochondrial DNA (type C synthetic ODN 2395; 0.1 mg). We were interested in mitochondria DNA since Zhang et al. (20) demonstrated that this molecule was increased in circulation in patients after trauma, and it was associated with inflammation. ODN2395, however, did not change hemodynamic parameter in rats (blood pressure: control, 80 ± 7 vs. ODN, 79 ± 8 mmHg; P > 0.05). These data suggest that F-MIT, but not mitochondrial DNA, have the ability to affect cardiovascular system.
F-MIT induce fever, vascular leakage, and blood clotting.
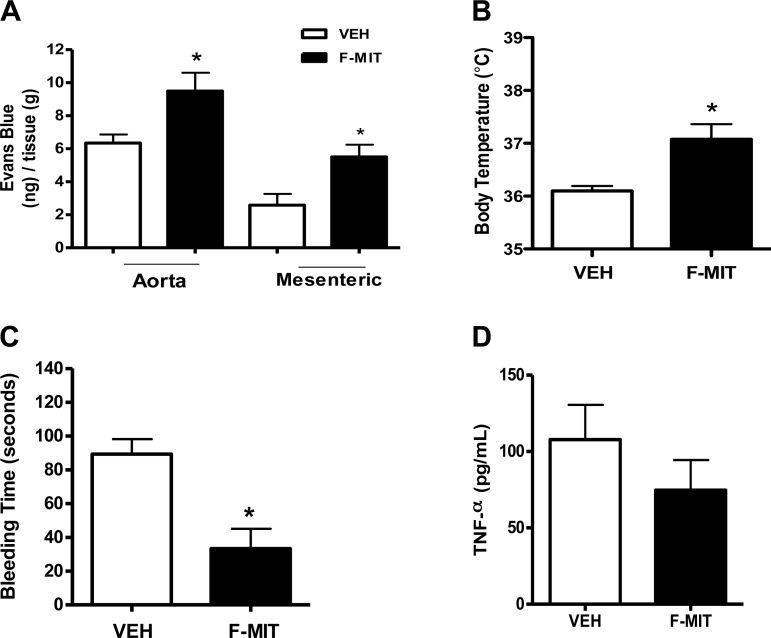
To evaluate whether F-MIT (0.02 mg/rat iv) were able to induce other manifestation of SIRS in addition to hypotension, some rats were infused with F-MIT (0.02 mg/rat iv). It was observed that F-MIT increased vascular permeability and body temperature (Fig. 2, A and B) as well as decreased bleeding time (Fig. 2C), indicating blood clotting. It is noteworthy that infusion of F-MIT did not increase systemic levels of TNF-α (Fig. 2D). These data infer that the effects observed after F-MIT infusion are not secondary to the production of proinflammatory mediators (e.g., cytokines).
Fig. 2.
Effects of F-MIT on vascular permeability (A), body temperature (B), bleeding time (C), and TNF-α production in plasma (D). Means ± SE; n = 4 to 5. t-test: *P < 0.05 vs. vehicle (Veh).
F-MIT induce organ damage.
Acute lung injury (ALI) is caused by any stimulus of local or systemic inflammation. ALI is a common characteristic between SIRS and sepsis (1, 2, 5, 12, 13). To answer the question as to whether F-MIT induce ALI, we used two different approaches. First, we tested the hypothesis that inhibition of FPR signaling would attenuate lung damage in a model of sterile injury, hemorrhagic shock. Second, we tested whether intraperitoneal injections of F-MIT (0.02 mg/rat) lead to lung damage after 6 h.
