Abstract
To what extent hypoxia alters the adenosine (ADO) system and impacts on cardiac function during embryogenesis is not known. Ectonucleoside triphosphate diphosphohydrolase (CD39), ecto-5′-nucleotidase (CD73), adenosine kinase (AdK), adenosine deaminase (ADA), equilibrative (ENT1,3,4), and concentrative (CNT3) transporters and ADO receptors A1, A2A, A2B, and A3 constitute the adenosinergic system. During the first 4 days of development chick embryos were exposed in ovo to normoxia followed or not followed by 6 h hypoxia. ADO and glycogen content and mRNA expression of the genes were determined in the atria, ventricle, and outflow tract of the normoxic (N) and hypoxic (H) hearts. Electrocardiogram and ventricular shortening of the N and H hearts were recorded ex vivo throughout anoxia/reoxygenation ± ADO. Under basal conditions, CD39, CD73, ADK, ADA, ENT1,3,4, CNT3, and ADO receptors were differentially expressed in the atria, ventricle, and outflow tract. In H hearts ADO level doubled, glycogen decreased, and mRNA expression of all the investigated genes was downregulated by hypoxia, except for A2A and A3 receptors. The most rapid and marked downregulation was found for ADA in atria. H hearts were arrhythmic and more vulnerable to anoxia-reoxygenation than N hearts. Despite downregulation of the genes, exposure of isolated hearts to ADO 1) preserved glycogen through activation of A1 receptor and Akt-GSK3β-GS pathway, 2) prolonged activity and improved conduction under anoxia, and 3) restored QT interval in H hearts. Thus hypoxia-induced downregulation of the adenosinergic system can be regarded as a coping response, limiting the detrimental accumulation of ADO without interfering with ADO signaling.
Keywords: anoxia-reoxygenation, arrhythmias, glycogen metabolism, hypoxia, adenosine signaling
low oxygen level is a prerequisite for normal embryonic and fetal growth including the cardiovascular system. However, hypoxia-induced imbalance between O2 supply and demand impacts gene expression, metabolism, growth, and function (13, 17, 20, 21, 33, 45, 48, 50, 53, 56). Oxygen deprivation during critical periods of embryogenesis impairs heart development and function with hemodynamic disturbances resulting in fetal growth retardation and increasing the risk of cardiovascular disease in adulthood, the so-called “fetal programming” (8, 12, 26, 38, 47, 57, 59). Maternal hypoxemia, reduction in umbilical blood flow, or placental insufficiency can rapidly lead to acute or chronic ischemia and/or hypoxia, which exacerbates ATP-derived adenosine (ADO) production. Since ADO represents an important regulator of the embryonic cardiovascular function (35), the present study focuses on the modulation of the adenosinergic system by hypoxia and its functional consequences in the developing heart. Generally ADO has negative chrono-, dromo-, and inotropic actions (28, 55, 62) and can be anti- or proarrhythmic both in adult and developing hearts (3, 5, 22, 25, 40, 51). We have recently shown in the embryonic chick heart model that ADO metabolism mainly relies on ectonucleoside triphosphate diphosphohydrolase (eNTPDase, apyrase, CD39), ecto-5′-nucleotidase (eNT, CD73), adenosine kinase (AdK), and adenosine deaminase (ADA). CD39 and CD73 sequentially convert ATP to ADO and ADA mediates conversion of ADO to inosine (INO). ADO is transported across cell membrane by equilibrative (ENT1,3,4) or concentrative (CNT3) transporters and interacts with the four subtypes of ADO receptors (AR), i.e., A1AR, A2AAR, A2BAR, and A3AR (5, 22, 40, 41, 51). Pharmacological disturbances of nucleosides metabolism and transport in the embryonic heart can rapidly lead to interstitial accumulation of ADO and INO and provoke arrhythmias in an autocrine/paracrine manner through A1AR and A2AAR stimulation and ERK2 activation (41). Activation of ADO receptors is also known to play a major role in protection of heart development against exposure to intrauterine hypoxia (39, 58); in regulation of heart rate, conduction, and contractility; and in modulation of substrate metabolism, in particular glucose uptake and utilization (18). During early cardiogenesis the myocardium relies mostly on glycolysis for its energy needs (17, 42). Glycogen is 10- to 20-fold more concentrated than in the adult heart, and when oxygen and glucose are lacking it represents an important source of glucose to maintain the production of glycolytically derived ATP with high, intermediate, and low glycogenolytic capacities in the atria (A), ventricle (V), and outflow tract (OT), respectively (42). The fact that glycogen synthase (GS) activity is specially high in the embryonic/fetal heart, accounting for the important glycogen stores (14), prompted us to investigate the role that ADO signaling could play in regulation of GS and glycogen content. Furthermore, to what extent a prolonged hypoxia during embryogenesis alters expression of the enzymes, transporters, and receptors of the adenosinergic system within the developing heart and affects its tolerance to a subsequent episode of anoxia/reoxygenation were examined. This work was aimed to decipher the mechanisms by which the developing myocardium can cope with an adverse hypoxic environment through ADO signaling, especially during a critical period of heart morphogenesis.
