Abstract
Vibrio cholerae has a single polar sheathed flagellum that propels the cells of this bacterium. Flagellar synthesis, motility, and chemotaxis have all been linked to virulence in this human pathogen. V. cholerae expresses flagellar genes in a hierarchy consisting of σ54- and σ28-dependent transcription. In other bacteria, σ28 transcriptional activity is controlled by an anti-σ28 factor, FlgM. We demonstrate that the V. cholerae FlgM homologue (i) physically interacts with σ28, (ii) has a repressive effect on some V. cholerae σ28-dependent flagellar promoters, and (iii) is secreted through the polar sheathed flagellum, consistent with anti-σ28 activity. Interestingly, FlgM does not have a uniform repressive effect on all σ28-dependent promoters, as determined by measurement of σ28-dependent transcription in cells either lacking FlgM (ΔflgM) or incapable of secretion (ΔfliF). Further analysis of a ΔfliF strain revealed that this flagellar assembly block causes a decrease in class III (FlrC- and σ54-dependent) and class IV (σ28-dependent), but not class II (FlrA- and σ54-dependent), flagellar transcription. V. cholerae flgM and fliA (encodes σ28) mutants were only modestly affected in their ability to colonize the infant mouse intestine, a measure of virulence. Our results demonstrate that V. cholerae FlgM functions as an anti-σ28 factor and that the sheathed flagellum is competent for secretion of nonstructural proteins.
Studies of bacterial flagellar assembly have revealed that the flagellum is assembled in a stepwise fashion that begins by insertion of a type III export apparatus into the cytoplasmic membrane (reviewed in reference 27). Flagellar components are then secreted through this export machinery to be added to the growing tip of the flagellum in the specific order in which they are assembled (reviewed in reference 42). The bulk of the flagellum is composed of flagellin subunits, which are only added to the flagellum after the basal-body-hook structure is completed. Transcription of flagellar genes generally occurs in a hierarchical fashion that mirrors assembly of the nascent flagellum; i.e., the genes encoding early flagellar components are transcribed prior to the genes encoding late flagellar components, such as flagellin subunits (24; reviewed in reference 41).
In Salmonella enterica serovar Typhimurium, transcription of the flagellin gene is repressed until the basal-body-hook structure is completed, through the action of an anti-sigma factor, FlgM. The flagellin gene is transcribed by RNA polymerase (RNAP) containing the alternate sigma factor σ28 (encoded by fliA) (34). FlgM binds to σ28 and prevents its association with RNAP, preventing flagellin gene transcription (23). However, once the basal-body-hook structure is assembled, FlgM is secreted through the flagellar export apparatus to the extracellular milieu, which allows σ28 to associate with RNAP and transcribe the flagellin gene (15). Thus, the function of FlgM is to couple flagellar assembly to appropriate temporal flagellar gene transcription.
Flagellar gene transcription in Vibrio cholerae, which possesses a single polar sheathed flagellum, is also organized into a transcription hierarchy (35). However, the four-tiered transcription hierarchy has notable differences from the three-tiered hierarchy of S. enterica serovar Typhimurium, which possess multiple peritrichous flagella. In V. cholerae, the genes encoding the early structural components of the flagellum (basal-body-hook) are transcribed in two distinct temporal classes (II and III) by RNAP containing the alternate sigma factor σ54; in contrast, transcription of the equivalent genes in S. enterica serovar Typhimurium occurs within the single “early” temporal class (II) by RNAP containing the housekeeping σ70 subunit. However, the last temporal class of flagellar genes in V. cholerae (class IV), as in S. enterica serovar Typhimurium (class III), is transcribed by RNAP containing σ28. The main σ28-dependent genes are four distinct flagellin genes, flaB, flaC, flaD, and flaE (20, 35). σ54-dependent transcription of a fifth flagellin gene, flaA, precedes transcription of the other four flagellin genes (20). The four-tiered σ54- and σ28-dependent flagellar transcription hierarchy of V. cholerae (35) is likely to be identical to the polar hierarchy of V. parahaemolyticus (18), is remarkably similar to those of Pseudomonas aeruginosa (3) and Campylobacter jejuni (14), and has similarities to that of Helicobacter pylori (1), suggesting that this flagellar hierarchy may be common among gram-negative bacteria with polar flagella.
The σ54-dependent FlaA flagellin is essential for V. cholerae motility and flagellar synthesis, while the σ28-dependent FlaB, FlaC, FlaD, and FlaE flagellins are largely dispensable both singly and in combination (20). A fliA strain (lacking σ28) is nonmotile and does not transcribe flaB, flaC, flaD, or flaE but produces a truncated flagellum, indicating that some FlaA subunits are assembled (35). Because FlaA transcription and assembly into the flagellum appear to precede the transcription and incorporation of the other flagellin subunits, it suggests that the checkpoint that controls σ28-dependent transcription may differ between V. cholerae and S. enterica serovar Typhimurium, where completion of the basal-body-hook structure causes the export of FlgM and σ28-dependent transcription (17). Also, the presence of a sheath enveloping the V. cholerae flagellum, which is contiguous with the outer membrane (7), could inhibit or prevent the secretion of nonstructural proteins, such as an anti-sigma factor, through the flagellum; S. enterica serovar Typhimurium flagella are not sheathed. These observations invited the question of whether V. cholerae FlgM functions similarly to FlgM of S. enterica serovar Typhimurium.
