Abstract
The cardiac insulin-like growth factor 2 receptor (IGF-2R) can induce cardiomyocyte hypertrophy in a heterotrimeric G protein receptor-coupled manner involving αq (Gαq) or αs (Gαs). We have previously shown increased left ventricular weight and cardiac IGF-2 and IGF-2R gene expression in low-birth-weight (LBW) compared with average-birth-weight (ABW) lambs. Here, we have investigated the cardiac expression of IGF-2 gene variants, the degree of histone acetylation, and the abundance of proteins in the IGF-2R downstream signaling pathway in ABW and LBW lambs. Samples from the left ventricle of ABW and LBW lambs were collected at 21 days of age. There was increased phospho-CaMKII protein with decreased HDAC 4 abundance in the LBW compared with ABW lambs. There was increased GATA 4 and decreased phospho-troponin I abundance in LBW compared with ABW lambs, which are markers of pathological cardiac hypertrophy and impaired or reduced contractility, respectively. There was increased histone acetylation of H3K9 at IGF-2R promoter and IGF-2R intron 2 differentially methylated region in the LBW lamb. In conclusion, histone acetylation of IGF-2R may lead to increased IGF-2R mRNA expression and subsequently mediate Gαq signaling early in life via CaMKII, resulting in an increased risk of left ventricular hypertrophy and cardiovascular disease in adult life.
Keywords: low birth weight, insulin-like growth factor 2 receptor, histone acetylation
there is a link between low birth weight (LBW) and an increased risk of death from cardiovascular disease (4), with individuals who are born small being more likely to have left ventricular hypertrophy (50). Animal models of intrauterine growth restriction and LBW in both rats and sheep have found not only increased relative heart weight but also left ventricular hypertrophy both before and after birth (5, 54, 57). This is important because left ventricular hypertrophy is the strongest predictor for progressive heart disease (21, 27) and occurs in the absence of hypertension. Therefore, understanding the mechanisms underlying left ventricular hypertrophy and the increased incidence of clinical events relating to cardiovascular disease may lead to successful therapies to prevent this pathogenesis (22, 35).
The IGF system regulates fetal and heart growth by activating nutritionally and oxygen-sensitive pathways (44), resulting in increased protein synthesis in cardiomyocytes, leading to proliferation and hypertrophy. The IGF-2 receptor (IGF-2R) has become a new focus for mechanistic studies in cardiac pathophysiology, because the IGF-2R can couple with the heterotrimeric G protein-coupled receptors and their αq subunits (Gαq) to induce cardiac hypertrophy via CaMKII, PKC-α, or p44/42 MAP kinase (ERK) (11, 51). Hypertrophy induced by activation of the Gαq signaling pathway can result in the expression of a “fetal gene program” (13), including increased atrial natriuretic peptide (ANP) and β-myosin heavy chain (β-MHC) abundance and reduced sarcoplasmic reticulum Ca2+ (SERCA2+) ATPase abundance (1, 7, 9, 15). GATA 4 is a regulator of cardiac gene expression, hypertrophy, stress compensation, and cardiomyocyte viability (43), which is involved in reexpression of the “fetal gene program” (39, 46). Activation of cardiac IGF-2R via Leu-27 IGF-2 infusion into the left circumflex coronary artery increases binucleated cardiomyocyte size and expression of the “fetal gene program” of the sheep fetus in a Gαs-dependent manner and subsequently increases phosphorylation of protein kinase A (PKA) (52).
The hearts of LBW lambs have increased IGF-2 and IGF-2R mRNA expression compared with hearts from average-birth-weight (ABW) lambs (54). There is also an inverse relationship between cardiac IGF-2R protein expression and left ventricle weight relative to body weight in the ABW lambs (54), which may reflect the traditional role of IGF-2R as a clearance receptor (31). This clearance role of IGF-2R in heart growth in late gestation has also been shown in a model of in vitro culture of the embryo (47). In contrast, a positive relationship between cardiac IGF-2R protein expression and relative left ventricular weight exists in LBW lambs (54), and this may indicate a role for IGF-2R in the LBW-induced left ventricular hypertrophy.
There was increased IGF-2 and IGF-2R mRNA expression, but no changes in the degree of methylation in the differentially methylated region (DMR) of the IGF-2/H19 locus and within intron 2 of IGF-2R in the hearts of LBW lambs compared with ABW lambs (54). In rat models of hypertrophy, there was an increase in IGF-2 and IGF-2R gene expression due to increased histone acetylation (10, 25, 26). Therefore, we aimed to determine the mRNA expression of four different exons of IGF-2 variants, downstream signaling of IGF-2R, specifically Gαq and PKA signaling pathways, and histone acetylation of IGF-2 promoter, IGF-2R promoter, and intron 2 DMR in the left ventricle of lambs after birth that may cause cardiac hypertrophy in the LBW lambs.
MATERIALS AND METHODS
Animals and Surgery
All procedures were approved by the University of Adelaide Animal Ethics Committee and complied with the Australian code of practice for the care and use of animals for scientific purposes.
Average birth weight lambs.
Ewes were naturally mated and delivered spontaneously at term. A frequency distribution curve of birth weights from 45 control Merino singleton lambs born during a 5-yr period in our laboratory was used to categorize lamb birth weights (16). The mean birth weight (± SD) of the control singleton cohort was 5.63 ± 0.67 kg. Thirteen Merino lambs were classified as average birth weight (ABW) (birth weight was within 2 SD of this mean value: 4.9–6.7 kg).
LBW lambs.
To induce placental restriction and a birth weight below 2 SD from the mean of controls (i.e., below 4.9 kg; low birth weight, LBW), nonpregnant ewes underwent surgery to remove the majority of endometrial caruncles from the uterus, leaving three to eight caruncles in each horn (14, 16, 17, 53). At least 10 wk later, the ewes were mated and delivered spontaneously. Lambs from both groups were fed by ewes for the first 3 wk of life.
Post Mortem and Tissue Collection
On postnatal day 21, lambs were humanely killed with an intravenous overdose of pentobarbital sodium delivered by intravenous injection (Virbac, Milperra, NSW, Australia). Left ventricle samples collected from each animal were frozen in liquid nitrogen and stored at −80°C. Information of the phenotype of the lambs (16) and study of cardiac growth (54) and metabolism (53) have been previously described. There were no changes in heart weight between groups, but the LBW lambs had a greater left ventricular weight relative to body weight [ABW: 3.34 ± 0.12 g/kg; LBW: 3.78 ± 0.12 g/kg; P < 0.05 (54)]. Analyses were performed subject to tissue availability and the exclusion of outliers (more than 2 SD), and, consequently, sample size was not identical for all measurements.