MATERIALS AND METHODS
Drugs, Antibodies, Reagents, and Medium
ADO, INO, specific agonists of A1AR (CCPA), A2AAR (CGS-21680), and A3AR (IB-MECA), and EHNA (inhibitor of ADA) were purchased from Sigma Aldrich. Goat anti-rabbit horseradish peroxidase-conjugated secondary antibody used for Western blotting determinations was from GE Healthcare. Antibodies against phosphorylated and total forms of AKT, GSK3β (Ser9), and GS were from Cell Signaling. Anti-HIF-1α was from Novus Biologicals and anti-GAPDH from Abcam. Immunoreactive bands were detected with enhanced chemiluminescent procedure. To assess apoptotic activity, the anti caspase-3 monoclonal antibody from Cell Signaling Technology was used to detect pro-caspase-3 and cleaved (active) caspase-3. Secondary antibodies were Alexa Fluor 680 goat anti-rabbit IgG (H+L) (Molecular Probes) and IRDye 800-conjugated affinity purified anti-Mouse IgG (H+L) (Rockland) for quantification with the Odyssey infrared imaging system. For ADO and INO determination by HPLC, all solvents were HPLC grade (Carlo Erba Reagenti). The standard HCO3−-CO2 buffered medium was composed of (in mmol/l) 99.25 NaCl, 0.3 NaH2PO4, 10 NaHCO3, 4 KCl, 0.79 MgCl2, 0.75 CaCl2, and 8 d-glucose. This culture medium was equilibrated in the chamber (see Anoxia-reoxygenation and electromechanical recordings) with 2.31% CO2 in air yielding a pH of 7.4.
Hypoxia Protocol
Fertilized eggs from Lohman Brown hens were incubated 96 h to stage 24HH according to the Hamburger-Hamilton (16) at an oxygen level of 21% O2 (normoxic group, N) or transiently at 10% O2 (hypoxic group, H) in a hypoxic chamber placed in an incubator maintained at 37.5°C and 80–90% humidity. The chamber containing 16 eggs positioned horizontally was equipped with an inlet and outlet and continuously flushed with a humidified calibrated gas (10% O2, rest N2). To assess the rate of ADO and INO accumulation in the myocardium and the kinetics of gene expression, the eggs were exposed to hypoxia during the last 1, 2, 3, 4, and 6 h of incubation. However, the standard duration of hypoxia used in this study was 6 h (Fig. 1A). This investigation fully conformed with the “Guiding Principles for Research Involving Animals and Human Beings” of the American Physiological Society and was approved by the veterinary authorities of the State of Vaud, Switzerland.
Fig. 1.
A: standard experimental protocol. Embryos were submitted in ovo to normoxia during 96 h or 90 h normoxia followed by 6 h of hypoxia. Normoxic (N) and hypoxic (H) hearts were then dissected for determination of adenosine (ADO), inosine (INO), protein, glycogen, and qRT-PCR in atria (A), ventricle (V), and outflow tract (OT), or subjected in vitro to anoxia-reoxygenation in absence or presence of ADO. Electrocardiogram (ECG) and ventricle shortening were recorded throughout in vitro experiments. B: investigated components of the adenosinergic system present in the embryonic heart. A1, A2A, A2B, and A3: subtypes of adenosine receptors (AR); AdK: adenosine kinase; APY/CD39: ecto-ATP apyrase; cNT, eNT/CD73: cytosolic, ecto-5′-nucleotidase; cADA and eADA: cytosolic and extracellular adenosine deaminase; ENT1,3,4 and CNT3: equilibrative and concentrative nucleoside transporters. No change, decrease and increase in gene expression (or in ADO and INO level) after 6 h hypoxia are indicated by = and downward and upward grey arrows, respectively. Hypoxia 1) increased ADO concentration without affecting INO level, 2) downregulated expression of A1AR and A2BAR but not A2AAR and A3AR, and 3) downregulated expression of all enzymes and transporters.