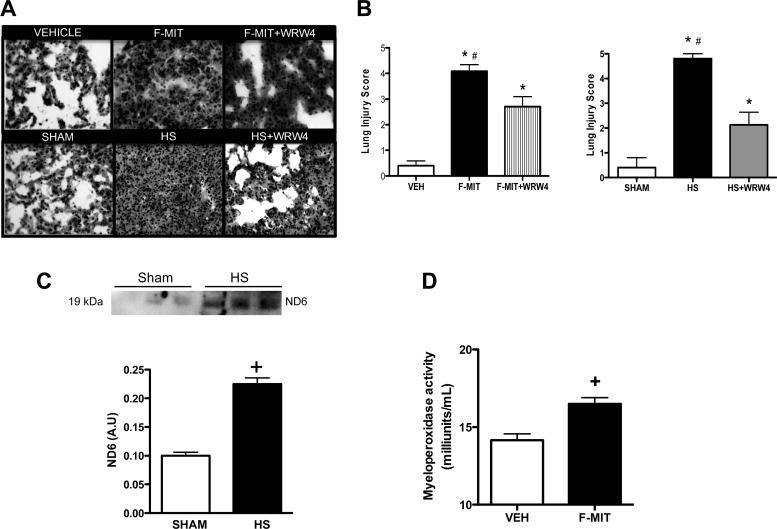
Following hemorrhagic shock or F-MIT treatment for 6 h, Wistar rats developed severe lung injury (Fig. 3, A and B), including edema, neuthrophil infiltration, alveolar septal thickening, and increased MPO activity (Fig. 3D). The preventive treatment with FPR-2 antagonist (WRW4) decreased hemorrhagic shock- and F-MIT-induced lung damage (Fig. 3 A and B). To support these data, which demonstrate FPR activation after trauma, it was observed that hemorrhagic shock induced the release of fragments of mitochondria ND6 in the circulation (Fig. 3C). It is noteworthy that the same F-MIT (peptide used in all experiments performed in the present study) were corresponding to the NH2-terminus of mitochondria ND6 (9). These results suggest that following trauma, fragments from mitochondria, including formylated peptides, activate FPR and lead to lung injury.
Fig. 3.
Representative hematoxylin-eosin staining of lung from rats treated with Veh, F-MIT, or F-MIT and formyl peptide receptor (FPR)-2 antagonist [Trp-Arg-Trp-Trp-Trp-Trp-NH2 (WRW4)] for 6 h (magnification, ×40) (A). Also, representative images of lung from rats that underwent hemorrhagic shock (HS) treated or not with FPR-2 antagonist (WRW4) (A). Graph represents lung injury score (B). C: protein expression (top) and densitometry analysis (bottom) from mitochondria NADPH dehydrogenase subunit 6 (ND6) in plasma from control rats (sham) and HS rats. AU, arbitrary units. Myeloperoxidase activity (D) in lung 6 h after Veh or F-MIT injection. Means ± SE; n = 4 to 5. One-way ANOVA: *P < 0.05 vs. Veh or Sham; #P < 0.05 vs. WRW4; t-test, +P < 0.05 vs. Veh.
Lung damage induced by FPR activation is due to MAPKs.