FlgM homologues have been identified and characterized in the gram-negative polar flagellates H. pylori (1, 16) and P. aeruginosa (6). These studies demonstrated that the FlgM homologues in these bacteria physically interact with σ28 and inhibit σ28-dependent transcription, but they did not address the question of whether FlgM is secreted through the flagellum. In fact, Josenhans et al. suggested that FlgM is not secreted through the sheathed H. pylori flagellum (16). A report on a flgM C. jejuni mutant (14) demonstrated that the FlgM of that organism has only weak repressive activity on σ28-dependent transcription but also did not address FlgM secretion. We report here that the V. cholerae FlgM homologue has the characteristics of the S. enterica serovar Typhimurium anti-σ28 factor: it physically interacts with σ28, it represses transcription from some σ28-dependent promoters, and it is secreted through the (polar sheathed) flagellum.
MATERIALS AND METHODS
Bacterial strains and media.
Escherichia coli strain DH5α (11) was used for cloning manipulations, SM10λpir (32) was used to transfer plasmids to V. cholerae by conjugation, and BL21(DE3) (Novagen) was used for protein expression. The V. cholerae strains used in this study are listed in Table 1. Construction of chromosomal deletions and insertions with pKEK229, a pCVD442 derivative with the sacB gene (4), has been described previously (2). Bacteriophage CP-T1ts-mediated transduction (12) was used to construct KKV1441(ΔflgM::Cmr); the correct construction of all strains was verified by PCR and sequencing.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotide primers used in this study
| Strain, plasmid, or primer | Relevant genotype description or sequence (5′-3′) | Source or reference |
|---|---|---|
| V. cholerae strains | ||
| O395 | Wild type classical Ogawa | 30 |
| KKV598 | O395, ΔlacZ | 2 |
| KKV1113 | O395 ΔfliA ΔlacZ | 35 |
| KKV1247 | O395 ΔfliF ΔlacZ | This study |
| KKV1365 | O395 ΔfliA ΔflgM::Cm ΔlacZ | This study |
| KKV1384 | O395 ΔflgM ΔlacZ | This study |
| KKV1441 | O395 ΔflgM::Cm ΔlacZ | This study |
| KKV1461 | O395 ΔflgM ΔlacZ | This study |
| Plasmids | ||
| pKEK229 | R6K ori sacB mob Ampr | 2 |
| pKEK389 | ΔflgM in pKEK229 | This study |
| pKEK424 | ΔfliF in pKEK229 | 25 |
| pKEK427 | ΔflgM::Cm in pKEK229 | This study |
| pET15b | ColE1 ori His tag T7lac promoter Ampr | Novagen |
| pKEK460 | FLAG-flgM in pET15b | This study |
| pKEK462 | fliA in pET15b | This study |
| pKEK470 | p15A ori T7lac promoter, FLAG-flgM Cmr | This study |
| pBAD24 | ColE1 ori AmpraraBAD promoter | 10 |
| pKEK474 | FLAG-flgM in pBAD24 | This study |
| pRS551 | Transcriptional lacZ fusion vector Ampr Kanr | 38 |
| pKEK72 | flrB promoter-lacZ fusion in pRS551 | 21 |
| pKEK76 | flaC promoter-lacZ fusion in pRS551 | 20 |
| pKEK77 | flaD promoter-lacZ fusion in pRS551 | 20 |
| pKEK79 | flaB promoter-lacZ fusion in pRS551 | 20 |
| pKEK81 | flaE promoter-lacZ fusion in pRS551 | 20 |
| pKEK80 | flaA promoter-lacZ fusion in pRS551 | 20 |
| pKEK327 | fliE promoter-lacZ fusion in pRS551 | 35 |
| pKEK329 | flhA promoter-lacZ fusion in pRS551 | 35 |
| pKEK331 | flgK promoter-lacZ fusion in pRS551 | 35 |
| pKEK332 | flgB promoter-lacZ fusion in pRS551 | 35 |
| pKEK415 | flaG promoter-lacZ fusion in pRS551 | 35 |
| Primers | ||
| FLGMDEL1 | GCGGATCCGTGTGGGTGATGTGATTGAAC | |
| FLGMDEL2 | GCGAATTCGCTGGCATCAGAAGAACGCAC | |
| FLGMDEL3 | GCGAATTCGGCTCTTACGTGGTTGACCCT | |
| FLGMDEL4 | GCGAAGCTTCGGATTTGACTCACCAACTCA | |
| FLIANDEI | GGGGAACCTTCATATGAATAAAGCGC | |
| FLIAUBAMHI | GCGGATCCTTAGTCATTCCGAGTCCAAGAAC | |
| FLGMUBAMHI | GCGGATCCAGGTTAGAAGCCGCCCAGTTC | |
| FLAGFLGM | CGCGCCATGGACTACAAGGATGACGACGACAAGCATATGGCAGGTATTGATAACATACGC |
Luria broth (LB) was used for both liquid and agar media. Antibiotics were added when appropriate at the following concentrations: ampicillin, 50 μg/ml; chloramphenicol, 2 or 20 μg/ml (for V. cholerae and E. coli, respectively); streptomycin, 100 μg/ml. Agar plates consisting of LB with 0.3% agar were used to measure motility. For counterselection with sacB-containing plasmids, LB without NaCl and with 10% sucrose was used.
