Abstract
Salt loading (SL) and water deprivation (WD) are experimental challenges that are often used to study the osmotic circuitry of the brain. Central to this circuit is the supraoptic nucleus (SON) of the hypothalamus, which is responsible for the biosynthesis of the hormones, arginine vasopressin (AVP) and oxytocin (OXT), and their transport to terminals that reside in the posterior lobe of the pituitary. On osmotic challenge evoked by a change in blood volume or osmolality, the SON undergoes a function-related plasticity that creates an environment that allows for an appropriate hormone response. Here, we have described the impact of SL and WD compared with euhydrated (EU) controls in terms of drinking and eating behavior, body weight, and recorded physiological data including circulating hormone data and plasma and urine osmolality. We have also used microarrays to profile the transcriptome of the SON following SL and remined data from the SON that describes the transcriptome response to WD. From a list of 2,783 commonly regulated transcripts, we selected 20 genes for validation by qPCR. All of the 9 genes that have already been described as expressed or regulated in the SON by osmotic stimuli were confirmed in our models. Of the 11 novel genes, 5 were successfully validated while 6 were false discoveries.
Keywords: transcriptome, supraoptic nucleus, water restriction, salt load, neuroendocrine
terrestrial life requires that the osmolality of the extracellular fluid (ECF) is strictly controlled. Increased ECF osmolality results in water leaving the cell, reducing intracellular fluid (ICF) volume, while increasing ICF osmolality, which will compromise the metabolic processes necessary for life. Chronic increases in ECF osmolality can be brought about experimentally by a high intake of salt (salt loading, SL) or by water deprivation (WD) (5). In the absence of drinking fluid (WD), extracellular and intracellular fluid volumes decrease, as water and sodium are inevitably lost in sweat and urine leading to hypovolemia and, as a consequence of dehydration-induced natriuresis, sodium depletion (14, 35). This depletion of sodium means that WD animals also display enhanced salt appetite (14). In contrast, SL increases body sodium content, causing an increase in ECF volume, but a decrease in the volume of the ICF (35).
Angiotensin II (ANG II), atrial natriuretic peptide (ANP), arginine vasopressin (AVP), and oxytocin (OXT) are the main hormones involved in the control of hydromineral homeostasis in mammals (5). ANP is synthesized and secreted into the bloodstream in response to stretching of the right atrial muscle cells by increased blood volume. Once in the bloodstream, ANP has a potent natriuretic effect, acting on the distal convoluted tubule of the nephron to inhibit sodium reabsorption (13, 44). ANP is an important indicator of blood volume. AVP and OXT release are stimulated by hypovolemia, hypertonicity, and hypernatremia, among other stimuli (36). Circulating levels of AVP correlate with plasma osmolality (4) and thirst perception (3, 42) and serves to increase permeability of the collecting duct in the kidney allowing water to be conserved and urine to be concentrated. In relation to osmotic control, OXT is a natriuretic hormone that is elevated early in response to osmotic challenge to reduce sodium appetite and influence sodium balance through increased excretion (59). ANG II exerts several effects on hydromineral balance, including the regulation of thirst and sodium appetite, AVP secretion, and renal water and sodium reabsorption (4, 37). The enzyme renin is responsible for the cleavage of angiotensinogen into angiotensin I, which in turn is cleaved into ANG II. Renin is stimulated by reducing the Na+ content that reaches the distal convoluted tubules of the nephron (31). The subfornical organ (SFO) expresses the angiotensin receptor 1A (At1A; Ref. 22), and the expression of this receptor changes following osmotic stimulation through water deprivation (7, 11). Moreover, the SFO directly projects to vasopressinergic magnocellular neurons of the supraoptic nucleus (SON; Ref. 61).
Evolution has thus ensured that cells are protected from fluctuations in the osmolality of the extracellular environment. Brain mechanisms involved in the regulation of osmolality are centered on the central osmoregulatory circuit. Osmosensitive neurons of the circumventricular SFO communicate chronic changes in plasma osmolality through direct projections to the magnocellular neurons (MCNs) of the hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei, thus modulating the synthesis of AVP and OXT, and their transport to, and release from, the posterior lobe of the pituitary (26, 61). When ECF osmolality rises, the SON responds with an increase in AVP synthesis and release that is accompanied by change in soma size (hypertrophy), the result of the intracellular organelle development required to respond to the stimulus in terms of hormone production. The SON experiences a function-related remodelling that is necessary for the optimal facilitation of hormone production and delivery (20, 50, 54). These morphological changes are accompanied by biochemical events such as a strong activation of the cAMP pathway (10) and transcriptional events that extend beyond simple AVP and OXT biogenesis (32, 46). We have previously shown that WD in both male and female rats is capable of modulating gene expression in the SON (23, 45) and that this modulation is a global transcriptome response (24). We have also recently demonstrated that many of the genes that are regulated in the SON in response to WD are also altered by lactation (45).
While SL and WD are often seen as experimentally similar stimuli, we demonstrate here that profound physiological differences exist between them at the level of drinking, feeding, weight loss, hormonal response, and responsiveness to water repletion at the end of the challenge. We wanted to ascertain whether these differences were managed at the level of the SON and have therefore profiled the transcriptome of this tissue after 7-days of SL and compared it wih our previous transcriptome data from the WD SON (23) to assess whether those genes that are similarly or differently regulated are relevant to the physiological changes we see.
METHODS
Animals.
Adult, 10- to 12-wk-old male Sprague-Dawley (Harlan Sera-lab, Loughborough, UK) and Wistar rats (Central Animal Facility University of São Paulo, Ribeirão Preto, Brazil) were maintained in standardized temperature (22 ± 1°C), humidity (50 ± 5%), and diurnal conditions (UK: 10 h light, 14 h dark; lights on at 0700, BR: 12 h light, 12 h dark). WD involved complete fluid deprivation for up to 3 days, whereas SL involved the replacement of drinking water with 2% (wt/vol in tap water) NaCl for up to 7 days; all animals had access to food (standard laboratory rat chow) ad libitum throughout the experiment. The euhydrated (EU) control group of animals had access to both food and normal drinking water for the duration of the experiment. Rats were euthanized (between the hours of 0900 and 1300), and the tissue was isolated and processed as described below. All experiments were carried out under the licencing arrangements of the UK Animals (Scientific Procedures) Act (1986) and Brazilian legislation (law number 11794, October 8, 2008) with both local ethics committee approval.
Metabolic cage experiments.
To enable precise daily measures of fluid intake, food intake, and urine output, animals (n = 6 per group) were individually housed in metabolic cages (Techniplast, Italy). Fluid intake, food intake, and urine output were recorded after 3 days acclimatization (control measure) and during 3 days WD and 7 days SL. Animals were euthanized by stunning (which involved a blow to the head), followed by decapitation before trunk blood collection. Plasma and urine osmolality was determined by freezing point depression using a Roebling micro-osmometer (Camlab).
Physiological assessment and water repletion.
In total, 20 rats were single housed and randomly assigned to one of three groups: 7 days SL (n = 7), 3 days WD (n = 7), or EU controls (n = 6). Cumulative fluid consumption by the EU and SL animals was measured by weight each day (between 10:00 and 11:00 AM) for 7 days. After 7 days of SL and 3 days of WD, animals were given free access to tap water, and fluid intake was recorded after 15, 30, and 45 min and then hourly until 6 h and then again at 24 h at which point the animal was euthanized. Cumulative water consumption per animal was normalized for spillage (calculated at 3 ml) and calculated as milliliters per kilogram based on starting body weight.
Hormone assessment.