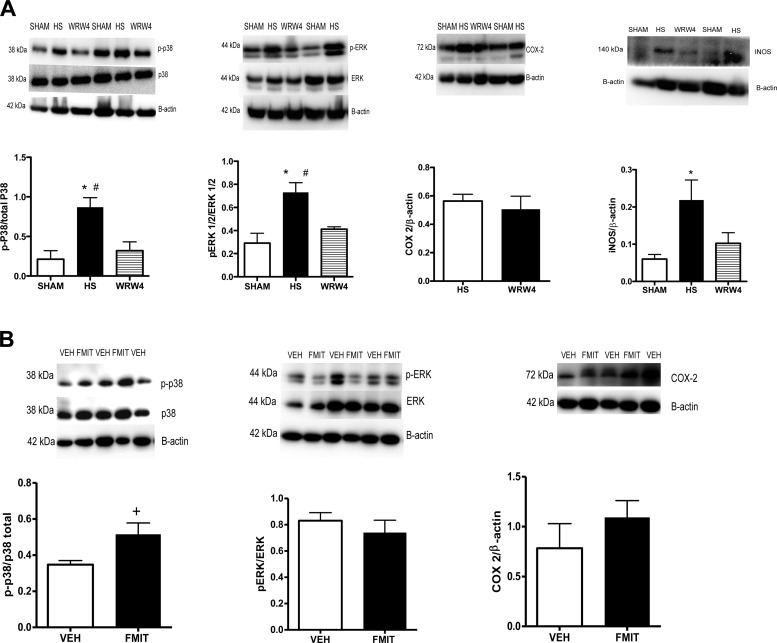
MAPK are serine/threonine-specific protein kinases involved in directing cellular responses to a diverse array of stimuli. As such, MAPKs have been implicated in a wide range of cellular responses including inflammation, cell death, cell differentiation, senescence, and tumorigenesis. Also, it is known that MAPK and iNOS are intracellular effectors following FPR activation (6). As shown in Fig. 4A, phosphorylation of p38 and ERK-1/2 (p44/42) MAPK and iNOS protein expression were decreased in animals that underwent hemorrhagic shock and were previously treated with WRW4 (FPR-2 antagonist). On the other hand, there was no difference between groups in the expression of FPR intracellular effector COX-2 (6). In addition, in lungs from rats treated with F-MIT for 6 h, we observed increased phosphorylation of p38 MAPK but not ERK-1/2 MAPK and COX-2 (Fig. 4B).
Fig. 4.
Representative blots and densitometric analysis from protein expression for MAPK [phospho (p)- and total-p38 and -ERK 1/2], inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) of lungs from HS rats treated or not with WRW4 (FPR-2 antagonist) (A), as well as animals treated with F-MIT for 6 h or Veh (B). Means ± SE; n = 4 to 5. One-way ANOVA: *P < 0.05 vs. sham; #P < 0.05 vs. WRW4; t-test, +P < 0.05 vs. Veh.
FMIT-induced hypotension is via FPR activation and NO release.
Since we demonstrated that F-MIT infusion leads to sepsis-like syndrome, including hypotension, hyperthermia, vascular permeability, blood clotting, and organ damage, our next step was to uncover the mechanisms associated with these effects.
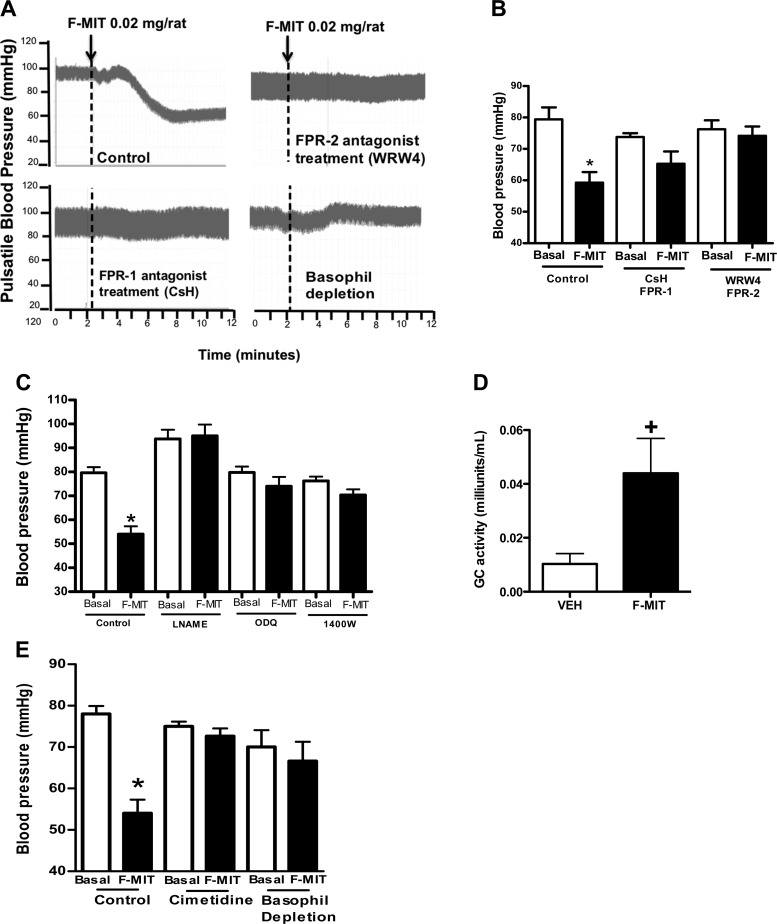
We treated some animals with cyclosporine H (FPR-1 antagonist), WRW4 (FPR-2 antagonist), l-NAME (unspecific NO synthase inhibitor), ODQ (soluble GC inhibitor), and 1400W (iNOS inhibitor) before F-MIT infusion (0.02 mg/rat iv). Both antagonists of FPR abolished the F-MIT-induced hypotension (Fig. 5, A and B). Additionally, F-MIT infusion did not have any effect on blood pressure in animals treated with l-NAME, ODQ, and 1400W (Fig. 5C). Finally, F-MIT infusion increased soluble plasma GC activity (Fig. 5D). Taken together, these data suggest that F-MIT, via FPR activation, lead to NO release and hypotension.