Plasmid construction.
All of the plasmids and oligonucleotide primers used are listed in Table 1. V. cholerae O395 chromosomal DNA was used as the template for PCR amplification. The in-frame deletion in flgM (ΔflgM) was constructed by first amplifying the 5′ fragment with oligonucleotides FLGMDEL1 and FLGMDEL2, digesting the amplified product with EcoRI and BamHI, and then ligating it into similarly digested pWSK30 (39) to form pKEK375. The 3′ fragment was PCR amplified with oligonucleotides FLGMDEL3 and FLGMDEL4, digested with EcoRI and HindIII, and ligated into similarly digested pKEK375 to form pKEK381 (ΔflgM). This results in the removal of 165 nucleotides that correspond to amino acids 29 to 83 of the predicted gene product. The chloramphenicol acetyltransferase gene from pACYC184 (37) was amplified by PCR with primers CAT1 and CAT2 (22), digested with MfeI, and ligated into pKEK381 digested with EcoRI to form pKEK411 (ΔflgM::Cmr). The ΔflgM and ΔflgM::Cmr mutations were moved into plasmid pKEK229 via NotI and SalI digestion and ligation to form pKEK389 and pKEK427, respectively.
To construct a plasmid for the expression of FlgM with an amino-terminal FLAG tag (FLAG-FlgM), flgM was PCR amplified with primers FLAGFLGM and FLGMUBAMHI, digested with NcoI and BamHI, and ligated into similarly digested pET15b (Novagen) to form pKEK460. pKEK460 was then digested with PshAI and HindIII and ligated with a PshAI-HindIII fragment of pACYC184 (37) to generate pKEK470. This plasmid still contains the T7lac promoter and FLAG-FlgM, but with p15A ori and Cmr from pACYC184. For expression of FLAG-FlgM in V. cholerae, pKEK470 was digested with NcoI and HindIII and ligated into similarly digested pBAD24 (10) to form pKEK474. The plasmid used for expression of σ28 with an amino-terminal His6 tag (His-σ28) was constructed by PCR amplification of fliA with primers FLIANDEI and FLIAUBAMHI, followed by digestion with NdeI and BamHI and ligation into similarly digested pET-15b to form pKEK462.
β-Galactosidase assays.
V. cholerae strains were transformed with the promoter-lacZ fusion-containing plasmids listed in Table 1, grown in LB plus antibiotic, and then harvested at an optical density at 600 nm of ∼0.2 to 0.4. Bacterial cells were permeabilized with chloroform and sodium dodecyl sulfate (SDS) and assayed for β-galactosidase activity by the method of Miller (31). All experiments were performed at least three separate times.
Electron microscopy.
Strains were grown to mid-log phase in LB, centrifuged, and then resuspended in 0.15 M NaCl. Samples were allowed to adhere to a carbon-coated grid and stained with 1% uranyl acetate before microscopy with a JEOL 1230 microscope. The AMT software was used to measure the lengths of flagella on wild-type and ΔflgM mutant V. cholerae strains (25 of each), which were determined to be significantly different by Student's two-tailed t test.
In vivo colonization assays.
The infant mouse colonization assay has been described previously (9). The inocula consisted of ∼106 CFU for both wild-type and mutant bacteria.
Protein detection.
For coprecipitation of His-σ28 and FLAG-FlgM, plasmids pKEK470 and/or pKEK462 were transformed into BL21(DE3) and expression of the proteins was induced by growth in LB with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 5 h at 37°C. Supernatants of sonicated cells were mixed with anti-His Dynabeads (Dynal Biotech), pelleted, and eluted in accordance with the manufacturer's instructions.
For detection of FLAG-FlgM secretion, KKV598 (wild type) and KKV1247 (ΔfliF) were transformed with pKEK474. These strains were grown in LB plus 0.1% arabinose at 37°C for 2 h. Samples were removed and centrifuged at 17,000 × g for 30 min. The supernatants were passed through a 0.2-μm syringe filter and concentrated with 10% trichloroacetic acid (TCA). Samples were then centrifuged (68,000 × g for 1.5 h), and the pellets were washed twice with cold acetone.
Samples were separated by SDS-15% polyacrylamide gel electrophoresis, probed by Western immunoblotting with either His-Tag (Novagen) or anti-FLAG M2 (Sigma) monoclonal antibody, and detected with ECL detection reagent (Amersham-Pharmacia).
RESULTS
Interaction of FlgM with σ28.
The gene encoding a FlgM homologue was identified in the V. cholerae genome sequence (VC2204) (13). The flgM gene is predicted to encode a protein of 11.3 kDa. As we noted previously (35), V. cholerae FlgM has homology with S. enterica serovar Typhimurium FlgM (28% identity). If V. cholerae FlgM functions as an anti-σ28 factor, it is predicted to physically interact with σ28 (FliA). To detect a physical interaction between FlgM and σ28, His-tagged V. cholerae σ28 and FLAG-tagged V. cholerae FlgM were coexpressed from different compatible plasmids within E. coli (Fig. 1). Magnetic metal chelation beads (anti-His; Dynal Biotech) were then used to precipitate His-σ28 from the E. coli lysate. Eluates from the magnetic beads were separated by SDS-polyacrylamide gel electrophoresis and then probed with either anti-His or anti-FLAG antibodies by Western immunoblotting.