Experimental Procedures
Glycogen metabolism.
To determine whether ADO could alter myocardial glycogen content, N or H spontaneously beating hearts were placed in 24 plastic wells in the absence or presence of ADO (1 mM) during 90 min at 37.5°C under normoxia. Hearts were then dissected in atria (A), ventricle (V), and outflow tract (OT), and all parts were stored at −20°C for subsequent biochemical determinations. As the Akt/GSK3β/GS cascade could be involved in glycogen metabolism via ADO receptors, phosphorylation level of the three enzymes was determined after 7, 15, 30, 60, and 90 min ± ADO. Furthermore, instead of ADO, specific agonists of A1AR, A2AAR, and A3AR were used to identify the receptor involved. The rate of glycogen depletion in glucose-free medium was also determined ± ADO in A, V, and OT.
ADO-induced arrhythmias.
The transient arrhythmogenic effect of exogenous ADO on activity of isolated N and H hearts was also investigated. The ratio of the number of arrhythmic hearts (all types of arrhythmias) to rhythmic hearts at a given time point was determined and reported as “percentage of arrhythmic hearts” as previously published (40).
Anoxia-reoxygenation and electromechanical recordings.
To determine to what extent the response of N and H hearts to anoxia-reoxygenation can be differently altered by ADO, spontaneously beating hearts were placed in the culture compartment of an airtight chamber maintained at 37.5°C. Electrocardiogram (ECG) and contractile activity were recorded as described elsewhere in detail (44). ECG of the ex vivo embryonic heart displayed characteristic P, QRS, and T components, which allowed to assess PP and PR intervals, QT duration, amplitude, and width of the QRS complex and T wave. Because of the variations of the ECG morphology from one heart to another, the isoelectric line was technically difficult to determine, making difficult the determination of the actual QT interval. Instead, the time interval between the Q wave and the peak (p) of the T wave was easily measurable and reported as QTp as described elsewhere (40). Simultaneously with ECG, ventricular shortening at the apex was determined using wall motion detection, which allowed to investigate the ventricular electromechanical delay (EMDv), reflecting excitation-contraction (E-C) coupling. After 45 min of stabilization, hearts were submitted to 30 min of anoxia (0% O2) followed by 60 min of reoxygenation (21% O2) in the absence or presence of 1 mM ADO (Fig. 1A). Arrhythmias induced by anoxia-reoxygenation were characterized mostly according to the classical diagnosis used in clinical human practice and were considered as present if documented at least once in a given recording (44). The efficiency of the atrioventricular (AV) propagation was calculated as the ratio of the ventricular to the atrial electrical activity duration and was expressed as a percentage, 100% representing one to one AV conduction (46).
Quantitative Real-Time PCR Analysis
The quantitative real-time PCR (qRT-PCR) has been performed as described in detail in our recent work (41). The following sets of oligonucleotides were purchased from Microsynth (Balgach, Switzerland): GAPDH, A1AR, A2AAR, A2BAR, A3AR, AdK, CD39, CD73, ADA, ENT1, ENT3, ENT4, CNT3, and HIF-1α (Table 1). The level of mRNA expression in A, V, and OT was investigated with GAPDH as a reference gene (41). The framework of the components of the adenosinergic system investigated in this study is represented in Fig. 1B.
Table 1.