In total, 21 rats were grouped housed and randomly assigned to one of three groups: SL (n = 7), 2 days WD (n = 7), or EU controls (n = 7). Animals were decapitated and blood was collected from the trunk into chilled tubes containing heparin for AVP and OXT or peptidase inhibitors for ANG II and ANP levels. Plasma was obtained after centrifugation (20 min, 1,600 g, 4°C) and stored at −20°C until specific extraction and radioimmunoassay procedures. The specific antibody for ANG II radioimmunoassay was obtained from Peninsula (ANG II, T4007; San Carlos, CA); and for AVP and OT radioimmunoassays were donated by W. K. Samson (University of St. Louis, St. Louis, MO) and for ANP was donated by J. Gutkowska (Univ. of Montreal, Montreal, Canada). The radioimmunoassay sensitivity and intra- and interassay coefficients of variation were 0.5 pg/ml, 8.7–11.2% for ANG II; 0.7 pg/ml, 4.1–7.8% for ANP; 0.1 pg/ml, 4.5–4.9% for AVP; and 0.1 pg/ml, 5.1–8.8% for OT.
Tissue collection and RNA extraction for microarray.
Rats were stunned (which involves a blow to the head) and then decapitated with a small animal guillotine (Harvard Apparatus, Holliston, MA). The brain was rapidly removed from the cranium and placed in an ice-cold brain matrix (ASI Instruments, Warren, MI). One section of ∼1-mm thickness was taken and the SON carefully dissected on a bed of ice under a dissecting microscope (Leica, Germany). After isolation, the samples were immediately placed in Eppendorf tubes containing RNAlater (Ambion, Huntingdon, UK). Tissue was mechanically homogenized in QIAzol Lysis Reagent (QIAgen, Crawley, UK) and the aqueous phase removed after centrifugation through a Phase Lock Gel column (Eppendorf, Cambridge, UK). Total RNA was purified with RNeasy Micro Kit MinElute Spin Columns (Qiagen) and eluted into 14 μl of RNase-free water. After quality control confirmation, total RNA was amplified using the Affymetrix IVT Express kit hybridized to Affymetrix Rat GeneChip 230 2.0 microarrays according to manufacturer's instructions (Affymetrix). Arrays were scanned using the GeneChip Scanner 3000 (Affymetrix). All data were analyzed in Genespring GX12 (Agilent); .CEL files from the SL and the EU arrays (n = 5) were uploaded subject to quartile RMA normalization where the median was used for baseline before statistical differences between the two conditions were determined using an unpaired t-test for unequal variance (Welch) with a Benjamini Hotchberg multiple test correction applied to maintain a corrected P value of <0.05. In a separate experiment, .CEL files from EU and WD microarray experiments (23) were remined in the same manner, and a list of regulated genes from this EU-WD experiment was generated. Importantly, the amplification procedure for these microarrays (23) differs from the protocols used in the present study. Briefly, cRNA synthesis and biotin labeling (single round of linear amplification achieved using the Message Amp II cRNA kit ; Ambion, Huntingdon, UK) resulted in different amplification efficiencies and therefore the data analyses for WD and SL were handled as independent experiments.
RNA extraction and cDNA synthesis for qPCR.
Rats (EU, 3 days DH, 7 days SL, n = 6 per group) were stunned and then decapitated, and brains were frozen on dry ice and stored at −80°C. With the use of a cryostat (Leica Microsystems CM1900 Cryostat), 60-μm rostral-caudal sections were taken and stained with Toluidine blue (0.1% wt/vol in 70% vol/vol EtOH; Sigma Aldrich) to map the hypothalamus. A 1-mm diameter micropunch (Fine Science Tools) was used to collect SON samples. Tissue samples were mechanically homogenized in QIAzol Lysis reagent (Qiagen) and allowed to stand for 5 min at room temperature before centrifugation (10 min, 10,000 g, 4°C). Extraction with chloroform was then performed (15 min, 12,000 g, 4°C). Total RNA was then precipitated with 1 volume EtOH (70% vol/vol) and purified using the RNeasy Mini Kit (Qiagen) according to manufacturer's protocol. RNA quality and yield were confirmed using the Implen Geneflow Nanophotometer. cDNA synthesis was performed using QuantiTect Reverse Transcription Kit (Qiagen) using 100 ng of input RNA. Primer sequences are provided in Table 1. The qPCRs were carried out in duplicate in 25-μl reaction volumes using 12.5 μl 2× SYBR green master mix buffer (Roche), optimum concentrations of primers, and first-strand cDNA template. The reactions were performed using an ABI 7500 Sequence Detection System (ABI, Warrington, UK), with universal cycling conditions. For relative quantification of gene expression the 2−ΔΔCT method was employed. RPL19 is a 60-s ribosomal protein L19 (RPL19) that is used as a housekeeping gene to allow normalization of gene expression between samples. RPL19 gives highly reproducible and stable expression measurements between different physiological challenges within the SON and PVN of the brain and so it acts as an excellent calibrator for our PCR experiments.
Table 1.
List of primers used in the quantitative PCR validation of microarray targets
| Probe | Forward | Reverse |
|---|---|---|
| hnOxt | TGAGCAGGAGGGGGCCTAGC | TGCAAGAGAAATGGGTCAGTGGC |
| hnAvp | GAGGCAAGAGGGCCACATC | CTCTCCTAGCCCATGACCCTT |
| Trpv2 | GAGTCACCATTCCAGAGGGA | GTTCAGCACAGCCTTCATCA |
| Arhgdib | Quantitect probes (Qiagen) | |
| Atf4 | Quantitect probes (Qiagen) | |
| Creb3l1 | GAGACCTGGCCAGAGGATAC | GTCAGTGAGCAAGAGAACGC |
| Giot1 | GACACTTCCGGTCCGTCATAG | GCCTCACTCAAGCACCCAGT |
| Opsin3 | ATGGCTATGGACACCTGGTC | CAGAGGAGTTGCAGAAGGGA |
| Vgf | ATGAGTTGCCGGACTGGAA | CGCGGCCGAATGTAGTTTG |
| Syt4 | CCGCGTGGAATTCGATGAAA | TCTTCTCTGGCAGCAGATCC |
| Rgs4 | CCTGAGGAGCGCAAA | TTTGAAAGCTGCCAG |
| Cd55 | CGCACAGGAAAATCA | CCAGCTTGTACCCTT |
| Nab1 | TACAGCATGTCAGGGGACAG | GGTAGAGCCTCTGGGTTCAA |
| Procr | AACGACGTGGTCTTTCCTCT | TATGGCAGTCTTTGGCTGGA |
| Atf5 | CAGTGCCTAGGGTACAGGAG | AATGGAGGGACAGGGTGAAG |
| Psph | TGGCCAAATTCTGTGGTGTG | AGCGCATCTTTGAAAGGCAA |
| Itgb1 | GTGGTTGCCGGAATT | GATTTTCACCCGTGT |
| Rgs2 | GACTGCGTACCCATGGACAA | GAAGTCTTCGCAAGCCAACC |
| Insig1 | CACGTCCCCAGATTTCCTCT | GGCTTTTCTGGAACACCCAT |
| Rgs5 | GCAACTACGGATTTGCCAGC | GTCAAAGCTGTGAGGGGAA |
See text for more details.
Statistical analysis.
With the exception of the microarray data, all analysis was performed using Prism Graphpad version 6.
RESULTS
Physiological responses are different in SL and WD rats.