FIG. 1.
Interaction of V. cholerae FlgM with σ28. His-σ28 and FLAG-FlgM were expressed separately and together in E. coli BL21(DE3), which contains T7 polymerase, from T7 promoters in plasmids pKEK462 and pKEK470, respectively, and magnetic anti-His beads (Dynal Biotech) were used to capture His-tagged protein from cell lysates as described in Materials and Methods. In the upper panel, samples were separated on Coomassie-stained polyacrylamide gel, and the lower panels show Western immunoblots of these same samples probed with anti-His tag and anti-FLAG tag antibodies. Masses of molecular size markers are noted on the left in kilodaltons, and arrows depict migration of His-σ28 and FLAG-FlgM. Lane numbers correspond to BL21(DE3) cells expressing His-σ28 (lane 1, whole-cell lysate; lane 2, anti-His bead eluate), FLAG-FlgM (lane 3, whole-cell lysate, lane 4, anti-His bead eluate), and His-σ28 and FLAG-FlgM (lane 5, whole-cell lysate, lane 6, anti-His bead eluate).
When His-σ28 is expressed alone, it is efficiently captured by the anti-His magnetic beads (lane 2); in contrast, when FLAG-FlgM is expressed alone, there is no detectable capture of this protein by the anti-His magnetic beads (lane 4), despite the presence of a large amount of FlgM protein in the lysate (lane 3). However, when His-σ28 and FLAG-FlgM are coexpressed within the same cell, capture of His-σ28 by magnetic beads causes the cocapture of a large amount of FLAG-FlgM (lane 6). These results demonstrate protein-protein interaction between His-σ28 and FLAG-FlgM, consistent with the predicted anti-σ28 activity of FlgM.
Secretion of FlgM through the V. cholerae flagellum.
The S. enterica serovar Typhimurium FlgM protein is secreted through the flagellar export apparatus. To monitor secretion of V. cholerae FlgM through the flagellar export apparatus, a FLAG tag was fused to the N terminus of FlgM to facilitate detection; a FLAG tag at the N terminus of S. enterica serovar Typhimurium FlgM does not inhibit secretion through the flagellum (K. Hughes, personal communication). To determine if the V. cholerae FlgM protein is likewise secreted through the flagellum, the FLAG-FlgM protein was expressed in a wild-type (flagellated) V. cholerae strain. FLAG-FlgM was also expressed within a ΔfliF V. cholerae strain, which lacks the MS ring, the first component assembled in the flagellum, and thus this strain lacks any flagellar structure or export apparatus. Protein in the filtered supernatant was concentrated by TCA precipitation, separated by SDS-polyacrylamide gel electrophoresis, and then subjected to Western immunoblot analysis with anti-FLAG antibodies.
FLAG-FlgM could be detected both in the supernatant (S) and in the cell pellet (P) of the wild-type (flagellated) strain (WT, Fig. 2). However, FLAG-FlgM was only found within the cell pellet (P) of the ΔfliF (nonflagellated) strain (fliF) and there was no detectable FLAG-FlgM present in the supernatant (S). These results indicate that there is a requirement for the flagellum to achieve secretion of FlgM into the supernatant, consistent with FlgM secretion through the V. cholerae polar (sheathed) flagellum. Interestingly, the FlgM protein in the supernatant has a slightly different mobility in the gel than the FlgM protein within the cells, suggesting either some C-terminal processing or an SDS-resistant conformational change.
FIG. 2.
FlgM is secreted through the V. cholerae flagellum. FLAG-FlgM was expressed from plasmid pKEK474 in V. cholerae strains KKV598 (wild type [WT]) and KKV1247 (fliF mutant). Bacterial pellets (P) and supernatants (S) were processed for Western immunoblot analysis with anti-FLAG antibodies as described in Materials and Methods.
Construction of a V. cholerae flgM mutant.
To study the function of V. cholerae FlgM, we constructed a strain with an in-frame deletion within the flgM gene. Construction of this strain was difficult; our initial attempts to introduce the ΔflgM mutation into the chromosome of a wild-type V. cholerae strain via suicide plasmid integration into the chromosome and subsequent sucrose counterselection to remove the plasmid were unsuccessful. Only wild-type (flgM+) strains were recovered, despite multiple attempts. We reasoned that this may be due to the deleterious effect of unregulated σ28 activity in a flgM strain, so the attempt at strain construction was repeated with a ΔfliA strain, KKV1113 (35). In the ΔfliA background, the ΔflgM chromosomal mutation was readily obtained; approximately one-half of the sucrose-resistant colonies contained this mutation. These results demonstrate that there is selective pressure against obtaining a ΔflgM mutation in a fliA+ strain background, suggesting that flgM exerts its effects through fliA.