Sequences of primers for quantitative PCR
| Sequences of Primers for Quantitative PCR |
|||
|---|---|---|---|
| Target | PubMed Accession No. | Forward primer | Reverse primer |
| A1AR | NM_204316.3 | GAAGATCCCGGTCAGGTATAAGAG (327–350) | GGTTATTCCAACCGAACATTGG (424–445) |
| A2AAR | XM_425280.4 | GCAGCTCAAGCAGATGGAGAAC (645–666) | TGGCTGCGTGGACTTCCTT (706–724) |
| A2BAR | NM_205087.1 | GCTGCTTTTCGTGTCTCTTTGAG (497–519) | CAGCCGAAGAAGTTGAAGTATACCA (542–566) |
| A3AR | NM_204151.1 | CCACATGACATTAAGCCCAAAA (1107–1128) | AGCGACAACCAAGCCAAATT (1167–1186) |
| CD39 | NM_204247.1 | GCT GGT CGA TGG CTA TAA GTT TG (1230–1252) | CTG CGT TTC CTG CCT TCT G (1285–1303) |
| CD73 | XM_419855 | TGC TTT GTG ATG CTA TGC TTT ATG (1052–1075) | AGT GAA ACA TGG TTC CAC GAC TT (1099–1121) |
| ADA | NM_001006290.1 | AAA GGG ATT TCC GCA TCA AA (425–444) | CTG GAG ACC AAC TTG GCA TAT (474–493) |
| AdK | NM_001006501.1 | GAGACGGAAGCTGCCACTTTT (658–678) | CGGGCTATCTCTTTAATGTCTTCAG (701–725) |
| ENT1 | XM_419491.3 | GGCCATCTTCTCCTACGTTCTTC (627–649) | GGTACTCTGTCTTGTCCTTCATGGA (679–703) |
| ENT3 | XM_421594.3 | AACCGGCCCCCTCTGA (218–233) | CGGTCGAAGCGATGGAGAT (259–277) |
| ENT4 | XM_003642144.1 | CGC ATC TCT GTG GGC TAC CT (352–371) | GTC GCA GAT GCT GAC GAA GC (392–411) |
| CNT3 | XM_425033.3 | GTG GGA GCA TGT ACG TAA ATG G (1295–1316) | AAA GAG CAT AGG TGG CAA TGG T (1348–1369) |
| GAPDH | NM_204305.1 | GTG GAG GGT CTT ATG ACC ACT GT (489–511) | GGG CCA TCC ACC GTC TTC (533–550) |
| HIF1α | NM_204297.1 | CACACCATGATATGTTCACGAAA (866–888) | AACCCAGACGTAGCCACCTT (929–948) |
See text for additional details.
Western Blotting
Because of the very small size of the hearts (∼70 μg protein), three hearts or 6 atria, 3 ventricles, and 6 outflow tracts were pooled for each determination. Briefly, samples were denatured, separated on SDS-polyacrylamide gels, and electroblotted on nitrocellulose membranes (41). Membranes were blocked and probed overnight with phosphorylated or total form antibodies. The membranes were then incubated with secondary anti-rabbit antibody. Immunoreactive bands were detected with enhanced chemiluminescent procedure.
Adenosine and Inosine Determination
ADO and INO myocardial content (i.e., in intracellular + interstitial compartments; expressed as nmol/mg protein) was determined by high performance liquid chromatography according to our previous work (41). Hearts from embryos exposed in ovo to normoxia or 2, 4, and 6 h of hypoxia were rapidly explanted at 0–4°C in the medium containing the ADA inhibitor (EHNA, 10 μM) to avoid ADO degradation into INO and stored at −80°C.
Protein and glycogen determination.
Protein and glycogen were determined according to our previous works (37, 42). Briefly, protein content was determined using BCA protein assay kit (Thermo Scientific Pierce) and BSA as the standard. Glycogen content was determined spectrofluorometrically according to Nahorski and Rogers (29), using an automated setup (Synergy MX, Biotek, 96 wells) and expressed as equivalent of glucose units (GU) normalized for protein content. Before dissection in A, V, and OT and storage at −20°C, all hearts were thoroughly rinsed at 0–4°C (60 min on a rotary shaker) in glucose-free solution to eliminate all traces of glucose remaining in the cardiac cavities and that could interfere with ulterior measurements. Glycogen and protein were determined in the same hearts or cardiac parts. After being thawed, all samples were sonicated (3× 2 s on an ice bath) for biochemical determinations.