Fig. 5.
Pretreatment with cyclosporine H (CsH, FPR-1 antagonist), WRW4 (FPR-2 antagonist), NG-nitro-l-arginine methyl ester (l-NAME; NOS inhibitor), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; guanylyl cyclase inhibitor), 1400W (iNOS inhibitor), or basophil depletion blocked F-MIT-induced hypotension. A: representative tracing and arrows show F-MIT infusion. B, C, and E: average of the values of blood pressure (in mmHg). D: guanylyl cyclase activity (GC) in plasma. Means ± SE; n = 4–8. One-way ANOVA: *P < 0.05 vs. basal; t-test, +P < 0.05 vs. Veh.
Basophil depletion but not neutrophil or mast cells depletion abolished FMIT-induced hypotension.
FPR-1 and -2 are expressed at high levels on leukocytes, where they mediate cell chemotaxis (6, 9). It is known that activation of FPR-1 in neutrophils leads to ROS generation, and iNOS and calcium increase (6, 9). In the present study, rabbit anti-rat polymorphonuclear neutrophil antiserum C48/80 compounds or anti-asialo GM1 antiserum were injected into the rats to deplete neutrophils, mast cells, or basophils, respectively, before F-MIT infusion.
Neither neutrophil (blood pressure: control, basal, 79 ± 2 vs. F-MIT infusion, 57 ± 4, mmHg; depletion, basal, 77 ± 2 vs. F-MIT infusion, 50 ± 5 mmHg, P < 0.05) nor mast cells depletion (blood pressure: control, basal, 79 ± 2 vs. F-MIT infusion, 57 ± 4; depletion, basal, 77 ± 1 vs. F-MIT infusion, 50 ± 5 mmHg, P < 0.05) blocked F-MIT-induced hypotension. However, basophil depletion abolished this response (Fig. 5, A and E). This suggests that F-MIT-induced hypotension is mediated by FPR activation in basophils.
Since stimulation of FPR induces histamine and NO release from basophil (3, 8), we treated some animals with cimetidine (histamine H2 receptor antagonist). Cimetidine also blocked F-MIT-induced hypotension (Fig. 5E). These results demonstrate that both NO and histamine are involved in F-MIT-induced hypotension.
F-MIT impair acetylcholine-induced relaxation in the presence of blood.