To obtain a fliA+ strain with the ΔflgM mutation, we first constructed a ΔflgM::Cmr chromosomal mutation in ΔfliA mutant strain KKV1113 via suicide plasmid pKEK427 (strain KKV1365) and then moved the ΔflgM::Cmr mutation into the wild-type (fliA+) strain (KKV1441) via CP-T1ts-mediated transduction (12). Cmr transductants in the fliA+ background took 2 days to form colonies on plates, compared to Cmr transductants in the fliA mutant background, which only took 1 day to form colonies. PCR analysis demonstrated that these Cmr transductants had a chromosomal ΔflgM::Cmr mutation (not shown). The ΔflgM::Cmr mutation was then replaced with ΔflgM (without any insertion) via suicide plasmid pKEK389. In this manner we constructed ΔflgM mutant V. cholerae strain KKV1461. Interestingly, although the ΔflgM strain took 2 days to form colonies on selective plates, its growth curve is indistinguishable from that of the wild-type strain in liquid LB (not shown).
Motility of the ΔflgM mutant strain was measured in motility agar plates (Fig. 3A). The swimming pattern of this strain was slightly reduced compared to that of the wild-type strain, indicating that the ΔflgM mutation has a modest negative effect on the ability of the bacteria to swim from the point of inoculation in this assay. In contrast, the ΔfliA mutation abolished motility, as we have shown previously (35). A ΔfliF mutant strain, which contains a mutation in the structural gene for the MS ring, the first component of the flagellum assembled (27), is also nonmotile in this assay, as expected. A flgM mutant of P. aeruginosa showed a significant motility defect in this assay (6), suggesting possible differences between the function of V. cholerae FlgM and that of P. aeruginosa FlgM.
FIG. 3.
Motility phenotype and electron micrograph of a V. cholerae flgM strain. (A) V. cholerae strains KKV598 (wild type [WT]), KKV1114 (fliA mutant), KKV1461 (flgM mutant), and KKV1247 (fliF mutant) were inoculated into motility agar and incubated at 30°C. Electron micrographs of V. cholerae strains KKV598 (wild type) (B) and KKV1461 (flgM) (C) are also shown. Bars are equivalent to 2 μm.
ΔflgM mutant V. cholerae cells were visualized by electron microscopy (Fig. 3C). The cells possessed single polar flagella that were noticeably longer than those of the wild-type strain (Fig. 3B). Measurement of flagella of the wild-type and ΔflgM mutant strains revealed a significantly longer flagellum (P = 0.001) in the ΔflgM strain (6.95 ± 1.18 μm) in comparison to that of the wild-type strain (5.71 ± 1.42 μm), consistent with the notion that FlgM plays a repressive role in flagellar length.
FlgM has repressive effects on σ28-dependent transcription.
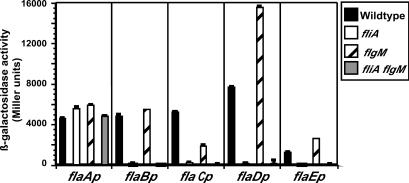
If FlgM functions as an anti-σ28 factor, its absence in a ΔflgM mutant would be predicted to cause an increase in σ28-dependent transcription. Transcription of the five V. cholerae flagellins was measured by promoter-lacZ transcriptional fusion plasmids (20) in the wild-type, ΔfliA, ΔflgM, and ΔfliA ΔflgM strains (Fig. 4). The flaA promoter is a σ54-dependent class III promoter, while the flaB, flaC, flaD, and flaE promoters are σ28-dependent class IV promoters (35).
FIG. 4.
Expression of flagellin promoters in fliA and flgM mutant V. cholerae strains. V. cholerae strains KKV598 (wild type), KKV113 (fliA mutant), KKV1461 (flgM mutant), and KKV1384 (fliA flgM mutant) carrying plasmids pKEK80 (flaAp-lacZ), pKEK79 (flaBp-lacZ), pKEK76 (flaCp-lacZ), pKEK77 (flaDp-lacZ), and pKEK81 (flaEp-lacZ) were assayed for β-galactosidase activity during logarithmic growth in LB. Assays were performed in triplicate, and standard deviations are shown.
As we have shown previously, the lack of σ28 (in a ΔfliA mutant) eliminates transcription of the flaB, flaC, flaD, and flaE promoters but has no effect on flaA transcription compared with transcription in a wild-type strain (35). The lack of FlgM (in the ΔflgM strain) had an approximately twofold stimulatory effect on flaD and flaE transcription, in comparison with transcription in the wild-type strain. Oddly, there was a decrease in flaC transcription in the ΔflgM strain, and transcription of the flaB promoter was unaffected by the lack of FlgM. The effect of the lack of FlgM seen at these four promoters is due to σ28-dependent transcription because introduction of a ΔfliA mutation into the ΔflgM background (the ΔfliA ΔflgM strain) eliminates flaB, flaC, flaD, and flaE transcription. There was no effect of the ΔflgM or ΔfliA ΔflgM mutations on flaA transcription. Our results demonstrate a repressive effect of FlgM on σ28-dependent transcription of flaD and flaE, as would be expected of an anti-σ28 factor. However, σ28-dependent transcription of the flaB and flaC promoters was either unaffected or increased in the presence of FlgM, indicating a lack of uniform repressive FlgM activity at all σ28-dependent promoters.
Lack of the MS ring causes decreases in class III and IV and increases in class II flagellar transcription.
Because the MS ring is the first structural component assembled in the nascent flagellum (27), absence of the MS ring in a ΔfliF V. cholerae mutant will prevent export and subsequent assembly of any flagellar component located exterior to the cytoplasm. To determine the effect on transcription of blocking flagellar assembly at the initial step, we measured the transcription of a number of class II, III, and IV promoters in the V. cholerae wild-type and ΔfliF mutant strains (Fig. 5).