Statistical Analysis
All values are reported as means ± SE unless otherwise indicated. The significance of any difference between groups was assessed using the Mann-Whitney test. The statistical significance was defined by a value of P ≤ 0.05 (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
RESULTS
Metabolic Consequences of Hypoxia
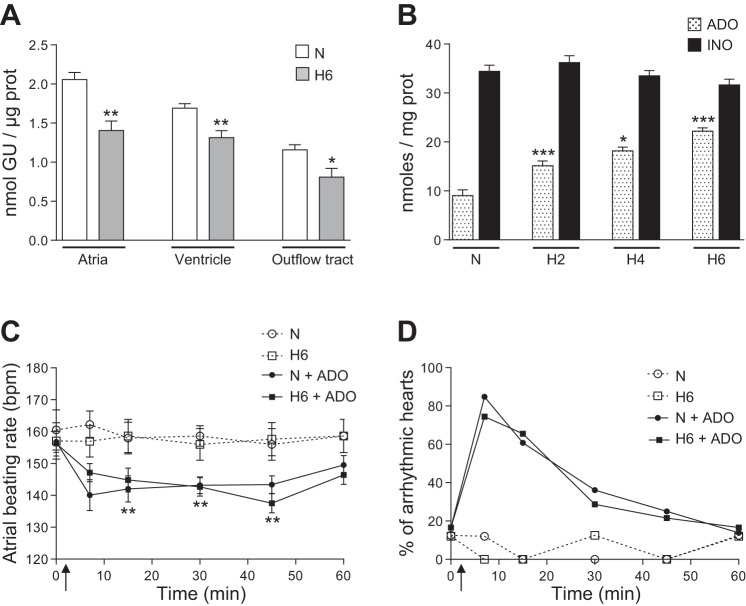
The myocardial growth was not altered by 6 h of hypoxia in ovo since the protein content of A, V, and OT was not affected, i.e., 17.2 ± 0.8 μg (n = 30) and 19.8 ± 1.9 μg (n = 16) in A, 31.5 ± 1.3 μg (n = 59) and 33.6 ± 2.1 μg (n = 31) in V, 12.1 ± 0.6 μg (n = 30) and 13.4 ± 1.4 μg (n = 16) in OT of normoxic and hypoxic hearts, respectively. Furthermore, the hearts of embryos submitted to hypoxia were quite resistant to apoptosis since the level of caspase-3 activation in A, V, and OT was not altered relative to the normoxic hearts (not shown). However, glycogen content was significantly decreased in all parts of the hearts after hypoxia indicating a metabolic adaptation to oxygen deficiency (Fig. 2A). During hypoxia ADO progressively accumulated in the heart but INO concentration did not change (Fig. 2B).
Fig. 2.
A: glycogen content in A, V, and OT of normoxic (N) and hypoxic (H6) hearts (GU: glucose unit; n = 6–28). B: myocardial ADO and INO levels throughout in ovo hypoxia (H2, H4 and H6: 2, 4 and 6 h hypoxia; n = 16–18). C and D: exogenous ADO (100 μM) induced bradycardia (C) and arrhythmias (D) to the same extent in both normoxic (N) and hypoxic (H6) hearts (**relative to the respective control N and H6; n = 8 hearts per condition; upward arrows indicate the time of ADO treatment). In A and B: *, **, *** versus normoxic conditions (N).
In both N and H hearts exposure to exogenous ADO rapidly and transiently induced bradycardia and arrhythmias to the same extent, showing that 6 h of hypoxia during cardiogenesis did not alter the initial response to an acute adenosinergic stimulation (Fig. 2, C and D).
ADO Preserved Glycogen Through Akt-GSK3β-GS Signaling Cascade
Exposure to ADO during 90 min in vitro in the presence of glucose (8 mM) preserved glycogen in the ventricle of both N and H6 hearts (Fig. 3A) but had no effect in A and OT (not shown) relative to untreated groups. However, in the absence of glucose, glycogen was rapidly depleted and ADO did not alter the high rate of glycogenolysis in neither V (Fig. 3B) nor in A and OT (not shown).
Fig. 3.
A: glycogen content in the ventricle of N and H hearts in absence or presence of ADO (1 mM) after 90 min in vitro under normoxia (n = 16 determinations per condition). B: progressive depletion of glycogen in the ventricle of the hearts beating in vitro without glucose. ADO (1 mM) had no effect on the rate of glycogenolysis. n = 8–10. C: effect of ADO (1 mM) on phosphorylation level of Akt, GSK3β, and GS in the whole heart during 90 min in vitro under normoxia (n = 3–10 determinations). D: after 10 min, ADO (100 μM) or the A1AR agonist (CCPA, 10 μM) significantly increased the phosphorylation level of Akt and GSK3β in the whole heart, whereas agonists of A2AAR (CGS-21680 10 μM) and A3AR (IB-MECA 10 μM) had no effect (n = 5–12 determinations). Representative Western blot (top) and quantification (bottom) are shown.