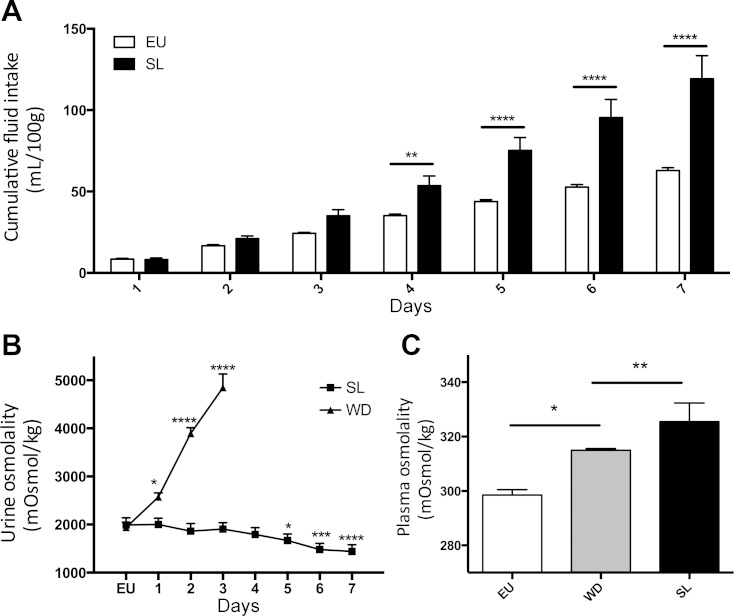
SL rats drank more than EU controls. When cumulative drinking was measured, the SL rats drank significantly more than EU rats from day 4 to 7, with the total mean cumulative fluid consumption of the SL group at day 7 nearly double that of the EU rats (RM two-way ANOVA, P < 0.05, Sidak's multiple comparison test; Fig. 1A). Urine osmolality progressively and significantly increased from 2,000 mosmol/kg H2O under EU conditions to 4,850 mosmol/kg H2O at 3 days in the WD animals, whereas urine osmolality in the SL group dropped to 1441.25 mosmol/kg H2O after 7 days; however, this only reached significance on day 5 (RM one-way ANVOA, P < 0.05, Sidak's multiple comparison test; Fig. 1B).
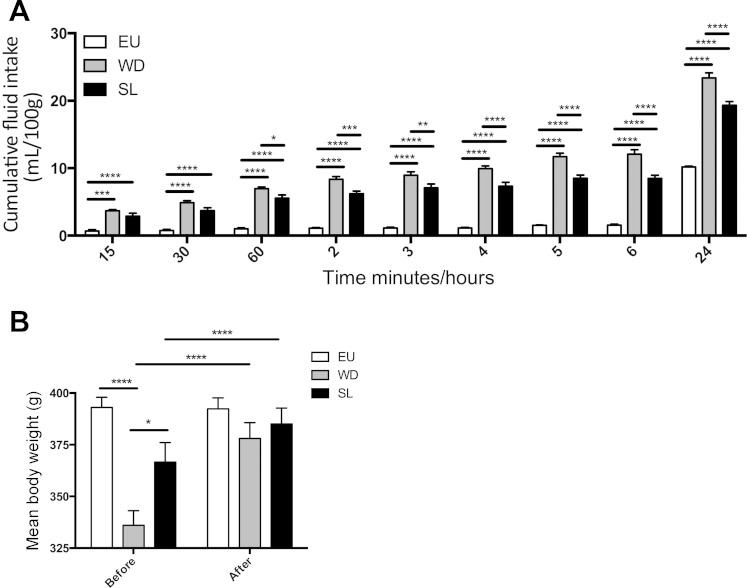
Fig. 1.
A: mean cumulative fluid intake (ml/100 g) of rats exposed to either 2% salt (SL; wt/vol) or tap water (EU) for 7 days. B: daily urine osmolality (mosmol/kg H2O) in water-deprived (WD; 3 days) and SL animals; C: plasma osmolality in EU animals after 3 days WD and after 7 days SL (means ± SE, significance: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
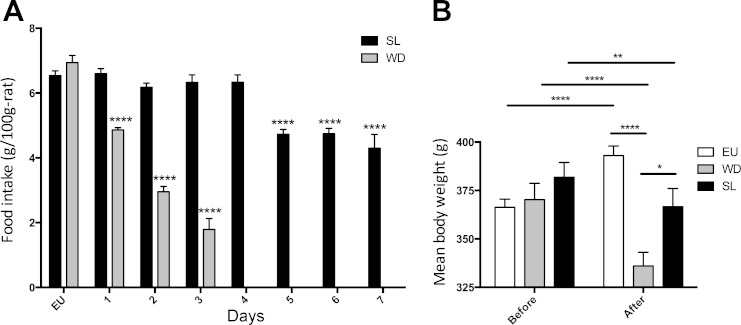
Both SL and WD resulted in a significant increase in plasma osmolality compared with EU controls (two-way ANOVA, P < 0.05, Tukey multiple test correction; Fig. 1C). During WD, food intake decreased compared wih EU control measures; 32% less after 1 day, 58% less after 2 days, and 75% after 3 days (one-way ANOVA, P < 0.05, Sidak's multiple comparison test; Fig. 2A). Food intake in SL animals remained similar to control measures until day 5, where intake dropped significantly (one-way ANOVA, P < 0.05, Sidak's multiple comparison test; Fig. 2A) below control measures and did not recover. Both WD and SL rats lost weight as a consequence of being subjected to their respective chronic osmotic stressors (RM two-way ANOVA, P < 0.05, Tukey multiple comparison test; Fig. 2B), whereas EU animals significantly gained weight during the same period.
Fig. 2.
A: mean food intake in rats exposed to either 2% SL (wt/vol) for 7 days or WD for 3 days (means ± SE, significance compared with EU control). B: mean weight (g) of rats before and after either SL or WD (means ± SE, significance: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
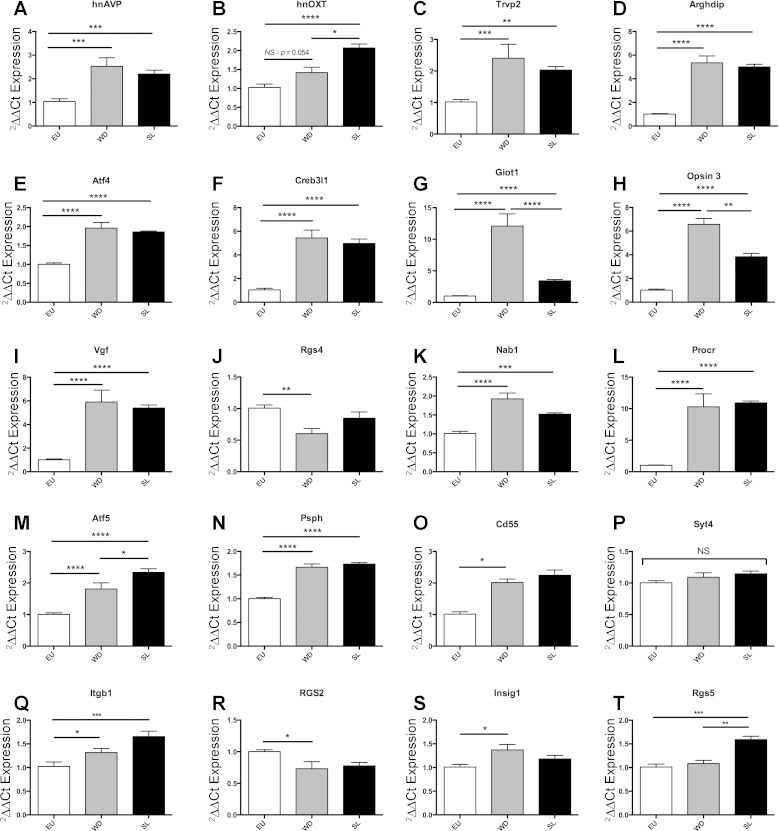
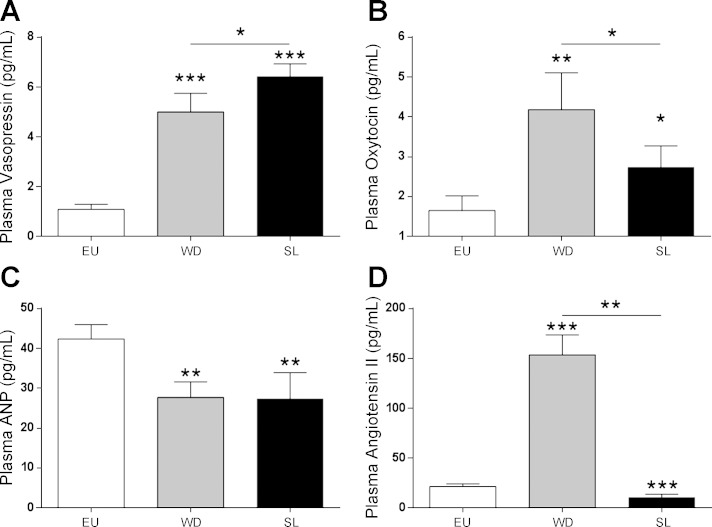
Heteronuclear (hn) AVP is significantly regulated in the SON of both the WD and the SL rat (see Fig. 7A for more details), whereas hnOXT is only significantly regulated in the SL SON (one-way ANOVA with Tukey multiple comparison test; see Fig. 7B). Plasma AVP levels increased in both WD and SL compared with EU animals, and there was a significant increase in AVP in SL animals compared with the WD animals (Fig. 3A). Plasma OXT was also higher in both SL and WD compared with EU controls, but OXT levels were significantly lower in SL compared with the WD animals (Fig. 3B). Plasma ANP levels were reduced in both WD and SL animals compared with EU (Fig. 3C). Plasma ANG II was increased by WD and decreased by SL compared with the control (Fig. 3D).