FIG. 5.
Expression of class II, III, and IV flagellar promoters in a fliF mutant V. cholerae strain. V. cholerae strains KKV598 (wild type) and KKV1247 (fliF mutant) carrying plasmids pKEK72 (flrBp-lacZ), pKEK327 (fliEp-lacZ), pKEK329 (flhAp-lacZ), pKEK332 (flgBp-lacZ), pKEK331 (flgKp-lacZ), pKEK80 (flaAp-lacZ), pKEK415 (flaGp-lacZ), pKEK79 (flaBp-lacZ), pKEK76 (flaCp-lacZ), pKEK77 (flaDp-lacZ), and pKEK81 (flaEp-lacZ) were assayed for β-galactosidase activity during logarithmic growth in LB. Assays were performed in triplicate, and standard deviations are shown.
The absence of the MS ring in the ΔfliF mutant caused an increase in transcription (up to threefold) of all three class II (FlrA- and σ54-dependent) flagellar promoters, flhA, fliE, and flrB, in comparison with that in the wild-type strain. In contrast, transcription of the four class III (FlrC- and σ54-dependent) flagellar promoters, flgK, flgB, flaG, and flaA, was decreased (up to threefold) in the ΔfliF mutant, compared to that in the wild-type strain. Finally, transcription of three class IV (σ28-dependent) flagellar promoters, flaC, flaD, and flaE, was reduced more than twofold in the ΔfliF mutant, in comparison to that in the wild-type strain. Oddly, transcription of the class IV flaB promoter was unaffected by the absence of the MS ring, just as it was unaffected by the absence of FlgM (see above). In general, these results demonstrate that blocking flagellar assembly at the initial step causes an increase in class II gene transcription and decreases in class III and IV gene transcription.
flgM and fliA have little effect on intestinal colonization.
Flagellar synthesis, regulation, and chemotaxis have all been linked to V. cholerae colonization defects in the infant mouse (2, 26, 40), but the connection between these phenomena remains unclear. To determine the effect of flgM and fliA mutations on V. cholerae intestinal colonization of the infant mouse, a competition assay was performed with an inoculum that consisted of both mutant and isogenic wild-type strains (see Materials and Methods). Colonization defects are recognized by a competitive index (CI) of less than 1.
The ΔfliA, ΔflgM, and ΔfliA ΔflgM mutant strains demonstrated modest colonization defects in this assay (Fig. 6). The ΔfliA and ΔfliA ΔflgM mutant strains were more affected for colonization (CI, ∼0.3) than the ΔflgM strain (CI, 0.601), indicating that loss of σ28 is more deleterious for colonization than is loss of FlgM. Still, these are modest colonization defects, demonstrating that σ28 and FlgM play little role in intestinal colonization. Control experiments showed wild-type growth rates of all strains in LB at 37°C; thus, no obvious in vitro growth defects account for the modest in vivo colonization defects of these strains.
FIG. 6.
Intestinal colonization of fliA and flgM mutant V. cholerae strains. Strains KKV1113 (fliA mutant), KKV1461 (flgM mutant), and KKV1384 (fliA flgM mutant) were coinoculated with O395 perorally into infant mice at a ratio of ∼1:1; intestinal homogenates were recovered at 24 h postinoculation, and CFU of wild-type and mutant strains were counted. The CI is given as the output mutant/wild-type ratio divided by the input mutant/wild-type ratio; each value shown is from an individual mouse.
DISCUSSION
The control of σ28-dependent transcription via anti-σ28 factor FlgM is a fascinating example of how temporal transcription within the bacterial cytoplasm can be controlled by the assembly of an external organelle. In S. enterica serovar Typhimurium, FlgM binds to σ28 and prevents its association with RNAP until completion of the basal-body-hook structure, at which point FlgM is secreted through the nascent flagellum (15). This allows σ28 holoenzyme to transcribe class III flagellar genes, the products of which are then assembled into the flagellar filament. The bulk of the filament is composed of flagellin subunits, and these are not needed until completion of the basal-body-hook structure; thus, FlgM prevents their expression until an appropriate point in flagellar assembly.
V. cholerae possesses a single polar sheathed flagellum that contains five distinct flagellin subunits; four of the flagellin genes are transcribed by σ28 holoenzyme, while a fifth flagellin gene is transcribed by σ54 holoenzyme (20). Transcription and assembly of the σ54-dependent flagellin, FlaA, appear to precede transcription and assembly of the σ28-dependent flagellins (35). This situation differs from that in S. enterica serovar Typhimurium, where there is a single flagellin gene transcribed by σ28 holoenzyme upon completion of the basal-body-hook structure, suggesting that control of σ28-dependent transcription differs between V. cholerae and S. enterica serovar Typhimurium. Also, the V. cholerae flagellum, unlike those of S. enterica serovar Typhimurium, is covered by a sheath that may impede the secretion of nonstructural proteins, such as FlgM.