Protein Extraction and Western Blot Analysis
Approximately 50 mg of left ventricle (ABW: n = 13; LBW: n = 8) was homogenized (PT-MR-3100; Kinematica, Lucerne, Switzerland) for protein extraction, and protein contents were determined, as previously described (54). Prior to Western blot analysis, 20 μg of protein was subjected to SDS-PAGE and stained with Coomassie blue reagent (Thermo Fisher Scientific, Hanover Park, IL) to ensure equal loading of the proteins (40, 41). Then, 5 and 10 μg of the same protein sample was loaded onto each gel to confirm linearity of the chemiluminescent signal (41).
Equal volumes of proteins were subjected to SDS-PAGE and transferred to membrane, as previously described (40, 52). Each Western blot was stained with Ponceau S to visualize equal protein loading on each well. The membranes were then incubated with the respective primary antibody: Erk1/Erk2 (Cell Signaling Technology, Danvers, MA), phospho-Erk1/2 (Thr-202/Tyr-204; Cell Signaling Technology), calcineurin A (Abcam, Cambridge, UK), nuclear factor of activated T-cells (NFATc3; Santa Cruz Biotechnology, Santa Cruz, CA), phospho-NFATc3 (Ser-265; Santa Cruz Biotechnology), CaMKII (Cell Signaling Technology), phospho-CaMKII (Thr-286; Santa Cruz Biotechnology), histone deacetylases (HDAC) 4 (Cell Signaling Technology), phospho-HDAC 4 (Ser-632)/HDAC 5 (Ser-498) (Cell Signaling Technology), PKC-α (Cell Signaling Technology), phospho-PKC-α (Thr-638; Santa Cruz Biotechnology), HDAC 5 (Cell Signaling Technology), GATA 4 (Santa Cruz Biotechnology), ANP (Abcam), β-MHC (Millipore, Billerica, MA), phospho-troponin I (Ser-23/24; Cell Signaling Technology), SERCA2+ ATPase (Pierce, Thermo Fisher Scientific, Rockford, IL), phospho-PKA α/β/γ catalytic subunits (Thr-198; Santa Cruz Biotechnology), phospho-PKAII α regulatory subunit (Ser-96; Santa Cruz Biotechnology), cAMP response element-binding (CREB) (Cell Signaling Technology), and phospho-CREB (Ser-133; Cell Signaling Technology) overnight with agitation at 4°C and subsequently detected by enhanced chemiluminescence, as previously described (52). Protein abundance was quantified by densitometry by subtracting the background of each individual lane for background correction.
RNA Extraction and Quantitative Real-Time RT-PCR
RNA was isolated from the left ventricle (∼100 mg) of each lamb (ABW: n = 12; LBW: n = 7), and cDNA was synthesized as previously described (54). Controls containing no Superscript III (NAC) and no RNA transcript (NTC) were used to test for genomic DNA and reagent contamination, respectively.
The reference genes [hypoxanthine phosphoribosyltransferase 1 (45), phosphoglycerate kinase 1 (45), and peptidylprolyl isomerase A (45)] were selected due to their high stability in expression across samples. The relative expression of mRNA transcripts of IGF-2 at exon 3, exon 4, exon 6, and exon 7 (Table 1), and the reference genes were measured by quantitative RT-PCR using fast SYBR Green master mix (Applied Biosystems, Foster City, CA) in a final volume of 6 μl on a ViiA7 fast real-time PCR system (Applied Biosystems), as previously described (49).
Table 1.
Sequences of oligonucleotide primers used for quantitative real-time RT-PCR
| Gene | Forward and Reverse Primer Sequences |
|---|---|
| IGF-2 at exon 3 | F: TCACTGTTCACCTTGAGGAC |
| R: TCACAGCAGCTGACTTCC | |
| IGF-2 at exon 4 | F: CTTGGATGCGGGAAGTTTCT |
| R: GCAATGCCCAGTCGTTCT | |
| IGF-2 at exon 6–8 | F: CGCCGCTGTTCGGTTTG |
| R: CATCGACTTTCCTGCTGTGAT | |
| IGF-2 at exon 7–8 | F: CTCCTCCTCATCCTCCTTCA |
| R: CATCGACTTTCCTGCTGTGAT |
F, forward; R, reverse.
Primers were validated and reagents were tested for contamination, as previously described (53). Melt curve/dissociation curves were also run to check for nonspecific product formation. Amplification efficiency reactions were performed and determined, as previously described (53). Each sample was run in triplicate for target and reference genes. The reactions were quantified by setting the threshold within the exponential growth phase of the amplification curve and obtaining corresponding Ct values. DataAssist Software v3.0 (Applied Biosystems) (24) was used to find the 2−ΔCt, which shows the abundance of each transcript relative to the abundance of the three stable reference genes and is expressed as mean normalized expression.
Chromatin Immunoprecipitation Assay
Genomic DNA associated with specific histone proteins was analyzed from the left ventricle of each lamb (ABW: n = 11; LBW: n = 7) by chromatin immunoprecipitation (ChIP) assay. Nuclei were extracted from frozen left ventricle tissue with a Sigma Nuclei Pure Prep isolation kit (Sigma-Aldrich, Sydney, Australia). The tissues were first homogenized using a Dounce homogenizer in a lysis master mix (nuclei PURE lysis buffer, 0.1 M DTT, and 10% Triton X-100) and then filtered through a 100-μm and 70-μm cell strainer and centrifuged at 30,000 g for 45 min at 4°C through a sucrose solution to obtain isolated nuclei. Nuclei were resuspended in a micrococcal nuclease (MNase) buffer and incubated with MNase enzyme for 6 min before stopping the enzyme with 0.5 M EDTA to obtain sheared chromatin fragments. Shearing size for chromatin of 150–600 bp was confirmed by agarose gel electrophoresis. Histone-specific DNA was then isolated from the chromatin extracts using a Qiagen ChIP OneDay kit (Qiagen). Chromatin was precleared and then incubated with the target or control antibody H4K8ac, H3K9ac, RNA Pol III (positive control), or Rabbit IgG (negative control; Merck Millipore, Bayswater, VIC, Australia), on a rotating wheel at 4°C for 2 h. Samples were then mixed with Protein A beads and were rotated again at 4°C for 1 h and then washed five times with IP wash buffer and stored at 4°C. DNA was then isolated and purified from the protein/DNA immunoprecipitated samples using DNA Spin Columns (Qiagen). The relative expression of IGF-2 promoters (Table 2), IGF-2R promoters (Table 2), IGF-2R intron 2 DMR (Table 2), β-2-microglobulin (B2M) promoter (Table 2), tyrosine 3-monooxygenase (YWAHZ) promoter (Table 2), and ubiquitin C (UBC) promoter (Table 2), which acted as housekeeper genes, associated with specific antibody binding to genomic DNA was then analyzed by quantitative PCR on a ViiA7 fast real-time PCR system (Applied Biosystems).