Fig. 7.
Quatitative PCR (qPCR) validation of mRNA expression (ΔΔCt) in the SON of the EU, 7-day SL, and the 3-day WD rat (means ± SE, significance = paired t-test, *P < 0.05). See text for specific details about A–T. Trvp, transient receptor potential cation channel V2; Arhgdib; Rho, GDP dissociation inhibitor (GDI) β; Atf4, activating transcription factor 4; Creb3l1, cAMP-responsive element binding protein 3-like 1; Giot1, gonadotrophin-inducible ovarian transcription factor 1; Opn3, Opsin3; Vgf, VGF nerve growth factor inducible; Syt4, synaptotagmin IV; Rgs4, regulator of G protein signaling 4; Cd55, Cd55 molecule/complement decay-accelerating factor; Nab1, nerve growth factor inducible-A-binding protein 1; Procr, endothelial protein C receptor; Atf5, activating transcription factor 5; Psph, phosphoserine phosphatase; Itgb1, integrin, β1; Rgs2, regulator of G protein signaling 2; Insig1, insulin-induced gene 1; Rgs5, regulator of G protein signaling 5. Significance: NS, not significant *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Fig. 3.
Plasma levels (pg/ml) of vasopressin (A), oxytocin (B), atrial natriuretic peptide (ANP, C), and angiotensin II (D) were measured from WD and SL animals (one-way ANOVA, *P < 0.05, **P < 0.005, ***P < 0.0001).
WD and SL rats differ in their response to water repletion.
After 7 days SL or 3 days WD, tap water was returned and fluid intake was recorded for 24 h. EU controls drank very little during the first 6 h (1.6 ± 0.15 ml, means ± SE) on account of this time falling within the light-phase (Fig. 4A). In contrast, both SL and WD rats drank more than the EU rats at every time point resulting in higher total fluid intake (two-way ANOVA, P < 0.05, Tukey multiple comparison test; Fig. 4A). After 30 min of water repletion, WD animals had drunk significantly more than SL animals and intake measures remained higher over the 24-h period (Fig. 4A). The weight loss caused by 7 days SL and 3 days WD (see Fig. 2B) was completely rescued by 24 h of water repletion (Fig. 4B). Both WD and SL rats gained weight as a result of 24-h repletion compared with their respective osmotic-challenged weight (RM two-way ANOVA, P < 0.05, Bonferroni multiple comparison test), whereas there was no significant change in EU animal weight over the 24-h.
Fig. 4.
A: mean cumulative fluid intake (ml/100 g) of rats replete for water following an osmotic challenge of either 2% salt (wt/vol; SL) for 7 days or WD for 3 days compared with EU animals (means ± SE) B: mean weight (g) of SL, WD, and EU animals after osmotic challenge and after repletion (means ± SE). Significance: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Transcriptome of the SON after SL or WD.
Microarray experiments were performed from tissue taken from the SON of animals subjected to 7 days SL and compared with the SON from EU animals. Separate microarrays comparing EU and WD SON (23) were reanalyzed in the same manner, and the two lists of genes were compared to decipher the overlap in the regulated transcriptome (i.e., genes that are regulated by both stimulus) as well as treatment unique transcripts (i.e., those genes regulated by one treatment but not the other). After 7 days SL, 9,843 genes are significantly regulated (Fig. 5; supplemental Fig. S1), 1,108 of which are regulated by greater than twofold (Fig. 5; supplemental Fig. S2). WD resulted in fewer regulated genes (4,480; Fig. 5; supplemental Fig. S3), of which 106 were regulated by greater than twofold (Fig. 5; supplemental Fig. S4). When the lists of genes that are significantly regulated by either SL or WD are compared, 2,783 genes are commonly regulated between the two conditions (Fig. 5; supplemental Fig. S5) representing ∼31% of the SL-regulated genes and ∼62% of the WD-regulated genes; 88% of the genes regulated by greater than twofold in the WD, SON were also regulated by SL (Fig. 5; supplemental Fig. S6). Raw .CEL files for all microarrays can be found in the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/; WD = GSE3110, SL = GSE65663).
Fig. 5.