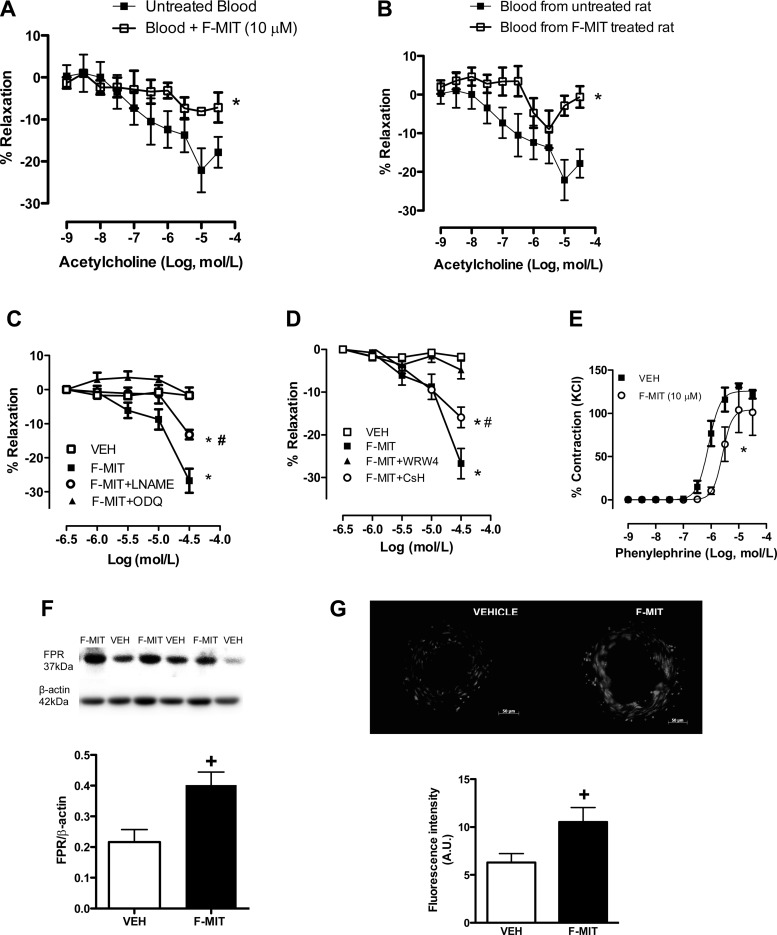
To analyze whether F-MIT cause endothelial dysfunction, we performed concentration-response curves to acetylcholine in mesenteric resistance arteries in the presence or absence of F-MIT (10 μmol/l). Also, some arteries were incubated with blood from naive animals in the presence or absence of F-MIT (10 μmol/l) or blood from treated animals with F-MIT for 6 h.
F-MIT did not change acetylcholine-induced relaxation [EC50: vehicle, 7.9 ± 0.10 vs. F-MIT, 7.8 ± 0.06; and maximum response (Emax): vehicle, 94 ± 2 vs. F-MIT: 97 ± 0.6%; P > 0.05] when incubated in Krebs-Henseleit solution. In contrast, arteries relaxed less when incubated in blood containing F-MIT (Emax: untreated blood, 24 ± 3 vs. blood + F-MIT, 7 ± 3%; P < 0.05) or blood from rats treated with F-MIT than arteries incubated with blood containing vehicle (Emax: blood from untreated rat, 24 ± 3 vs. blood from F-MIT treated rat, 10 ± 0.6%; P < 0.05) (Fig. 6, A and B).
Fig. 6.
Acetylcholine-induced relaxation in blood incubated with F-MIT (A) or in blood from rats treated with F-MIT (B) for 6 h. Concentration-responses curves to F-MIT (C and D) or phenylephrine (E) in the presence or absence of l-NAME (C), ODQ (C), WRW4 (D), CsH (D), or F-MIT (E). Representative blots and densitometric analyses from protein expression for FPR of resistance arteries from Wistar rats treated with F-MIT or Veh for 15 min (F). Effect of F-MIT on reactive oxygen species generation (G). G, top: representative fluorescence photomicrographs. G, bottom: densitometric analysis. Means ± SE; n = 4 to 5. Two-way ANOVA: *P < 0.05 vs. blood from untreated rats, untreated blood, or Veh; #P < 0.05 vs. F-MIT; t-test, +P < 0.05vs. Veh.