Table 2.
Sequences of Oligonucleotide Primers Used for ChIP Assay
| Gene | Forward and Reverse Primer Sequences |
|---|---|
| IGF-2 promoter 1 | F: ACTGGACCCGGAAGAGGAG |
| R: CACCCCTGCCCCAATCTG | |
| IGF-2 promoter 2 | F: CCAAGTTCCACACTGAGGATT |
| R: TGAGGACCCAGGCATGTA | |
| IGF-2 promoter 4 | F: CGGCCCAGACATAAAAGCTG |
| R: AGGATGAGGAGGAGGAGGAG | |
| IGF-2R promoter | F: CCTGGTGTTACTTGGGTCATT |
| R: CACTGGACTCGGATGCAATTA | |
| IGF-2R intron 2 DMR | F: TTTAGACAGGCAAGGCAGAG |
| R: CGCCAATGGAGAGGAGATAATAA | |
| B2M promoter | F: TGATGTACAGGCAGCGAAGG |
| R: ATTCAGGCAGCCAATCGGAA | |
| YWAHZ promoter | F: GAAGGGTTGCGGGACATC |
| R: ACTTCTCTACTCCTGTCCTGAG | |
| UBC promoter | F: TTGTGCCTCAGAGCAGACAC |
| R: TTCTGCGGTGATTTTCCCGA |
Statistical Analysis
The effect of treatment (ABW vs. LBW) and sex on the expression of left ventricle proteins was determined using two-way ANOVA [Statistical Package for Social Scientists (SPSS) 18 for Windows, SPSS, Chicago, IL]. There was no significant effect of sex and no interaction between treatment and sex for any of the measured parameters, which may be due to the study not having enough power to measure sex effect (ABW: males, n = 8; females, n = 5; LBW: males, n = 3; females, n = 5). Thus, a Student's t-test was used to determine the effect of treatment (ABW vs. LBW). Data are presented as means ± SE. A probability of <5% (P < 0.05) was considered statistically significant.
RESULTS
Changes in mRNA Expression of IGF-2 Variants in ABW and LBW Lambs
There was increased mRNA expression of IGF-2 at exon 4 but not at exons 3, 6, and 7 (Table 3).
Table 3.
mRNA expression of IGF-2 variants in ABW and LBW lambs
| IGF-2 Variants (MNE) | ABW (n = 12) | LBW (n = 7) |
|---|---|---|
| Exon 3 | 0.0027 ± 0.0003 | 0.0033 ± 0.0007 |
| Exon 4 | 0.0047 ± 0.0004 | 0.0071 ± 0.0012* |
| Exon 6 | 3.3164 ± 0.2912 | 4.0178 ± 0.1746 |
| Exon 7 | 1.9355 ± 0.1134 | 2.2403 ± 0.1128 |
Values are expressed as means ± SE
Significantly different from ABW lambs (P < 0.05).
ABW, average birth weight; LBW, low birth weight; MNE, mean normalized expression.
Decreased Histone Acetylation at IGF-2 Promoter 2 Site
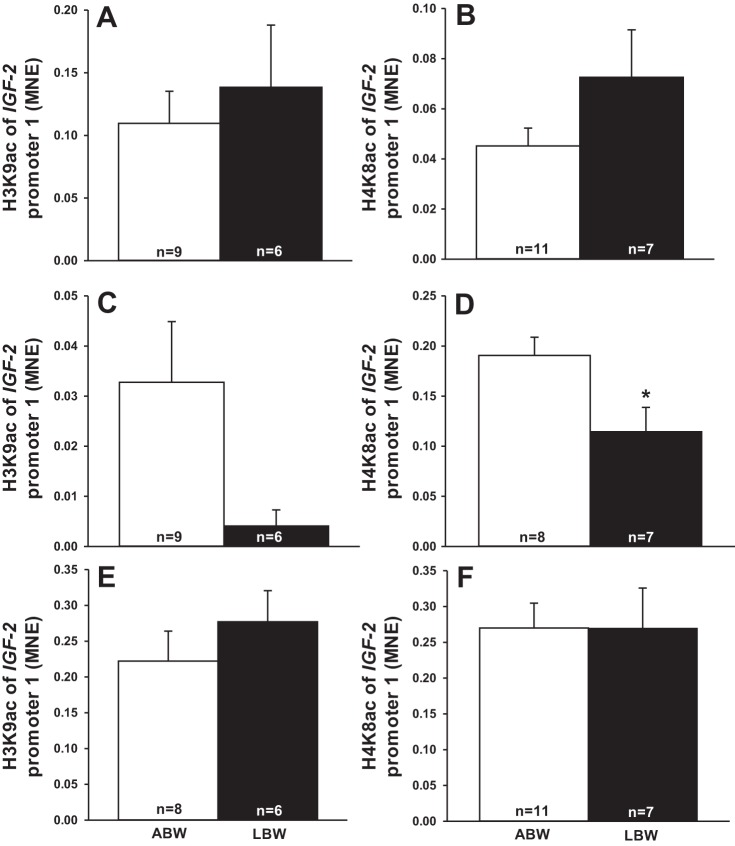
There was no change in acetylation of histone H3K9 (Fig. 1C) but decreased acetylation of histone H4K8 (Fig. 1D) of IGF-2 promoter 2. There were no changes in the acetylation of histone H3K9 and H4K8 at IGF-2 promoter 1 (Fig. 1, A and B) and IGF-2 promoter 4 (Fig. 1, E and F).
Fig. 1.