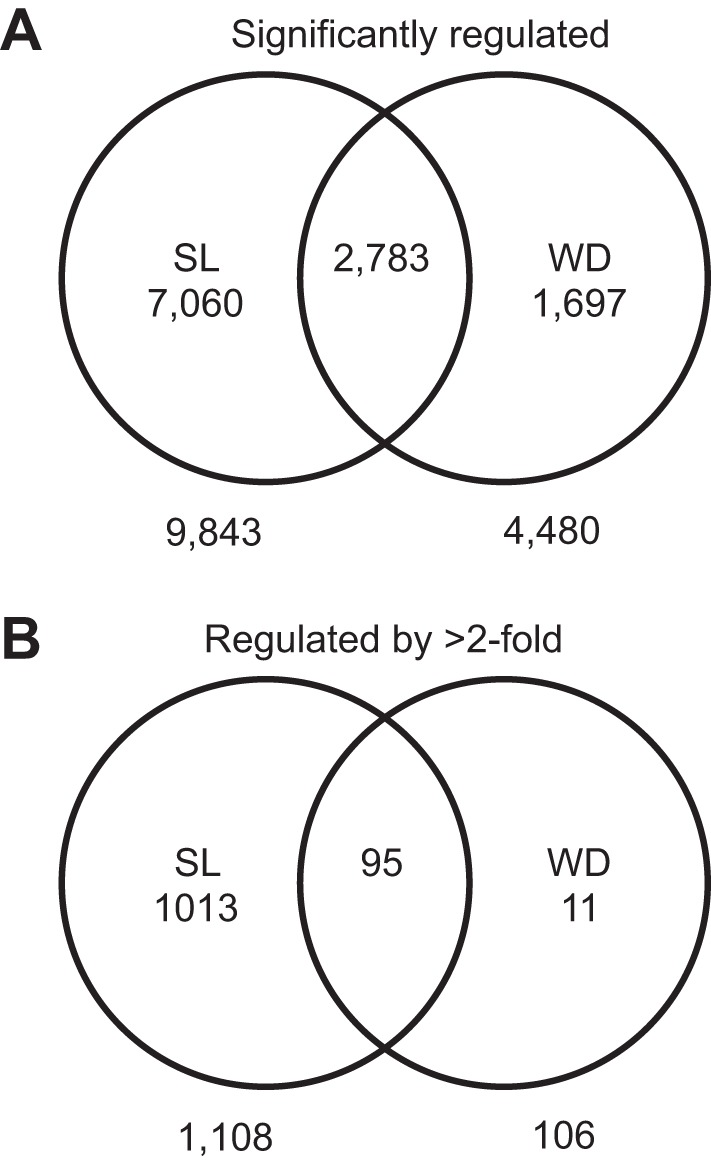
Affymetrix microarrays were used to interrogate supraoptic nucleus (SON) from EU vs. 7-day SL animals (2% wt/vol) and revealed 9,843 genes (A) significantly regulated genes (Welch t-test, corrected using Benjamini-Hochberg to keep P < 0.05, S1), of which 1,108 genes (B) were regulated by greater than twofold (S2) between EU and SL. Microarrays comparing EU and 3-day WD in the SON were also compared, and 4,480 genes (S3) were regulated of which 106 genes (S4) were regulated by greater than twofold. In total, 2,783 genes (S5) were commonly regulated by both SL and WD in the rat SON, and 95 genes (S6) that were commonly regulated by greater than 2-fold (see supplementary Figs. S1–S6).
Validation of mRNA targets by qPCR.
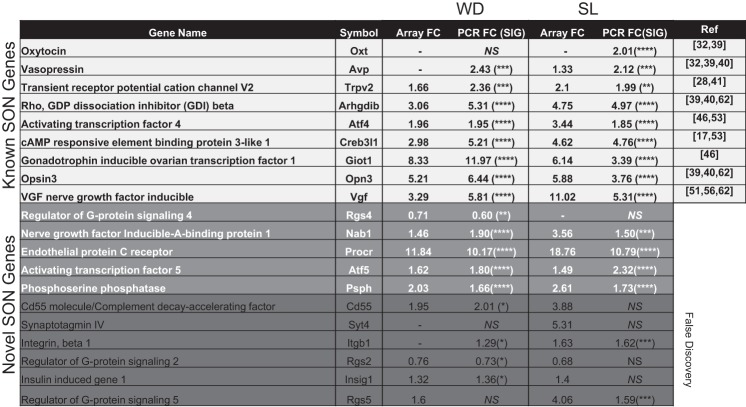
From the microarray data, we identified 20 genes for validation (Fig. 6). We first looked at 7 genes that the literature previously had reported to be expressed in the SON and that are known to be regulated in response to an osmotic stimulus (Fig. 6). All of these genes were positively confirmed by qPCR as being regulated in the SON following either SL or WD compared with EU (one-way ANOVA, P < 0.05, Tukey multiple test correction, performed in Prism, Graphpad using ddCT): AVP (Fig. 7A), OXT (Fig. 7B), Trpv2 [Fig. 7C, (28, 41)], Arhgdib [Fig. 7D, (39, 40, 62)], Atf4 [Fig. 7E, (46, 53)], Creb3l1 [Fig. 7F, (17, 53)], Giot1 [Fig. 7G, (46)], Opsin 3 [Fig. 7H, (39, 40, 62)], and Vgf [Fig. 7I, (51, 56, 62)]. We then validated five novel genes that the array data predicted as being regulated in the SON following both SL and WD: Nab1 (Fig. 7K), Procr (Fig. 7L), Atf5 (Fig. 7M), Psph (Fig. 7N), and Cd55 (Fig. 7O). Of these, all but Cd55 validated appropriately by ANOVA, though Cd55 was significantly different when analyzed by t-test (data not shown). Additionally, we revealed that Oxt, Giot1, Opsin, Atf5, and Rgs5 show significant differences in expression between WD and SL (Fig. 7, B, G, H, M, and T). We also validated two novel genes that were regulated differently by WD or SL according to the microarray: Syt4, which was not regulated according to our PCR validation (Fig. 7P), and Rgs4 is 1.65-fold downregulated by WD but not SL (Fig. 7J). Finally, we present some qPCR data on gene transcripts that did not entirely corroborate the array results (Fig. 6): Itgb1, which was not regulated by WD according to the array data, but we showed this transcript to be significantly regulated by both WD and SL (Fig. 7Q); Rgs2, which was significantly regulated following WD but not following SL (Fig. 7R); Insig1, which was confirmed for WD but not SL (Fig. 7S); and Rgs5, which was confirmed in SL but not WD (Fig. 7T).
Fig. 6.
Probes identified by the array and validation by PCR in the SON. FC, Fold change, NS, not significant, Ref, references to support gene expression in the SON. Significance: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
DISCUSSION
We have investigated the neuroendocrine, hydromineral, behavioral, and transcriptomic responses to two overlapping chronic osmotic stimuli in the rat, namely SL for up to 7 days, and complete WD for up to 3 days. First, we have chronicled the impact of SL and WD on fluid and food intake and measured changes in both urine and plasma osmolality in both SL and WD animals. Levels of the circulating hormones ANG II, AVP, OXT, and ANP were also recorded after WD and SL. Animals were then given back tap water, and their drinking responses were measured.
Rats subjected to an SL paradigm drink more than EU controls, experience a drop in urine osmolality throughout the protocol, and have a final plasma osmolality that is >20 mosmol/kg H2O higher than EU animals. The high-sodium intake of SL animals significantly increases plasma osmolality. In response to elevated plasma sodium, renal excretion of sodium is induced to stabilize plasma levels. As a consequence the renal excretion of water also significantly increases resulting in excretion of high volumes of dilute urine. WD animals also experience an elevated plasma osmolality, but urine osmolality increases with dehydration.
We have shown here that hnOxt expression in both the SON and circulating OXT levels are elevated in both SL and WD, corroborating previous data (6). In addition to circulating OXT, we also show that both circulating levels of AVP and hnAvp expression in the SON are higher in response to both SL and WD. A significant increase in plasma AVP is recorded following both SL and WD, in line with previous reports that the circulating concentration of these peptides correlates with plasma osmolality (6, 19). There is also a significant difference between the WD and SL with respect to both AVP and OXT, the former being higher and the latter lower after SL than WD. Circulating ANP levels are lower than those under EU conditions (2). Although ANP levels correlate well with AVP and hypertonicity, ANP is regulated principally by plasma volume, whereas AVP is primarily modulated by a change in plasma osmolality and secondarily by volume change (27). The increased concentration of ANG II in WD animals is also well reported in the literature (15) and is the result of juxtaglomerular renin secretion stimulation in response to their three main stimuli, all present in this model: reduction of renal perfusion pressure, the reduced content of Na+ and Cl− in the distal convoluted tubule, and the β1-adrenergic stimulation (31). In the animals subjected to SL, however, a large increase of Na+ and Cl− in the distal convoluted nephron tubule is seen (13, 44), which should be the main contributing factor in reducing renin secretion and ANG II plasma levels in SL.