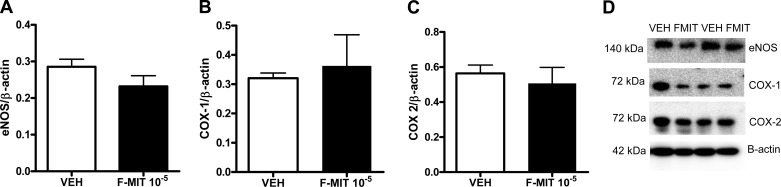
In another set of experiments, F-MIT induced relaxation in a concentration-dependent manner in phenylephrine-constricted arteries incubated in Krebs-Henseleit solution (absence of blood). This response was abolished by ODQ (100 μmol/l) and WRW4 (100 μmol/l), whereas l-NAME (100 μmol/l) and CsH (1 μmol/l) only partially inhibited the relaxation (Fig. 6, C and D). These data suggest that F-MIT induce vascular relaxation in the absence of blood via FPR activation and partially via NO release. However, F-MIT-induced relaxation was independent of changes in total eNOS and COX protein expression (Fig. 7). Finally, it was also observed that F-MIT decreased phenylephrine-induced contraction (Fig. 6E), suggesting that F-MIT attenuate vascular responsiveness to activation of α1-adrengeric receptor.
Fig. 7.
Representative blots and densitometric analysis from protein expression for endothelial nitric oxide synthase (eNOS; A and D), COX-1 (B and D), COX-2 (C and D), and β-actin from mesenteric resistance arteries treated with F-MIT (10 μmol/l) or Veh for 15 min. Means ± SE; n = 4 to 5. t-test, P > 0.05.
It is known that FPR activation can lead to oxidative stress via MAPK and NAD(P)H oxidase (6). Using Western blot analysis and dihydroethidum fluorescent staining, we observed that mesenteric resistance arteries incubated with F-MIT increased FPR protein expression and ROS generation (Fig. 6, F and G).
DISCUSSION
Our results demonstrate for the first time that N-formylated peptides of mitochondria origin produced severe hypotension associated with vasodilatation, hyperthermia, lung damage, blood clotting, and vascular leakage. Since human SIRS is associated with all these signs, we suggest that F-MIT may be the DAMP that leads to cardiovascular collapse as seen in sepsis-like syndrome or SIRS.
In 2010, Zhang and colleagues (20) demonstrated that injury releases mitochondrial DNA into the circulation with immune system activation. We observed that infusion of F-MIT, and not a mimetic of the biological structure of mitochondrial DNA, caused significant disturbances in the cardiovascular system. In support of these results, we also observed that trauma induces the release of mitochondrial ND6 in the circulation of rats that underwent hemorrhagic shock, a model of sterile trauma. In normal conditions, this protein is absent from plasma; however, we observed for the first time that after cell trauma and injury, this protein is unintentionally released into the circulation. It should also be reiterated that the formylated peptide used to perform the experiments was derived from mitochondria ND6 (9).
Lung injury, present in sepsis and SIRS, is characterized by pulmonary edema and inflammation. Hauser et al. (4) observed that fragmented mitochondria induced pulmonary inflammation via neutrophil activation. Accordingly, F-MIT were able to cause edema, neuthrophil infiltration, and alveolar septal thickening associated with increased MPO activity. Interestingly, hemorrhagic shock was able to cause lung damage via FPR activation. Based on these results, after trauma or cell damage, formylated peptides/proteins from mitochondria released into the circulation, leading to FPR activation and further lung injury.
West et al. (18) have shown that MAPKs in neutrophils are activated by injury. Zhang et al. (21) also observed that mitochondrial DNA, released by shock, induced neutrophil activation via p38 MAPK. Here, we observed that both exogenous and endogenous F-MIT (released after hemorrhagic shock-induced trauma) lead to lung injury via FPR and MAPK signaling.