Low birth weight decreased histone acetylation at IGF-2 promoter 2. Acetylation of histone H3K9 and H4K8 at IGF-2 promoter 1 (A and B) in low birth weight (LBW) compared with average birth weight (ABW) lambs. Acetylation of histone H3K9 and H4K8 at IGF-2 promoter 2 (C and D) in LBW compared with ABW lambs. Acetylation of histone H3K9 and H4K8 at IGF-2 promoter 4 (E and F). Sample size for each group is indicated in the bar. *Significantly different from ABW lambs (P < 0.05). ABW, average birth weight (white bar); LBW, low birth weight (black bar); MNE, mean normalized expression.
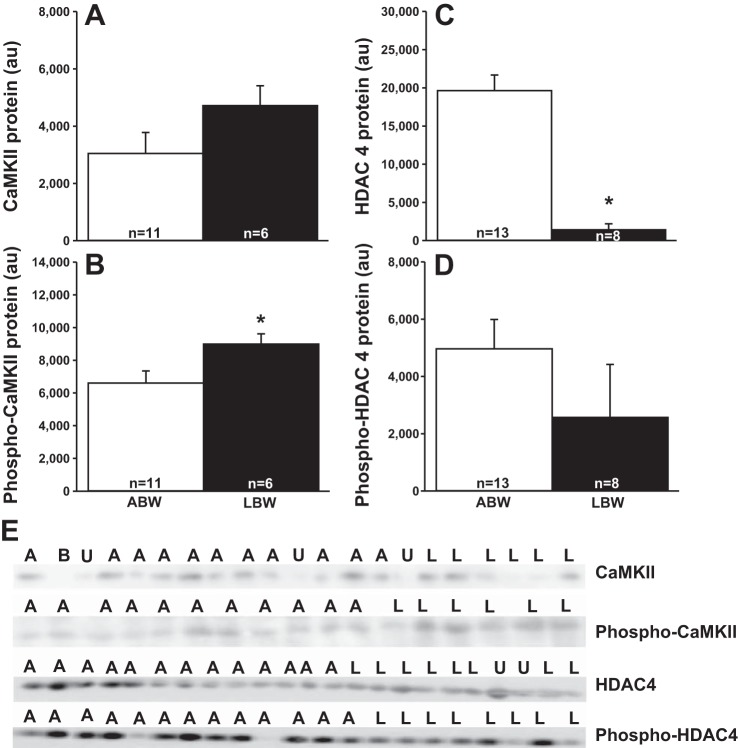
Cardiac CaMKII/HDAC 4 Signaling Pathway Is Activated in LBW Lambs
There was no difference in the cardiac CaMKII protein abundance (Fig. 2A) but increased phospho-CaMKII protein abundance (Fig. 2B) in LBW compared with ABW lambs. There was a reduction in HDAC 4 (Fig. 2C), but not phospho-HDAC 4 (Fig. 2D) protein abundance in LBW compared with ABW lambs.
Fig. 2.
Low birth weight activates a CaMKII and HDAC 4 pathway. Cardiac CaMKII (A), phospho-CaMKII (B), HDAC 4 (C) and phospho-HDAC 4 (D) protein in LBW compared with ABW lambs. Images of the Western blots for cardiac CaMKII, phospho-CaMKII, HDAC 4, and phospho-HDAC 4 abundance in ABW and LBW lambs (E). Sample size for each group is indicated in the bar. *Significantly different from ABW lambs (P < 0.05). ABW or A, average birth weight (white bar); LBW or L, low birth weight (black bar); B, blank; U, unanalyzable; au, arbitrary units.
There Was no Change in the Cardiac ERK, Calcineurin A/NFAT or PKC-α/HDAC 5 Signaling Pathways in LBW Lambs
There was no difference in calcineurin A, NFATc3, phospho-NFATc3, ERK, and phospho-ERK or PKC-α, phospho-PKC-α, HDAC 5, and phospho-HDAC 5 (Table 4) in the hearts of LBW and ABW lambs.
Table 4.
Protein abundance of downstream targets of Gαq in the hearts of LBW and ABW lambs
| Protein Abundance, au |
||
|---|---|---|
| ABW | LBW | |
| ERK | 93,387 ± 7,404 (12) | 10,6586 ± 6,566 (8) |
| Phospho-ERK | 5,959 ± 1,301 (12) | 4,300 ± 1,040 (8) |
| Calcineurin A | 2,693 ± 978 (5) | 1,907 ± 578 (5) |
| NFATc3 | 105 ± 20 (8) | 189 ± 61 (5) |
| Phospho-NFATc3 | 18,643 ± 2,706 (9) | 17,738 ± 3,639 (5) |
| PKC-α | 15,747 ± 4,331 (9) | 25,116 ± 8,825 (5) |
| Phospho-PKC-α | 6,130 ± 1,252 (9) | 7,459 ± 396 (5) |
| HDAC 5 | 334 ± 56 (13) | 350 ± 84 (8) |
| Phospho-HDAC 5 | 17,761 ± 2,307 (13) | 12,821 ± 2,042 (8) |
Values are expressed as means ± SE; n equals number within parentheses.
au, arbitrary units.
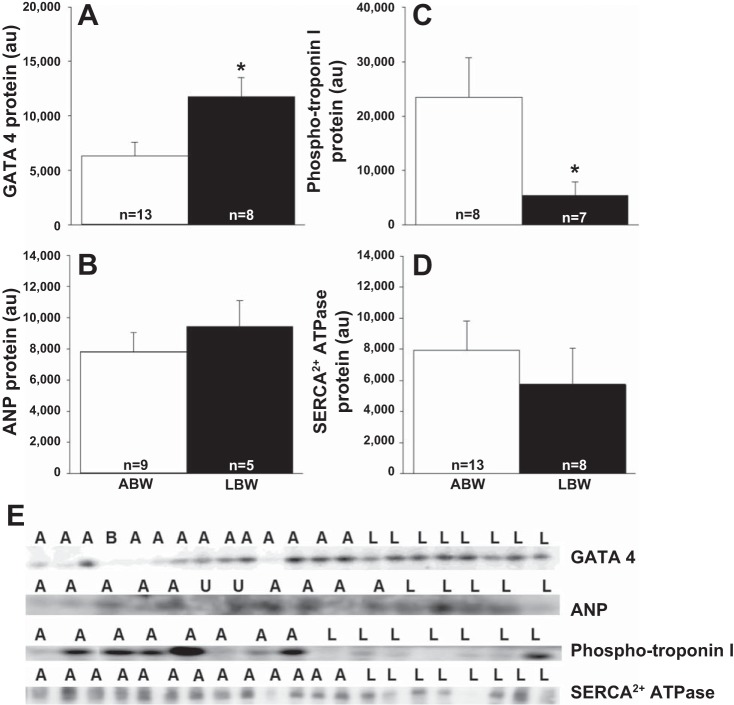
Markers of Pathological Cardiac Hypertrophy and Impaired Contractility Are Present in LBW Lambs
There was increased cardiac GATA 4 protein (Fig. 3A) in the LBW compared with ABW lambs; but no difference in ANP (Fig. 3B) or β-MHC abundance (ABW: 5,775.92 ± 636.50 au; LBW: 4,191.75 ± 788.41 au). There was a decreased phospho-troponin I (Fig. 3C) protein in the LBW compared with ABW lambs, but no difference in SERCA2+ ATPase protein (Fig. 3D).