As glycogen synthesis depends on GS activity we assessed in the absence or presence of ADO the level of phosphorylation of Akt, GSK3β, and GS known to be sequentially involved in glycogen regulation (Fig. 3C). Within the first 15 min of exposure to ADO, Akt was activated (phosphorylated) and GSK3β was inactivated (phosphorylated). As expected, GS phosphorylation decreased, showing that GS was activated, a trend that was maintained during 90 min. In the presence of ADO, Akt and GSK3β were phosphorylated mainly via A1AR activation since only the specific agonist of A1AR (CCPA) increased Akt and GSK3β phosphorylation, whereas the agonists of A2AAR and A3AR (CGS-21680 and IB-MECA, respectively) had no effect (Fig. 3D).
Expression of Genes Involved in ADO Signaling Was Strongly Altered by Hypoxia
Distribution of gene expression within the normoxic embryonic heart.
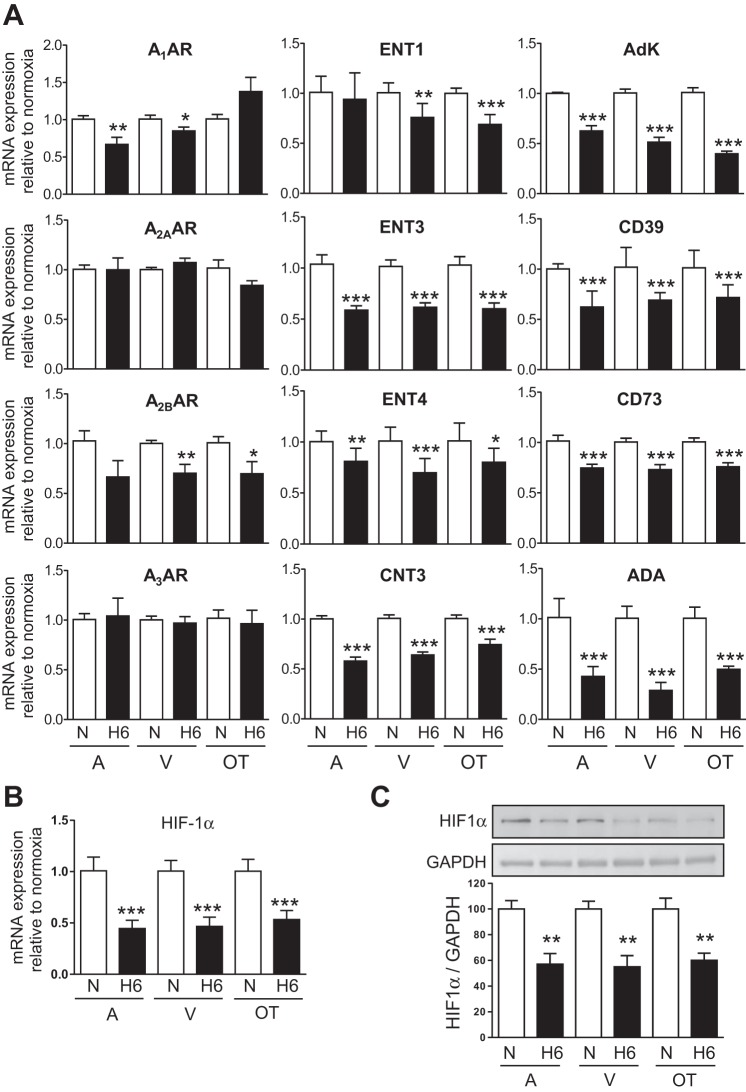
In the present work we found that A1AR, A2BAR, A3AR, and AdK mRNA levels were the highest in A, whereas expression of A2AAR was the highest in A and OT (Fig. 4). Our recent complementary study also shows that the highest expression of CD39, ADA, and CNT3 is found in A and that of CD73 in V. ENT1 and ENT3 are homogeneously distributed, whereas ENT4 expression is the highest in the OT (41).
Fig. 4.

Spatial variations of basal gene expression in the embryonic heart of the four adenosine receptors (AR) and adenosine kinase (AdK) n = 6–9 determinations. *, ***ventricle (V) or outflow tract (OT) versus atria (A), respectively.