A discrepancy exists between the circulating OXT hormone measurement, which is highest in following WD and the hnRNA measurement that just misses significance. While transcript, pituitary content, and circulating hormone levels of AVP are all closely coupled, the same is not true for OXT. Because a large amount of mature peptide is stored in the pituitary, a rise in plasma OXT has been observed in response to SL that peaks at 2 days and then drops and remains stable for days 3 and 4, while OXT peptide content in the NL drops throughout a 5-day challenge (62). In contrast, hnRNA level have been demonstrated to progressively rise through time, with a peak and plateau at 3 days, markedly different from the sharp and sustained elevation of AVP (62). There are also differences in terms of transcript expression that exist between the SON and the PVN, a structure that also contributes to the circulating OXT content. In the SON, WD induces a large elevation of AVP and OXT that is commensurate with cFos expression. In contrast, in the PVN, the AVP and OXT expression change in response to WD is not as steep as for cFos and peaks at a different time point (12). Because the PVN is out of the scope of this paper, we cannot at this point comment on the role of the PVN in the maintenance of circulating OXT peptide levels and therefore we can only speculate on the role of this structure in the mediation of sustained peptide release from the pituitary and subsequent circulating levels.
Animals that are osmotically challenged eat less food during the insult. Both WD and SL animals consumed significantly less food and weighed less compared with their EU state, a finding in line with previous studies (12, 21, 49), but the magnitude of the drop was the largest in the WD group where eating was quickly attenuated. Animals undergoing WD quickly lose their appetite, presumably because the salt in food will exacerbate the problem of rising ECF sodium and because water is required for salivation and digestion. At the end of the 3 days of WD or 7 days SL, animals have a lower weight than before the challenge. OXT is not only able to modulate osmotic status [by stimulating sodium excretion and limiting sodium intake (59)] but also nutritional status. Interestingly, both WD and SL are used as a model of anorexia (8), and dehydration-induced anorexia is attenuated in OXT-deficient mice (47). Whereas this OXT effect is directed from the parvocellular neurons (from the PVN projecting elsewhere), there is some evidence that the SON is responsive to the anorectic molecules nesfatin and cholecystokinin (9, 34), and that MCN themselves are capable of acting as metabolic and glucose sensors that participate in appetite regulation through the stimulation of OXT and AVP release (52). The SON is also responsive to the adipokine leptin, the receptor for which is expressed (18); peripheral application of leptin rescues the fasting-induced drop in PVN OXT expression (43). It is, however, likely that the regulation of feeding after SL and WD is not confined to this single tissue or any single mechanism.
Our physiological assessment of WD and SL demonstrates that although these two osmotic challenges overlap, they manifest themselves differently in terms of urine osmolality, drinking and feeding behavior, and hormone response. Because these differences suggested a role for the hypothalamus, we wanted to investigate what role the SON, a major hub in the osmotic control circuitry, played in these two challenges. We already know that the SON is highly responsive to WD in terms of transcriptome expression (23), and we wanted to establish whether the SON transcriptome was also similarly responsive to SL and, importantly, what overlap in SON transcriptome patterning was evident when comparing SL and WD. We thus profiled the transcriptome of the SON in EU animals and in 7-day SL animals using Affymetrix microarrays and compared the data with a reanalyzed comparison of previously published EU and WD microarrays (23).
The compared SL and WD datasets revealed that twice the number of genes are regulated after SL than after WD. There are two possible reasons for this disparity. First, there may be a biological explanation. It is possible that SL engender more changes in gene expression that does WD. We have observed in previous work that WD regulates fewer transcripts than does other stimuli in the same tissue, for example, food restriction (22, 25), and that the transcriptome of the SON becomes more organized after a survival threat. When the transcriptome response to WD is modelled, the emerging distribution is different from that found in the EU transcriptome, a trend we have previously placed within the context of enhanced transcriptome organization (24). Because a different physiological response must be mounted to different survival threats, global transcriptome responses to either WD or SL in the SON may also be different.
The second possibility is a technical rather than biological one. Different amplification procedures have been utilized in the WD and the SL experiments, and because of this improved linear range, a larger number of transcripts can be identified in the latter. The highly expressed AVP transcript is revealed as being significantly regulated in the SL array but not the WD array but is qPCR validated in both SL and WD (Figs. 3A and 6). To ensure that any technical differences did not limit discovery, we ensured that the raw data from the older, WD arrays were subjected to the same normalization and statistical analysis as the newer SL arrays were. Importantly, we did not normalize and analyze these two different studies together. We treated EU versus WD and EU versus SL as separate experiments and compared the lists of genes that resulted from each experiment.
There are a large number of genes that are commonly and significantly regulated as a consequence of the two challenges, and up to 90% of those genes that are highly expressed (>twofold) in the WD SON are also regulated by SL. We chose 20 genes to validate by qPCR in SON from EU, SL, and WD animals. The choice of probes for this validation was based on genes that were significantly different (no fold change cut-off applied) according to the microarray, their previous description by the literature that they were responsive to either SL or WD, or their differential regulation between these stimuli (17, 28, 39–41, 53, 56, 62). We note that this is the first time that Trpv2 has been shown to be regulated in the SON by osmotic stimuli. We also selected some novel mRNAs to validate from the list of genes that were regulated by either SL and WD or both according to the microarray, and whose specific function in osmotic control is less understood including regulator of G protein signaling (Rgs) proteins that provide a negative modulation of G protein-mediated signal transduction, acting to dampen G protein activity as necessary (1). Specifically, Rgs4 is known to be downregulated in the hypothalamus by acute restraint stress (30); we show that Rgs4 is downregulated in the SON after WD but not SL. Complement decay-accelerating factor (Cd55) inhibits complement activation and reduces neuronal cell death after hypoxia and inflammation in the brain (57, 60). Cd55 is significantly regulated in the SON after WD but not SL, despite the higher magnitude of change in the latter. Nerve growth factor inducible-A-binding protein 1 (Nab1) is capable of repressing the activity of the immediate early gene Ngfi-A (a zinc-finger transcription factor) through direct binding (48). Previous reports show that Ngfi-A is upregulated in the SON of animals subjected to intraperitoneal injections of hypertonic saline (29) and under the control of cAMP Response Element Binding Protein (Creb; Ref. 33). Not only is Nab1 upregulated here in both SL and WD but also Creb3l1, a gene we have previously shown to directly regulate AVP expression within the SON (17). Endothelial protein C receptor (Procr) regulates the protein C anticoagulant and anti-inflammatory pathways (38, 58) and is upregulated in the SON in response to both SL and WD. Activating transcription factor 5 is a basic-leucine-zipper transcription factor induced by endoplasmic reticulum stress (55) and is regulated by both SL and WD in the SON. Finally, phosophoserine phosphatase (Psph) catalyzes the final step of l-serine biogenesis (16) and is confirmed as regulated by osmotic stress in this study.
Our validation presented here reveals a degree of false discovery that it is important to discuss. Of the 20 genes we attempted to validate with PCR, 6 genes demonstrated either type I or type II errors, though at least one of these, Rgs2, only just missed significance. We can partly account for these differences in terms of differences in the tissue collection methods between the array and the PCR experiments; the latter were punched rather than hand dissected, thus the qPCR validation is more structure specific than the array. Also, probe design was against the full transcript, rather than being biased toward the 3′ end of the RNA, where the Affymetrix probes are most likely to reside. Therefore, our qPCR validation represents the validation of the transcript rather than validation of the Affymetrix probe.