Fig. 3.
Low birth weight affects cardiac pathological hypertrophy and contractility markers. Cardiac GATA 4 (A), ANP (B), phospho-troponin I (C), and SERCA2+ ATPase (D) in LBW compared with ABW lambs. Images of the Western blots for cardiac GATA 4, ANP, phospho-troponin I, and SERCA2+ ATPase abundance in ABW and LBW lambs (E). Sample size for each group is indicated in the bar. *Significantly different from ABW lambs (P < 0.05). ABW or A, average birth weight (white bar); LBW or L, low birth weight (black bar); B, blank; U, unanalyzable; au, arbitrary units.
Decreased Cardiac Phospho-Troponin I in LBW Lambs Is not Due to Activation of the PKA Signaling Pathway
There were no differences in phospho-PKA αβγ catalytic subunits, phospho-PKAII α regulatory subunit, CREB, or phospho-CREB proteins (Table 5) in the hearts of LBW and ABW lambs.
Table 5.
Protein abundance of downstream targets of Gαs in the hearts of LBW and ABW lambs
| Protein Abundance, au |
||
|---|---|---|
| ABW | LBW | |
| Phospho-PKA αβγ catalytic subunits | 47,622 ± 4,079 (8) | 54,775 ± 5,391 (7) |
| Phospho-PKAII α regulatory subunit | 840 ± 90 (12) | 648 ± 117 (8) |
| CREB | 570 ± 126 (8) | 914 ± 136 (7) |
| Phospho-CREB | 590 ± 78 (8) | 446 ± 85 (7) |
Values are expressed as means ± SE; n = number within paretheses.
ABW, average birth weight; LBW, low birth weight; au, arbitrary units.
Increased Histone Acetylation at IGF-2R Promoter and Intron 2 Sites
There was increased acetylation of histone H3K9 of IGF-2R promoter (Fig. 4A) and IGF-2R intron 2 DMR (Fig. 5A) but no changes in the acetylation of histone H4K8 (Figs. 4B and 5B).
Fig. 4.
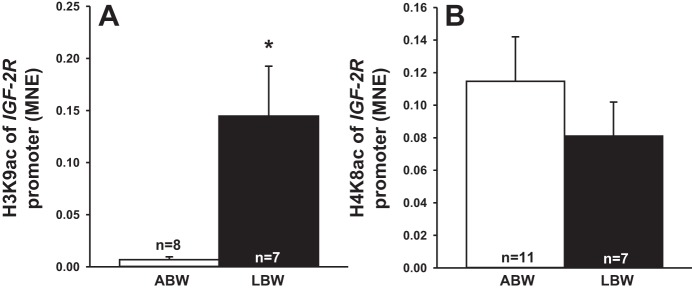
Low birth weight increased histone acetylation at IGF-2R promoter. Acetylation of histone H3K9 (A) and H4K8 (B) of IGF-2R promoter (A) in LBW compared with ABW lambs. Sample size for each group is indicated in the bar. *Significantly different from ABW lambs (P < 0.05). ABW, average birth weight (white bar); LBW, low birth weight (black bar).
Fig. 5.
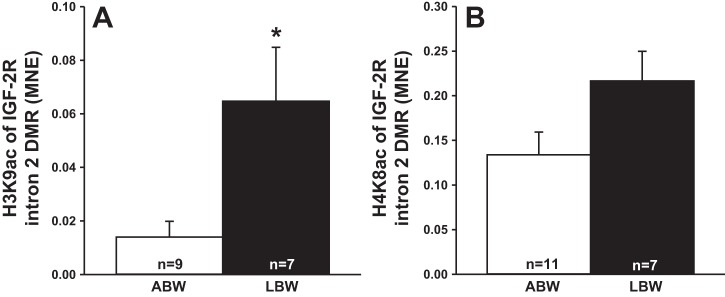
Low birth weight increased histone acetylation at IGF-2R intron 2 DMR. Acetylation of histone H3K9 (A) and H4K8 (B) of IGF-2R intron 2 DMR in LBW compared with ABW lambs. Sample size for each group is indicated in the bar. *Significantly different from ABW lambs (P < 0.05). ABW, average birth weight (white bar); LBW, low birth weight (black bar).
DISCUSSION
The IGF-2R is involved in the intracellular transport of lysosomal enzymes to late endosomes/lysosomes and is important for clearing IGF-2 (31). Although the IGF-2R does not contain tyrosine kinase activity or an autophosphorylation site, there is evidence for the ability of cardiac IGF-2R to couple to Gαq and induce cardiac hypertrophy (19, 23). In the heart of the LBW, the elevated expression of the IGF-2R was associated with increased histone acetylation in critical regions of the IGF-2R gene. Furthermore, the IGF-2R downstream pathway was activated in a Gαq-dependent, but not a Gαs-dependent manner, and this may lead to increased vulnerability to pathological cardiac hypertrophy in adult life.
IGF-2R Increased Gαq Signaling, But not Gαs Signaling in the Heart of LBW Lambs
The Gαq signaling pathway can induce cardiac hypertrophy effectors, and in rat cardiomyocytes, the IGF-2R can activate phospholipase C-β in a Gαq-dependent manner, which, in turn, causes phosphorylation of PKC-α and CaMKII, leading to pathological cardiac hypertrophy associated with the production of ANP and BNP (11). There are multiple Gαq downstream targets, but in the hearts of LBW lambs, neither the ERK nor PKC-α signaling pathways were activated, although there was increased abundance of phospho-CaMKII.