Differential gene regulation by hypoxia in atria, ventricle, and outflow tract.
Exposure of the embryos to 6 h of hypoxia differentially decreased the level of mRNA expression of A1AR and A2BAR within the heart but did not affect expression of A2AAR and A3AR (Fig. 5A). Expression of the investigated equilibrative (except ENT1 in A) and concentrative nucleoside transporters as well as expression of all intracellular and extracellular enzymes involved in ADO metabolism were significantly downregulated by hypoxia. The hypoxia-specific transcription factor HIF-1α was stable during the first 3 h of hypoxia in A, V, and OT (not shown) and downregulated afterward at the mRNA and protein levels (Fig. 5, B and C).
Fig. 5.
A: effect of 6 h of hypoxia (H6) on expression of the subtypes of adenosine receptors (A1AR, A2AAR, A2BAR, and A3AR), equilibrative (ENT1, 3 and 4), and concentrative (CNT3) nucleoside transporters and enzymes (Adk, CD39, CD73, and eADA) relative to normoxia (N) in A, V, and OT. n = 6–9 determinations for each gene. *, **,*** versus respective N heart. Decrease of HIF-1α mRNA (B) and protein (C) after 6 h hypoxia (H6) relative to N. n = 5–7 determinations.
Gene regulation of ADA and CD73 after 1, 2, 3, and 4 h hypoxia in A, V, and OT showed a characteristic spatiotemporal pattern (Fig. 6). Interestingly, of the two major enzymes regulating production (CD73) and degradation (ADA) of ADO, the most rapid (1 h) and marked hypoxia-induced downregulation was found for ADA in A, whereas CD73 was transiently upregulated after 2 h in A (Fig. 6) and downregulated after 6 h hypoxia in all parts of the heart (Fig. 5A).
Fig. 6.
Spatio-temporal pattern of gene regulation. Expression of ADA and CD73 in A, V, and OT after 1, 2, 3, and 4 h hypoxia (H1, H2, H3, and H4, respectively) relative to N. n = 3 determinations for each gene investigated. *, *** versus N.
Effects of hypoxia and ADO on cardiac activity during anoxia-reoxygenation.
Under preanoxia, all N hearts (± ADO) were rhythmic, whereas 50% (n = 6) and 33% (n = 6) of the H6 hearts presented spontaneous arrhythmias in the absence and presence of ADO, respectively. Anoxia and reoxygenation transiently and significantly altered electromechanical parameters and induced several types of arrhythmias in both N and H hearts. The principal types of arrhythmias were transient atrial tachycardia (range 180–300 beats/min) and bradycardia (range 110–140 beats/min), atrial ectopy, second degree AV blocks (2:1 to 8:1), Wenckebach phenomenon (Mobitz type I), third degree AV blocks (ventricular escape beats), and intermittent cardioplegia as previously documented (44, 45). Some of these arrhythmias are illustrated in Fig. 7. Hypoxic hearts showed the lowest atrial and ventricular tolerance to anoxia and the most delayed recovery at reoxygenation relative to N hearts (Fig. 8). Under anoxia, in both the N and H6 groups ADO significantly prolonged electrical activity of A and V (Fig. 8A) and improved the efficiency of the AV propagation (Fig. 8B). At reoxygenation, 20% and 33% of the untreated N and H6 hearts presented third-degree AVB, whereas the presence of ADO increased these percentages to 100% and 62%, respectively (see Fig. 7B at 10 min reoxygenation). Moreover, in the N group ADO delayed ventricular but not atrial recovery (Fig. 8C), which also suggests that ADO tended to dissociate atrial and ventricular activity only during reoxygenation while ADO improved AV conduction under anoxia (Fig. 8B). However, electromechanical activity of all investigated hearts fully recovered after 60 min reoxygenation, whatever the treatment.
Fig. 7.
ECG recordings of ex vivo hearts showing the P, QRS, and T components and the principal arrhythmias observed in N (A and B) and H (C and D) hearts under preanoxia (basal), after 10 min anoxia and after 10 and 60 min reoxygenation (Reox) in absence (A and C) or presence (B and D) of ADO (1 mM). Atrial ectopy combined with second degree AVB (mainly 2:1) were observed after 10 min of anoxia and reoxygenation in all experimental groups. However, all N hearts (B) and 2/3 of H hearts (D) treated with ADO displayed third degree AVB (A and V beating at their intrinsic rate) after 10 min reoxygenation. QRS amplitude decreased during anoxia and upon reoxygenation (10 min) in all hearts. Note that ECG morphology strongly fluctuated throughout anoxia/reoxygenation but fully recovered after 60 min reoxygenation. Horizontal bar represents 200 ms. Vertical bar represents 50 μV.