Perspectives and Signficance
We present here data that compares two discrete osmotic challenges, SL and WD. We have measured the response in terms of drinking and feeding behavior, physiological assessment of blood and urine, and quantification of circulating hormone levels in the blood. We also performed transcriptomic experiments on the SON of the hypothalamus after SL and compared this with our previously published data from the WD animal. Despite several fundamental differences between SL and WD in terms of natriuresis, drinking behavior, and circulating levels of the hormones AVP and OXT, the transcriptional response by the SON is very stable and we hypothesize that either the SON is not able to discriminate between these two osmotic cues or that this tissue displays a core plastic response that allows it to respond to different stimuli with a similar transcriptional program.
GRANTS
This work was supported by funding from the British Heart Foundation (RG/11/28714, to M. Greenwood, J. F. R. Paton, D. Murphy), the Biotechnology and Biological Sciences Research Council (BB/J005452/1, to C. C. T. Hindmarch, J. F. R. Paton, D. Murphy), and a High Impact Research Chancellory Grant (UM.C/625/1/HIR/MOHE/MED/22 H-20001-E000086) from the University of Malaya (to C. C. T. Hindmarch, S. Ziau Hoe, A. S. Mecawi, M. R. Mustafa, D. Murphy). This research was also supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke (for H. Gainer and K. R. Johnson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P.G., A.S.M., S.Z.H., M.R.M., G.A.A.-M., and C.C.T.H. performed experiments; M.P.G., A.S.M., S.Z.H., M.R.M., and C.C.T.H. analyzed data; M.P.G., A.S.M., H.G., D.M., and C.C.T.H. interpreted results of experiments; M.P.G., A.S.M., K.R.J., L.L.E., J.F.P., J.A.-R., H.G., D.M., and C.C.T.H. edited and revised manuscript; M.P.G., A.S.M., S.Z.H., K.R.J., L.L.E., J.F.P., J.A.-R., H.G., D.M., and C.C.T.H. approved final version of manuscript; A.S.M. and C.C.T.H. prepared figures; H.G., D.M., and C.C.T.H. conception and design of research; D.M. and C.C.T.H. drafted manuscript.
Supplementary Material
REFERENCES
- 1.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal 18: 579–591, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Adem A, Al Haj M, Benedict S, Yasin J, Nagelkerke N, Nyberg F, Yandle TG, Frampton CM, Lewis LK, Nicholls MG, Kazzam E. ANP and BNP responses to dehydration in the one-humped camel and effects of blocking the renin-angiotensin system. PLos One 8: e57806, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amabebe E, Idu F, Obika LF. Relationship between thirst perception and plasma arginine vasopressin concentration in man. Niger J Physiol Sci 27: 3–10, 2012. [PubMed] [Google Scholar]
- 4.Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev 84: 169–208, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Antunes-Rodrigues J, Ruginsk SG, Mecawi AS, Margatho LO, Reis WL, Ventura RR, da Silva AL, Vilhena-Franco T, Elias LLK. Frontiers in neuroscience neuroendocrinology of hydromineral homeostasis. In: Neurobiology of Body Fluid Homeostasis: Transduction and Integration, edited by De Luca LA, Menani JV, and Johnson AK. Boca Raton, FL: CRC, 2014. [Google Scholar]
- 6.Balment RJ, Brimble MJ, Forsling ML. Release of oxytocin induced by salt loading and its influence on renal excretion in the male rat. J Physiol 308: 439–449, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barth SW, Gerstberger R. Differential regulation of angiotensinogen and AT1A receptor mRNA within the rat subfornical organ during dehydration. Brain Res Mol Brain Res 64: 151–164, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Boyle CN, Lorenzen SM, Compton D, Watts AG. Dehydration-anorexia derives from a reduction in meal size, but not meal number. Physiol Behav 105: 305–314, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caquineau C, Douglas AJ, Leng G. Effects of cholecystokinin in the supraoptic nucleus and paraventricular nucleus are negatively modulated by leptin in 24-h fasted lean male rats. J Neuroendocrinol 22: 446–452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter DA, Murphy D. Cyclic nucleotide dynamics in the rat hypothalamus during osmotic stimulation: in vivo and in vitro studies. Brain Res 487: 350–356, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Morris M. Differentiation of brain angiotensin type 1a and 1b receptor mRNAs: a specific effect of dehydration. Hypertension 37: 692–697, 2001. [DOI] [PubMed] [Google Scholar]
- 12.da Silveira LT, Junta CM, Monesi N, de Oliveira-Pelegrin GR, Passos GA, Rocha MJ. Time course of c-fos, vasopressin and oxytocin mRNA expression in the hypothalamus following long-term dehydration. Cell Mol Neurobiol 27: 575–584, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28: 89–94, 1981. [DOI] [PubMed] [Google Scholar]
- 14.De Luca LA Jr, Pereira-Derderian DT, Vendramini RC, David RB, Menani JV. Water deprivation-induced sodium appetite. Physiol Behav 100: 535–544, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583–686, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Furuya S. An essential role for de novo biosynthesis of l-serine in CNS development. Asia Pac J Clin Nutri 17, Suppl 1: 312–315, 2008. [PubMed] [Google Scholar]
- 17.Greenwood M, Bordieri L, Greenwood MP, Rosso Melo M, Colombari DS, Colombari E, Paton JF, Murphy D. Transcription factor CREB3L1 regulates vasopressin gene expression in the rat hypothalamus. J Neurosci 34: 3810–3820, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakansson ML, Meister B. Transcription factor STAT3 in leptin target neurons of the rat hypothalamus. Neuroendocrinology 68: 420–427, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Hammer M, Ladefoged J, Olgaard K. Relationship between plasma osmolality and plasma vasopressin in human subjects. Am J Physiol Endocrinol Metab 238: E313–E317, 1980. [DOI] [PubMed] [Google Scholar]
- 20.Hatton GI. Function-related plasticity in hypothalamus. Annual Rev Neurosci 20: 375–397, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Heilig CW, Stromski ME, Blumenfeld JD, Lee JP, Gullans SR. Characterization of the major brain osmolytes that accumulate in salt-loaded rats. Am J Physiol Renal Fluid Electrolyte Physiol 257: F1108–F1116, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Hindmarch C, Fry M, Yao ST, Smith PM, Murphy D, Ferguson AV. Microarray analysis of the transcriptome of the subfornical organ in the rat: regulation by fluid and food deprivation. Am J Physiol Regul Integr Comp Physiol 295: R1914–R1920, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Hindmarch C, Yao S, Beighton G, Paton J, Murphy D. A comprehensive description of the transcriptome of the hypothalamoneurohypophyseal system in euhydrated and dehydrated rats. Proc Natl Acad Sci USA 103: 1609–1614, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hindmarch CC, Franses P, Goodwin B, Murphy D. Whole transcriptome organisation in the dehydrated supraoptic nucleus. Braz J Med Biol Res 46: 1000–1006, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hindmarch CC, Fry M, Smith PM, Yao ST, Hazell GG, Lolait SJ, Paton JF, Ferguson AV, Murphy D. The transcriptome of the medullary area postrema: the thirsty rat, the hungry rat and the hypertensive rat. Exp Physiol 96: 495–504, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Hindmarch CC, Murphy D. The transcriptome and the hypothalamo-neurohypophyseal system. Endocr Develop 17: 1–10, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Kamoi K, Sato F, Arai O, Ishibashi M, Yamaji T. Effects of plasma volume and osmolality on secretion of atrial natriuretic peptide and vasopressin in man. Acta Endocr 118: 51–58, 1988. [DOI] [PubMed] [Google Scholar]