The decreased cardiac abundance of HDAC 4 in LBW lambs without changes in phospho-HDAC 4 (Ser-632) abundance may be due to the fact that CaMKII can also phosphorylate HDAC 4 at two other sites: Ser-467 (3) and Ser-210 (37), for which there are no commercially available antibodies. Therefore, we were unable to determine which site is phosphorylated by CaMKII leading to the subsequent reduced amount of HDAC 4 protein in hearts of the LBW lambs. However, CaMKII and HDAC 4 protein have been associated with cardiac pathology in human and animal models of cardiac hypertrophy (2, 28, 33, 58) and heart failure with an upregulation of CaMKII expression (29, 61).
In vitro IGF-2R activation leads to an increase in the cross-sectional area of binucleated cardiomyocytes from the late-gestation normally grown sheep fetus, in a CaMKII-dependent manner (51). We have shown that treating binucleated cardiomyocytes from the normally grown sheep fetus in late gestation with KN-93 (an inhibitor of CaMKII) alone or with Leu-27 IGF-2 (an IGF-2R agonist) inhibited the Leu-27 IGF-2-induced increase in cardiomyocyte size (51). Moreover, when CaMKII is inhibited, either by pharmacological or genetic approaches, there is a reduction in arrhythmias (30, 56) and hypertrophy (59); thus, placing CaMKII as a possible clinical target for LBW individuals with cardiac hypertrophy.
In the normally grown sheep fetus that had left circumflex coronary infusion of Leu-27 IGF-2, cardiac IGF-2R was upregulated, leading to cardiomyocyte hypertrophy in a Gαs-dependent manner via phosphorylation of PKA and CREB in late gestation (52). However, in the context of the impact of LBW, cardiac IGF-2R did not activate the Gαs-signaling pathway. Further studies investigating the triggering factor of cardiac IGF-2R on subsequent activation of Gαq (CaMKII) or Gαs (PKA) signaling would help to isolate the subsequent consequences of different downstream molecules.
Effect of LBW on Cardiac GATA 4, Pathological Hypertrophy, and Marker of Impaired Contractility
LBW did not have an effect on the calcineurin/NFAT pathway, which is supported by a lack of change in SERCA2+ ATPase protein between LBW and ABW lambs. Calcineurin/NFAT signaling is involved in the transcription and expression of SERCA2+ ATPase (48), a marker of heart failure and hypertrophy. In the heart, NFATs bind to the transcription factor GATA 4 and activate the transcription of genes involved in hypertrophy (55). GATA 4 has the ability to induce cardiac hypertrophy, as demonstrated by overexpression of GATA 4 in cultured cardiomyocytes and transgenic mice with GATA 4 overexpression (36). In adult mice, GATA 4 is required to maintain controlled cardiomyocyte hypertrophy and survival (8). In response to hypertrophic stimuli, GATA 4 can regulate reexpression of cardiac structural genes, such as ANP, β-MHC, and troponin I in the adult heart (32, 39, 46), which are widely accepted as markers for pathological cardiac hypertrophy (7) and a failing heart (6).
The increased abundance of GATA 4 was not associated with an increase in ANP or β-MHC abundance, all of which are markers of hypertrophy and known collectively as the fetal genotype. However, we observed a decrease in phospho-troponin I abundance that may indicate impaired cardiac contractility, which may contribute to diastolic and systolic dysfunction in heart failure by reducing cross-bridge-cycling rates (34). Cardiac hypertrophy and reduced contractility have previously been shown in the 3-mo-old offspring of dams fed a low-sodium diet during pregnancy, another model of intrauterine growth restriction (5). Therefore, there is a need to investigate lambs at an older age to further establish the relationship between LBW, pathological cardiac hypertrophy, and impaired contractility.
Role of IGF-2 and IGF-2R Histone Acetylation in Cardiac Programming
The persistent increase in both IGF-2 and IGF-2R mRNA expression before and after birth due to restriction of fetal substrate supply (54) leads us to propose that IGF-2 and IGF-2R are programmed in the fetus due to exposure to a suboptimal intrauterine environment. We have now shown that IGF-2 mRNA expression is increased at exon 4 and IGF-2R in the LBW lamb. Both IGF-2 and IGF-2R DNA methylation and histone acetylation are important regulators of their gene expression (25, 26, 38, 60). Although we have previously reported no difference in the degree of methylation of both IGF-2 and IGF-2R DMRs (54), we found decreased histone acetylation of H4K8 of IGF-2 promoter 2 and increased histone acetylation of H3K9 of IGF-2R promoter and IGF-2R intron 2 DMR. IGF-2 promoter 2 is linked to IGF-2 at exon 4 (18, 20, 42), so upregulation of IGF-2 at exon 4 mRNA expression may be linked to acetylation of IGF-2 promoter 2. The conflicting data between IGF-2 histone acetylation and gene expression may be due to the presence of transcription factors/enhancers that could influence the final outcome of gene transcription. The IGF-2 promoter 2 in bovine cardiac tissue is expressed in fetal tissues and completely absent in adults (12), suggesting that IGF-2 promoter 2 is normally silent in the heart due to epigenetics. The changes in acetylation of histone H4K8 of IGF-2 promoter 2 observed in LBW lambs may be opening IGF-2 mRNA exon 4 to allow translation that should be mostly silent. The IGF-2R data are consistent with a previous finding in that it is histone acetylation rather than the degree of methylation of IGF-2R that is essential for mediating IGF-2R gene expression (10).