The FPR family has been most extensively investigated in the context of leukocyte recruitment and activation, where FPR promotes cell motility and mediates host defense (6, 9). Also, neutrophil FPR activation with the bacterial peptide (fMLP) induces NADPH oxidase activation and ROS generation (6). However, the effects of FPR activation on the cardiovascular system have not been previously investigated. We have observed that both F-MIT and fMLP induce vasodilatation in resistance arteries and that FPR-1 antagonist inhibits this response (15). In the present study, FPR-1 or -2 antagonist and NO pathway inhibition abolished F-MIT-induced hypotension. These results demonstrate that F-MIT induce NO release via FPR activation. Since iNOS inhibitor abolished F-MIT-induced hypotension and FPR antagonist decreased iNOS protein expression in lung from rats subjected to hemorrhagic shock, it is possible to imply that iNOS is the specific intracellular effector following FPR activation. As FPR is expressed on neutrophils, mast cells, and basophils (3, 6, 8, 9), it is possible that one, if not all, of these cell types contributes to the F-MIT-induced sepsis-like syndrome. In fact, Zhang et al. (20) demonstrated neutrophil activation and chemotaxis after stimulation with mitochondrial DAMPs. However, we observed that neutrophils did not contribute to F-MIT-induced hypotension, as neutrophil depletion did not block this response. This suggests that neutrophil activation is only involved in F-MIT-induced lung and organ injury but not in F-MIT-induced hypotension.
In addition to neutrophils, it is known that stimulation of FPR by fMLP induces basophil and mast cells to release immunogenic compounds such as histamine and NO (3, 8). Furthermore, we demonstrated that histamine plays a role in F-MIT-induced hypotension since cimetidine completely abolished F-MIT-induced hypotension. To investigate the cellular origin of histamine, we selectively depleted animals of granulocytes (basophils or mast cells, independent of each other). We observed that basophil, but not mast cell, depletion abolished F-MIT-induced hypotension. These results infer that F-MIT, via FPR activation, may release both mediators NO and histamine from basophils, which subsequently decrease blood pressure and increase vascular permeability.
The major pathophysiological characteristic of sepsis-like syndrome is vascular collapse. F-MIT did not change acetylcholine-induced relaxation. However, arteries relaxed less when incubated in blood containing F-MIT or blood from rats treated with F-MIT compared with their respective controls. These results demonstrate that F-MIT induce endothelial dysfunction in a blood-dependent manner. The reason why F-MIT led to endothelial dysfunction in the presence of blood is unknown and needs to be the focus of future experiments. However, it is possible that F-MIT may activate FPR on leukocytes (neutrophil, basophils, and mast cells), and these leukocytes may cause vascular injury. In the absence of blood, F-MIT induced vasodilatation via mainly FPR-2 activation and partially via NO release. This result is interesting, since pronounced vasodilatation is a common characteristic from SIRS and sepsis. Moreover, F-MIT increased ROS generation in resistance arteries. We do not know why F-MIT induce vasodilatation associated with ROS generation. It is possible to speculate that FPR may lead to these responses via NAD(P)H activation, specifically via Nox4 since this isoform generates predominantly H2O2 (vasodilator in resistance arteries) rather than superoxide (11). Taken together, FPR is able to create an inflammatory environment in response to injury or pathogens and promote an immune response.
One of the common characteristics of both SIRS and sepsis is a fatal unresponsiveness to vasoconstrictive drugs, including adrenergic agonists (2, 12). We observed that F-MIT decreased phenylephrine-induced contraction in resistance arteries. These results further support the role that F-MIT cause sepsis-like syndrome and cardiovascular collapse (including hypotension, hyporeactivity to vasoconstrictors, vasodilatation, and endothelial dysfunction) as well as hyperthermia, vascular leakage, blood clotting, and lung injury. F-MIT release is increased in sterile shock and basophil depletion, blockade of histamine and FPR receptors, and the inhibition of NO pathway abolished the hypotensive effects of F-MIT. Future studies are warranted into the effects of chronic exposure to F-MIT, the differential involvement of FPR receptors subtypes, and chronic diseases associated with cell damage.