Our results demonstrate that V. cholerae FlgM interacts with σ28, is secreted through the flagellum, can inhibit transcription of some σ28-dependent promoters, and represses flagellar length; all of these functions are consistent with anti-σ28 activity, as initially characterized in S. enterica serovar Typhimurium. One would therefore anticipate that the absence of FlgM would result in increased σ28-dependent transcription at all of the σ28-dependent flagellin promoters, and in fact flaD and flaE transcription increased in the ΔflgM strain. However, flaC transcription actually decreased in a ΔflgM strain while flaB transcription was unaffected. Perhaps unregulated σ28 activity causes sequestration of a limited amount of σ28 holoenzyme at certain promoters (flaD, flaE), causing a decrease in transcription at other promoters (flaC).
It is difficult to rationalize the unresponsiveness of the flaB promoter to both the absence of FlgM and the absence of the MS ring. This promoter is clearly σ28 dependent, yet its activity is not diminished in the absence of secretion (ΔfliF mutant) nor increased in the absence of FlgM; thus, this promoter behaves differently than other σ28- and FlgM-controlled promoters. These differences in transcriptional responsiveness of the individual flagellin genes undoubtedly lead to differences in the composition of the flagellin subunits within the flagellum under different conditions, and we predict that this leads to altered swimming behavior. Complex flagella (i.e., composed of multiple distinct flagellin subunits) are present in many bacteria with polar flagella (e.g., Vibrio, Pseudomonas, and Campylobacter spp., etc.), but no studies have been performed yet that link the flagellin composition under different conditions to specific attributes of flagellar performance, such as rotation rate, speed, etc.
The lack of the MS ring in V. cholerae (ΔfliF mutant) led to general decreases in class III and IV flagellar transcription. We have already shown that transcription of class III genes is dependent on phosphorylation of FlrC (encoded by a class II gene) (2, 21), which suggests that the lack of secretion and/or lack of the MS ring inhibits phosphorylation of FlrC. Although FlrB, the kinase that phosphorylates FlrC, has been identified (2), it is not clear what modulates FlrB activity; these results suggest that it may be either an early structural component or a functional secretion apparatus. Transcription of the analogous C. jejuni class III genes, which are regulated by a FlrC homologue, FlgR, have been shown to depend upon the formation of the flagellar secretory apparatus (14), so we favor a similar functional, rather than structural, checkpoint in V. cholerae.
Although a number of bacteria possess sheathed flagella (e.g., Vibrio and Helicobacter spp.), very little is known about the sheath itself. The sheath is hypothesized to mask the proinflammatory flagellin subunits from the host immune system (28). Limited studies of V. cholerae demonstrated that the sheath possesses lipopolysaccharide, indicating that it is contiguous with the outer membrane (7). Additional studies of V. alginolyticus and V. anguillarum have identified a specific protein and lipopolysaccharide associated with the flagellar sheath in these species (8, 33). Analysis of mutations in hook-associated proteins that connect the flagellin filaments to the basal body in V. parahaemolyticus suggested that the sheath acts as a barrier to the secretion of flagellin subunits to the extracellular milieu (29), raising the question of the competence of the sheathed Vibrio flagellum to secrete FlgM (28).
Our studies demonstrate that V. cholerae FlgM is secreted through the sheathed flagellum, thus opening up the possibility that other nonstructural proteins could also be secreted through the flagellar export machinery. Type III secretion systems (TTSS) are frequently used by pathogenic bacteria to alter host physiology during infection, but the only TTSS present in the V. cholerae genome is the flagellar export apparatus (13). However, Yersinia enterocolitica is known to use the flagellar TTSS to secrete a virulence-associated phospholipase (43). Various studies have linked motility and flagellar synthesis to aspects of V. cholerae virulence (2, 5, 9, 26, 36, 40), but the phenomena are frequently strain and animal model dependent so it has been difficult to elucidate the contribution of the flagellum to cholera pathogenesis. The studies presented here demonstrate that the lack of σ28 and/or FlgM has a modest impact on the ability of a classical V. cholerae strain (O395) to colonize the infant mouse intestine; this animal model has been useful in predicting colonization behavior in humans (19). The effect of these mutations in other V. cholerae strains and on other aspects of virulence (intoxication, dissemination, reactogenicity, etc.) may be more profound.
Acknowledgments
We thank Kelly Hughes for helpful discussions.
This work was supported by NIH AI43486 to K.E.K.