Perspectives and Significance
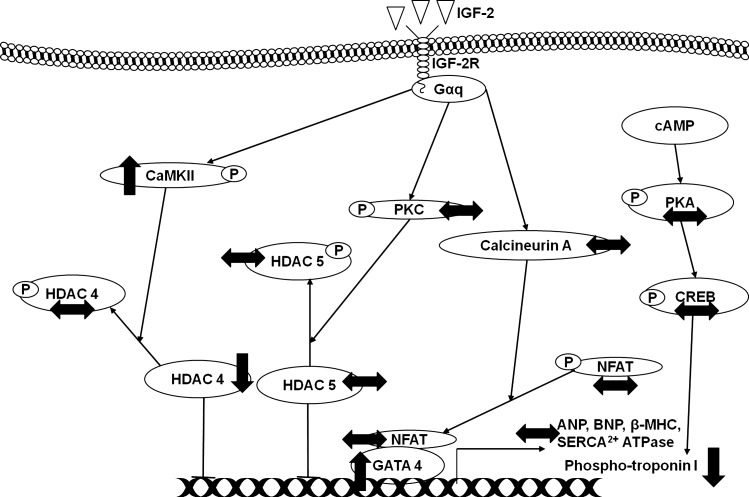
Our study has demonstrated, for the first time, that LBW increases phospho-CaMKII and GATA 4, but decreases HDAC 4 protein abundance, all markers of Gαq activation of pathological hypertrophy, in the hearts of LBW lambs (Fig. 6). Decreased phospho-troponin I abundance, indicative of impaired cardiac contractility, was also observed in the hearts of LBW lambs (Fig. 6). These data provide the first evidence that LBW leads to changes in IGF-2R and upregulation of the cardiac Gαq signaling pathway, which may contribute to a vulnerability to left ventricular hypertrophy and an increased risk of cardiovascular disease in adult life.
Fig. 6.
Activation of cardiac IGF-2R increases phospho-CaMKII and GATA 4 with decreased of HDAC 4 protein abundance but does not increase any of the pathological cardiac hypertrophy markers in the LBW lambs compared with ABW lambs at 21 days after birth. ↑, increased protein expression compared with ABW lambs; ↓, decreased protein expression compared with ABW lambs; ↔, no changes in protein expression compared with ABW lambs; P, phospho-protein.
GRANTS
The animal component of this project was funded by an National Health and Medical Research Council of Australia (NHMRC) Program Grant (to I. C. McMillen). The molecular analysis component of this project and J. L. Morrison were funded by a South Australian Cardiovascular Research Network Fellowship (CR10A4988). D. A. Brooks was supported by a Senior Research Fellowship from the NHMRC (349405).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.C.W.W., I.C.M., J.A.D., D.A.B., and J.L.M. conception and design of research; K.C.W.W., D.N.T., I.C.M., J.A.D., and J.L.M. performed experiments; K.C.W.W., D.N.T., S.Z., I.C.M., D.A.B., and J.L.M. analyzed data; K.C.W.W., D.N.T., S.Z., I.C.M., D.A.B., and J.L.M. interpreted results of experiments; K.C.W.W. prepared figures; K.C.W.W., D.A.B., and J.L.M. drafted manuscript; K.C.W.W., D.N.T., S.Z., I.C.M., J.A.D., D.A.B., and J.L.M. edited and revised manuscript; K.C.W.W., D.N.T., S.Z., I.C.M., J.A.D., D.A.B., and J.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Laura O'Carroll and Anne Jurisevic for their expert assistance during sheep surgery and the conduct of the protocols using the pregnant ewes and lambs in this study. We also thank Stacey Dunn for her assistance with the ChIP assay.
REFERENCES
- 1.Adams J, Sakata Y, Davis M, Sah V, Wang Y, Liggett S, Chien K, Brown J, Dorn IIGW. Enhanced Gaq signaling: A common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA 95: 10,140–10,145, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson ME. CaMKII and a failing strategy for growth in heart. J Clin Invest 119: 1082–1085, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 116: 1853–1864, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 327: 1077–1081, 1986. [DOI] [PubMed] [Google Scholar]
- 5.Battista MC, Calvo E, Chorvatova A, Comte B, Corbeil J, Brochu M. Intra-uterine growth restriction and the programming of left ventricular remodelling in female rats. J Physiol 565: 197–205, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 26: 2433–2441, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol Ther 128: 191–227, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, Rajagopal S, Son JK, Ma Q, Springer Z, Kang PM, SI, Pu WT. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci USA 103: 14,471–14,476, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien KR, Knowlton KU, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J 5: 3037–3046, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Chu CH, Lo JF, Hu WS, Lu RB, Chang MH, Tsai FJ, Tsai CH, Weng YS, Tzang BS, Huang CY. Histone acetylation is essential for ANG-II-induced IGF-IIR gene expression in H9c2 cardiomyoblast cells and pathologically hypertensive rat heart. J Cell Physiol 227: 259–268, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Chu CH, Tzang BS, Chen LM, Kuo CH, Cheng YC, Chen LY, Tsai FJ, Tsai CH, Kuo WW, Huang CY. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Gαq interaction and protein kinase C-α/CaMKII activation in H9c2 cardiomyoblast cells. J Endocrinol 197: 381–390, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Curchoe C, Zhang S, Bin Y, Zhang X, Yang L, Feng D, O'Neill M, Tian XC. Promoter-specific expression of the imprinted IGF2 gene in cattle (Bos taurus). Biol Reprod 73: 1275–1281, 2005. [DOI] [PubMed] [Google Scholar]
- 13.D'Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn IIGW. Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci USA 94: 8121–8126, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danielson L, McMillen IC, Dyer JL, Morrison JL. Restriction of placental growth results in greater hypotensive response to alpha-adrenergic blockade in fetal sheep during late gestation. J Physiol 563: 611–620, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorn IIGW, Robbins J, Sugden PH. Phenotyping hypertrophy: eschew obfuscation. Circ Res 92: 1171–1175, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Duffield JA, Vuocolo T, Tellam R, McFarlane JR, Kauter KG, Muhlhausler BS, McMillen IC. Intrauterine growth restriction and the sex-specific programming of leptin and peroxisome proliferator-activated receptor gamma (PPARγ) mRNA expression in visceral fat in the lamb. Pediatr Res 66: 59–65, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Dyer JL, McMillen IC, Warnes KE, Morrison JL. No evidence for an enhanced role of endothelial nitric oxide in the maintenance of arterial blood pressure in the IUGR sheep fetus. Placenta 30: 705–710, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Ekström TJ, Cui H, Li X, Ohlsson R. Promoter-specific IGF2 imprinting status and its plasticity during human liver development. Development 121: 309–316, 1995. [DOI] [PubMed] [Google Scholar]
- 19.El-Shewy HM, Luttrell LM. Insulin-like growth factor-2/mannose-6 phosphate receptors. Vitam Horm 80: 667–697, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Gebert C, Wrenzycki C, Herrmann D, Gröger D, Reinhardt R, Hajkova P, Lucas-Hahn A, Carnwath J, Lehrach H, Niemann H. The bovine IGF2 gene is differentially methylated in oocyte and sperm DNA. Genomics 88: 222–229, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Ghali JK, Liao Y, Simmons B, Castaner A, Cao G, Cooper RS. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med 117: 831–836, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 32: 1454–1459, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Harris LK, Westwood M. Biology and significance of signalling pathways activated by IGF-II. Growth Factors 30: 1–12, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu JF, Oruganti H, Vu TH, Hoffman AR. The role of histone acetylation in the allelic expression of the imprinted human insulin-like growth factor II gene. Biochem Biophys Res Commun 251: 403–408, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Hu JF, Pham J, Dey I, Li T, Vu TH, Hoffman AR. Allele-specific histone acetylation accompanies genomic imprinting of the insulin-like growth factor II receptor gene. Endocrinology 141: 4428–4435, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Kannel WB, Levy D, Cupples LA. Left ventricular hypertrophy and risk of cardiac failure: insights from the Framingham Study. J Cardiovasc Pharmacol 10: S135–S140, 1987. [PubMed] [Google Scholar]