Evolutionarily, FPR activation in the arteries does not occur in physiological conditions, given that its agonists are peptides from bacteria and mitochondria. Therefore, it is possible that the vasodilatation and vascular permeability induced by FPR is not to control moment-to-moment blood pressure but to create an inflammatory environment in response to injury or pathogens and promote an immune response. Collectively, our findings provide a new and different way of considering the role of F-MIT in trauma-induced SIRS, vascular collapse, and vascular pathophysiology. As such, these peptides could be considered a putative target for the treatment of sterile inflammation.
GRANTS
This work was supported by grants from American Heart Association, National Institutes of Health, National Council for Scientific and Technological Development (CNPq-Brazil), and The Society for Women's Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.F.W. and R.C.W. conception and design of research; C.F.W. and C.G.M. performed experiments; C.F.W. and C.G.M. analyzed data; C.F.W. interpreted results of experiments; C.F.W. prepared figures; C.F.W. drafted manuscript; C.F.W., C.G.M., T.S., S.G., and R.C.W. edited and revised manuscript; C.F.W., C.G.M., T.S., S.G., and R.C.W. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of S. Goulopoulou: Dept. of Integrative Physiology and Anatomy, Univ. of North Texas Health Science Ctr., 3500 Camp Bowie Blvd., Fort Worth, TX.
REFERENCES
- 1.Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med 29, Suppl: S109–S116, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J. The immunopsthogenesis of sepsis. Nature 420: 885–891, 2002. [DOI] [PubMed] [Google Scholar]
- 3.de Paulis A, Prevete N, Fiorentino I, Walls AF, Curto M, Petraroli A, Castaldo V, Ceppa P, Fiocca R, Marone G. Basophils infiltrate human gastric mucosa at sites of Helicobacter pylori infection, and exhibit chemotaxis in response to H. pylori-derived peptide Hp(2–20). J Immunol 172: 7734–7743, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Hauser CJ, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, Itagaki K. Mitochondrial DAMPs from femoral reamings activate neutrophils via formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma 24: 534–538, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Le Y, Murphy PM, Wang JM. Formyl peptide receptors revisited. Trends Immunol 23: 541–548, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Peters K, Unger RE, Brunner J, Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res 60: 49–57, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Pundir P, Catalli A, Leggiadro C, Douglas SE, Kulka M. Pleurocidin, a novel antimicrobial peptide, induces human mast cell activation through the FPRL1 receptor. Mucosal Immunol 7: 177–187, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol 35: 2486–2495, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. J Vis Exp 73: e50062, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Remick DG. Pathophysiology of sepsis. Am J Pathol 170: 1435–1444, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med 9: 517–524, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg 258: 591–596, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenceslau CF, McCarthy CG, Goulopoulou S, Szasz T, NeSmith EG, Webb RC. Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis. Med Hypotheses 81: 532–535, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC. Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J 35: 1172–1179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenceslau CF, Rossoni LV. Rostafuroxin ameliorates endothelial dysfunction and oxidative stress in resistance arteries from deoxycorticosterone acetate-salt hypertensive rats: the role of Na+K+-ATPase/cSRC pathway. J Hypertens 32: 542–554, 2014. [DOI] [PubMed] [Google Scholar]
- 18.West MA, Koons A, Crandall M, Skinner R, Worley M, Shapiro MB. Whole blood leukocyte mitogen activated protein kinases activation differentiates intensive care unit patients with systemic inflammatory response syndrome and sepsis. J Trauma 62: 805–811, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Yaghi A, Paterson NA, McCormack DG. Vascular reactivity in sepsis: importance of controls and role of nitric oxide. Am J Respir Crit Care Med 151: 706–712, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock, and activates neutrophils via p38 map kinase. Shock 34: 55–59, 2010. [DOI] [PubMed] [Google Scholar]