REFERENCES
- 1.Colland, F., J. C. Rain, P. Gounon, A. Labigne, P. Legrain, and H. De Reuse. 2001. Identification of the Helicobacter pylori anti-σ28 factor. Mol. Microbiol. 41:477-487. [DOI] [PubMed] [Google Scholar]
- 2.Correa, N. E., C. M. Lauriano, R. McGee, and K. E. Klose. 2000. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol. Microbiol. 35:743-755. [DOI] [PubMed] [Google Scholar]
- 3.Dasgupta, N., M. C. Wolfgang, A. L. Goodman, S. K. Arora, J. Jyot, S. Lory, and R. Ramphal. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50:809-824. [DOI] [PubMed] [Google Scholar]
- 4.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freter, R., and P. C. M. O'Brien. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect. Immun. 34:222-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisk, A., J. Jyot, S. K. Arora, and R. Ramphal. 2002. Identification and functional characterization of flgM, a gene encoding the anti-σ28 factor in Pseudomonas aeruginosa. J. Bacteriol. 184:1514-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuerst, J. A., and J. W. Perry. 1988. Demonstration of lipopolysaccharide on sheathed flagella of Vibrio cholerae O1 by protein A-gold immunoelectron microscopy. J. Bacteriol. 170:1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuno, M., K. Sato, I. Kawagishi, and M. Homma. 2000. Characterization of a flagellar sheath component, PF60, and its structural gene in marine Vibrio. J. Biochem. 127:29-36. [DOI] [PubMed] [Google Scholar]
- 9.Gardel, C. L., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 64:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:577-580. [DOI] [PubMed] [Google Scholar]
- 12.Hava, D. L., and A. Camilli. 2001. Isolation and characterization of a temperature-sensitive generalized transducing bacteriophage for Vibrio cholerae. J. Microbiol. Methods 46:217-225. [DOI] [PubMed] [Google Scholar]
- 13.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, H. O. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrixson, D. R., and V. J. DiRita. 2003. Transcription of σ54-dependent but not σ28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol. Microbiol. 50:687-702. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, K. T., K. L. Gillen, M. J. Semon, and J. E. Karlinsey. 1993. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science 262:1277-1280. [DOI] [PubMed] [Google Scholar]
- 16.Josenhans, C., E. Niehus, S. Amersbach, A. Horster, C. Betz, B. Drescher, K. T. Hughes, and S. Suerbaum. 2002. Functional characterization of the antagonistic flagellar late regulators FliA and FlgM of Helicobacter pylori and their effects on the H. pylori transcriptome. Mol. Microbiol. 43:307-322. [DOI] [PubMed] [Google Scholar]
- 17.Karlinsey, J. E., S. Tanaka, V. Bettenworth, S. Yamaguchi, W. Boos, S. I. Aizawa, and K. T. Hughes. 2000. Completion of the hook-basal body complex of the Salmonella typhimurium flagellum is coupled to FlgM secretion and fliC transcription. Mol. Microbiol. 37:1220-1231. [DOI] [PubMed] [Google Scholar]
- 18.Kim, Y. K., and L. L. McCarter. 2000. Analysis of the polar flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 182:3693-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klose, K. E. 2000. The suckling mouse model of cholera. Trends Microbiol. 8:189-191. [DOI] [PubMed] [Google Scholar]
- 20.Klose, K. E., and J. J. Mekalanos. 1998. Differential regulation of multiple flagellins in V. cholerae. J. Bacteriol. 180:303-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klose, K. E., and J. J. Mekalanos. 1998. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol. Microbiol. 28:501-520. [DOI] [PubMed] [Google Scholar]
- 22.Klose, K. E., and J. J. Mekalanos. 1997. Simultaneous prevention of glutamine synthesis and high-affinity transport attenuates Salmonella typhimurium virulence. Infect. Immun. 65:587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutsukake, K., S. Iyoda, K. Ohnishi, and T. Iino. 1994. Genetic and molecular analyses of the interaction between the flagellum-specific sigma and anti-sigma factors in Salmonella typhimurium. EMBO J. 13:4568-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutsukake, K., Y. Ohya, and T. Iino. 1990. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J. Bacteriol. 172:741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauriano, C. M., C. Ghosh, N. E. Correa, and K. E. Klose. The sodium-driven flagellar motor controls exopolysaccharide expression in Vibrio cholerae. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 26.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macnab, R. M. 2003. How bacteria assemble flagella. Annu. Rev. Microbiol. 57:77-100. [DOI] [PubMed] [Google Scholar]
- 28.McCarter, L. 2001. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65:445-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarter, L. L. 1995. Genetic and molecular characterization of the polar flagellum of Vibrio parahaemolyticus. J. Bacteriol. 177:1595-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mekalanos, J. J., R. J. Collier, and W. R. Romig. 1979. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J. Biol. Chem. 254:5855-5861. [PubMed] [Google Scholar]
- 31.Miller, J. H. 1992. A short course in bacterial genetics, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 32.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norqvist, A., and H. Wolf-Watz. 1993. Characterization of a novel chromosomal virulence locus involved in expression of a major surface flagellar sheath antigen of the fish pathogen Vibrio anguillarum. Infect. Immun. 61:2434-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohnishi, K., K. Kutsukake, H. Suzuki, and T. Iino. 1990. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol. Gen. Genet. 221:139-147. [DOI] [PubMed] [Google Scholar]
- 35.Prouty, M. G., N. E. Correa, and K. E. Klose. 2001. The novel σ54- and σ28-dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol. Microbiol. 39:1595-1609. [DOI] [PubMed] [Google Scholar]
- 36.Richardson, K. 1991. Roles of motility and flagellar structure in pathogenicity of Vibrio cholerae: analysis of motility mutants in three animal models. Infect. Immun. 59:2727-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose, R. E. 1988. The nucleotide sequence of pACYC184. Nucleic Acids Res. 16:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 39.Wang, R. F., and S. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 40.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development, and virulence in Vibrio cholerae O139. Mol. Microbiol. 39:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, J., and A. Newton. 1997. Regulation of the Caulobacter flagellar gene hierarchy; not just for motility. Mol. Microbiol. 24:233-239. [DOI] [PubMed] [Google Scholar]
- 42.Yonekura, K., S. Maki-Yonekura, and K. Namba. 2002. Growth mechanism of the bacterial flagellar filament. Res. Microbiol. 153:191-197. [DOI] [PubMed] [Google Scholar]
- 43.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]