- 28.Kee HJ, Kook H. Roles and targets of class I and IIa histone deacetylases in cardiac hypertrophy. J Biomed Biotechnol 2011: Article ID 928326, 10 p., 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Acitivity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res 42: 254–261, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kirchhof P, Fabritz L, Kilic A, Begrow F, Breithardt G, Kuhn M. Ventricular arrhythmias, increased cardiac calmodulin kinase II expression, and altered repolarization kinetics in ANP receptor-deficient mice. J Mol Cell Cardiol 36: 691–700, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Kornfeld S. Structure and function of the mannose 6-phosphate/insulin-like growth factor II receptors. Annu Rev Biochem 61: 307–330, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Ku PM, Chen LJ, Liang JR, Cheng KC, Li YX, Cheng JT. Molecular role of GATA binding protein 4 (GATA-4) in hyperglycemia-induced reduction of cardiac contractility. Cardiovasc Diabetol 10: 57, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurdi M, Booz GW. Three 4-letter words of hypertension-related cardiac hypertrophy: TRPC, mTOR, and HDAC. J Mol Cell Cardiol 50: 964–971, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 66: 12–21, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem 276: 30,245–30,253, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Little GH, Bai Y, Williams T, Poizat C. Nuclear calcium/calmodulin-dependent protein kinase II preferentially transmits signals to histone deacetylase 4 in cardiac cells. J Biol Chem 282: 7219–7231, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Long JE, Cai X. Igf-2r expression regulated by epigenetic modification and the locus of gene imprinting disrupted in cloned cattle. Gene 388: 125–134, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem 275: 38,949–38,952, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Muhlhausler BS, Duffield JA, Ozanne SE, Pilgrim C, Turner N, Morrison JL, McMillen IC. The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signalling in skeletal muscle. J Physiol 587: 4199–4211, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholas LM, Rattanatray L, Maclaughlin SM, Ozanne SE, Kleemann DO, Walker SK, Morrison JL, Zhang S, Muhlhäusler BS, Martin-Gronert MS, McMillen IC. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J 27: 3786–3796, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Ohlsson R, Hedborg F, Holmgren L, Walsh C, Ekström TJ. Overlapping patterns of IGF2 and H19 expression during human development: biallelic IGF2 expression correlates with a lack of H19 expression. Development 120: 361–368, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res 98: 837–845, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Owens JA, Kind KL, Carbone F, Robinson JS, Owens PC. Circulating insulin-like growth factors-I and -II and substrates in fetal sheep following restriction of placental growth. J Endocrinol 140: 5–13, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Passmore M, Nataatmadja M, Fraser JF. Selection of reference genes for normalisation of real-time RT-PCR in brain-stem death injury in Ovis aries. BMC Mol Biol 10: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pikkarainen S, Tokola H, Kerkela R, Ruskoaho H. GATA transcription factors in the developing and adult heart. Cardiovasc Res 63: 196–207, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Powell K, Rooke JA, McEvoy TG, Ashworth CJ, Robinson JJ, Wilmut I, Young LE, Sinclair KD. Zygote donor nitrogen metabolism and in vitro embryo culture perturbs in utero development and IGF2R expression in ovine fetal tissues. Theriogenology 66: 1901–1912, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Prasad AM, Inesi G. Silencing calcineurin A subunit reduces SERCA2 expression in cardiac myocytes. Am J Physiol Heart Circ Physiol 300: H173–H180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soo PS, Hiscock J, Botting KJ, Roberts CT, Davey AK, Morrison JL. Maternal undernutrition reduces P-glycoprotein in guinea pig placenta and developing brain in late gestation. Reprod Toxicol 33: 374–381, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Vijayakumar M, Fall CH, Osmond C, Barker DJ. Birth weight, weight at one year, and left ventricular mass in adult life. Br Heart J 73: 363–367, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang KC, Brooks DA, Botting KJ, Morrison JL. IGF-2R-mediated signaling results in hypertrophy of cultured cardiomyocytes from fetal sheep. Biol Reprod 86: 183, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Wang KC, Brooks DA, Thornburg KL, Morrison JL. Activation of IGF-2R stimulates cardiomyocyte hypertrophy in the late gestation sheep fetus. J Physiol 590: 5425–5437, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang KC, Lim CH, McMillen IC, Duffield JA, Brooks DA, Morrison JL. Alteration of cardiac glucose metabolism in association to low birth weight: experimental evidence in lambs with left ventricular hypertrophy. Metabolism 62: 1662–1672, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Wang KC, Zhang L, McMillen IC, Botting KJ, Duffield JA, Zhang S, Suter CM, Brooks DA, Morrison JL. Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J Physiol 589: 4709–4722, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkins BJ, Molkentin JD. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol 541: 1–8, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation 106: 1288–1293, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J 20: 1251–1253, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110: 479–488, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE Jr, Thiel W, Guatimosin S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med 11: 409–417, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Zhang S, Rattanatray L, MacLaughlin SM, Cropley JE, Suter CM, Molloy L, Kleemann D, Walker SK, Muhlhausler BS, Morrison JL, McMillen IC. Periconceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. FASEB J 24: 2772–2782, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res 63: 476–486, 2004. [DOI] [PubMed] [Google Scholar]