- 28.Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K, Sokabe M, Iida H. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science 285: 882–886, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki M, Yamaguchi K, Saito J, Ozaki Y, Mera T, Hashimoto H, Fujihara H, Okimoto N, Ohnishi H, Nakamura T, Ueta Y. Expression of immediate early genes and vasopressin heteronuclear RNA in the paraventricular and supraoptic nuclei of rats after acute osmotic stimulus. J Neuroendocrinol 17: 227–237, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Kim G, Lee Y, Jeong EY, Jung S, Son H, Lee DH, Roh GS, Kang SS, Cho GJ, Choi WS, Kim HJ. Acute stress responsive RGS proteins in the mouse brain. Mol cells 30: 161–165, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz A. Control of renin synthesis and secretion. Am J Hypertens 25: 839–847, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Lightman SL, Young WS 3rd. Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol 394: 23–39, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lubelski D, Ponzio TA, Gainer H. Effects of A-CREB, a dominant negative inhibitor of CREB, on the expression of c-fos and other immediate early genes in the rat SON during hyperosmotic stimulation in vivo. Brain Res 1429: 18–28, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh IS, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10: 355–365, 2009. [DOI] [PubMed] [Google Scholar]
- 35.McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci 19: 1–6, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Mecawi AS, Vilhena-Franco T, Araujo IG, Reis LC, Elias LL, Antunes-Rodrigues J. Estradiol potentiates hypothalamic vasopressin and oxytocin neuron activation and hormonal secretion induced by hypovolemic shock. Am J Physiol Regul Integr Comp Physiol 301: R905–R915, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Mecawi AS, Vilhena-Franco T, Fonseca FV, Reis LC, Elias LL, Antunes-Rodrigues J. The role of angiotensin II on sodium appetite after a low-sodium diet. J Neuroendocrinol 25: 281–291, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Mohan Rao LV, Esmon CT, Pendurthi UR. Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood 124: 1553–1562, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutsuga N, Shahar T, Verbalis JG, Brownstein MJ, Xiang CC, Bonner RF, Gainer H. Selective gene expression in magnocellular neurons in rat supraoptic nucleus. J Neurosci 24: 7174–7185, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutsuga N, Shahar T, Verbalis JG, Xiang CC, Brownstein MJ, Gainer H. Regulation of gene expression in magnocellular neurons in rat supraoptic nucleus during sustained hypoosmolality. Endocrinology 146: 1254–1267, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Nedungadi TP, Dutta M, Bathina CS, Caterina MJ, Cunningham JT. Expression and distribution of TRPV2 in rat brain. Exp Neurol 237: 223–237, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obika LO, Amabebe E, Ozoene JO, Inneh CA. Thirst perception, plasma osmolality and estimated plasma arginine vasopressin concentration in dehydrated and oral saline loaded subjects. Niger J Physiol Sci 28: 83–89, 2013. [PubMed] [Google Scholar]
- 43.Perello M, Raingo J. Leptin activates oxytocin neurons of the hypothalamic paraventricular nucleus in both control and diet-induced obese rodents. PLos One 8: e59625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollock DM, Banks RO. Effect of atrial extract on renal function in the rat. Clin Sci 65: 47–55, 1983. [DOI] [PubMed] [Google Scholar]
- 45.Qiu J, Hindmarch CC, Yao ST, Tasker JG, Murphy D. Transcriptomic analysis of the osmotic and reproductive remodeling of the female rat supraoptic nucleus. Endocrinology 152: 3483–3491, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu J, Yao S, Hindmarch C, Antunes V, Paton J, Murphy D. Transcription factor expression in the hypothalamo-neurohypophyseal system of the dehydrated rat: upregulation of gonadotrophin inducible transcription factor 1 mRNA is mediated by cAMP-dependent protein kinase A. J Neurosci 27: 2196–2203, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinaman L, Vollmer RR, Karam J, Phillips D, Li X, Amico JA. Dehydration anorexia is attenuated in oxytocin-deficient mice. Am J Physiol Regul Integr Comp Physiol 288: R1791–R1799, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Russo MW, Sevetson BR, Milbrandt J. Identification of NAB1, a repressor of NGFI-A- and Krox20-mediated transcription. Proc Natl Acad Sci USA 92: 6873–6877, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scalera G, Tarozzi G. Sapid solutions and food intake in repeated dehydration and rehydration periods in rats. Exp Physiol 86: 489–498, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Sharman G, Ghorbel M, Leroux M, Beaucourt S, Wong LF, Murphy D. Deciphering the mechanisms of homeostatic plasticity in the hypothalamo-neurohypophyseal system–genomic and gene transfer strategies. Prog Biophysics Molec Biol 84: 151–182, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Snyder SE, Salton SR. Expression of VGF mRNA in the adult rat central nervous system. J Comp Neurol 394: 91–105, 1998. [PubMed] [Google Scholar]
- 52.Song Z, Levin BE, Stevens W, Sladek CD. Supraoptic oxytocin and vasopressin neurons function as glucose and metabolic sensors. Am J Physiol Regul Integr Comp Physiol 306: R447–R456, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart L, Hindmarch CC, Qiu J, Tung YC, Yeo GS, Murphy D. Hypothalamic transcriptome plasticity in two rodent species reveals divergent differential gene expression but conserved pathways. J Neuroendocrinol 23: 177–185, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Theodosis DT, El Majdoubi M, Pierre K, Poulain DA. Factors governing activity-dependent structural plasticity of the hypothalamoneurohypophysial system. Cell Mol Neurobiol 18: 285–298, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Torres-Peraza JF, Engel T, Martin-Ibanez R, Sanz-Rodriguez A, Fernandez-Fernandez MR, Esgleas M, Canals JM, Henshall DC, Lucas JJ. Protective neuronal induction of ATF5 in endoplasmic reticulum stress induced by status epilepticus. Brain 136: 1161–1176, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Toshinai K, Saito T, Yamaguchi H, Sasaki K, Tsuchimochi W, Minamino N, Ueta Y, Nakazato M. Neuroendocrine regulatory peptide-1 and -2 (NERPs) inhibit the excitability of magnocellular neurosecretory cells in the hypothalamus. Brain Res 1563: 52–60, 2014. [DOI] [PubMed] [Google Scholar]
- 57.van Beek J, van Meurs M, t Hart BA, Brok HP, Neal JW, Chatagner A, Harris CL, Omidvar N, Morgan BP, Laman JD, Gasque P. Decay-accelerating factor (CD55) is expressed by neurons in response to chronic but not acute autoimmune central nervous system inflammation associated with complement activation. J Immunol 174: 2353–2365, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Vassiliou AG, Kotanidou A, Mastora Z, Maniatis NA, Albani P, Jahaj E, Koutsoukou A, Armaganidis A, Orfanos SE. Elevated soluble endothelial protein C receptor levels at ICU admission are associated with sepsis development. Minerva Anestesiol 81: 125–134, 2015. [PubMed] [Google Scholar]
- 59.Verbalis JG, Mangione MP, Stricker EM. Oxytocin produces natriuresis in rats at physiological plasma concentrations. Endocrinology 128: 1317–1322, 1991. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Li Y, Dalle Lucca SL, Simovic M, Tsokos GC, Dalle Lucca JJ. Decay accelerating factor (CD55) protects neuronal cells from chemical hypoxia-induced injury. J Neuroinflammation 7: 24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss ML, Hatton GI. Collateral input to the paraventricular and supraoptic nuclei in rat. I. Afferents from the subfornical organ and the anteroventral third ventricle region. Brain Res Bull 24: 231–238, 1990. [DOI] [PubMed] [Google Scholar]
- 62.Yue C, Mutsuga N, Verbalis J, Gainer H. Microarray analysis of gene expression in the supraoptic nucleus of normoosmotic and hypoosmotic rats. Cell Mol Neurobiol 26: 959–978, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.