Fig. 8.

Duration of atrial and ventricular electrical activities (including rhythmic and arrhythmic) (A) and efficiency of the atrioventricular (AV) propagation expressed in percentage under anoxia (B) in N and H6 hearts in absence (untreated) or presence of ADO (1 mM). Time to resume atrial and ventricular actvities on reoxygenation (C) of N and H hearts untreated or exposed to ADO. n = 5–8 hearts investigated in each experimental group.
ADO had, as expected, 1) a bradycardic effect associated with a decrease of PR interval under preanoxia in N hearts but not in H hearts, 2) restored QTp interval in H6 hearts under preanoxia and during reoxygenation, and 3) suppressed the bradycardia observed during the first 10 min of reoxygenation in N hearts (Fig. 9).
Fig. 9.
Changes in PP, PR, and QTp intervals in N and H6 hearts in presence or absence of ADO (1 mM) under preanoxia, after 10 min anoxia and after 10 and 60 min reoxygenation. PP, PR, and QTp fully recovered after 60 min reoxygenation. n = 5–8 hearts investigated in each experimental group.
The width of the QRS complex (preanoxic 11.1 ± 0.3 ms, n = 20) and EMDv (preanoxic 15.7 ± 0.6 ms, n = 19) were transiently and significantly increased (P ≤ 0.01) after 10 min anoxia and after 10 min reoxygenation by 27% and 33% for QRS and 65% and 110% for EMDv, respectively, with no difference between the four experimental groups. These data indicate that intraventricular conduction and E-C coupling were impaired reversibly during anoxia-reoxygenation and, in particular, that ADO had no effect on these parameters.
Discussion
Metabolic Consequences of Hypoxia
Under our experimental conditions the level of oxygenation was strictly controlled since in the chicken hypoxia acts directly on the embryo developing in ovo without any maternal influence, by contrast with mammalian embryo developing in utero. The protein content in the A, V, and OT did not change after 6 h hypoxia relative to normoxia showing that the growth rate was not affected. Our data also show that on the basis of caspase-3 activation, the programmed cell death required for normal cardiac morphogenesis was not increased by 6 h hypoxia in neither A and V nor in OT, which displays the highest apoptotic activity (2, 11). Thus, like the embryonic mouse heart (ED9.5) (57), the 4-day-old embryonic chick heart appears to be quite resistant to hypoxia-induced apoptosis.
The high myocardial concentration of ADO found in ovo under normoxia appears to be a metabolic characteristic for the embryonic heart developing normally in an environment poor in oxygen. This is in line with the important role that ADO plays in regulation of the embryonic cardiovascular function (35). It should be noticed that the ratio INO/ADO of 4 under normoxia is close to the ratio INO/ADO >3 found in hypoxic embryonic and adult cardiomyocytes (1, 27) and that INO can also alter cardiac activity in an autocrine/paracrine manner as we recently showed (41). In the present work, the time-dependent myocardial accumulation of ADO under hypoxia with no effect on INO level (Fig. 2A) can be partly explained by the marked downregulation of both AdK and ADA expression since AdK and eADA are the principal enzymes responsible for the removal of intra- and extracellular ADO, respectively (32).
Activation of the Adenosine A1 Receptor Played a Role in Glycogen Metabolism
After 6 h hypoxia, glycogen content significantly decreased in all cardiac parts, indicating that this endogenous substrate was mobilized to maintain cardiac energy metabolism and electromechanical activity. As it was technically difficult to assess in ovo the effect of ADO on glycogen metabolism without disturbing embryogenesis, we investigated this issue in ex vivo hearts. Under our experimental conditions, in the presence of glucose, ADO activated the Akt-GSK3β-GS cascade through A1AR favoring glycogen synthesis (and/or limiting glycogenolysis). By contrast, in glucose-free medium ADO did not affect the high glycogenolytic rate in A, V, and OT. These observations strongly suggest that accumulation of ADO during 6 h of hypoxia in ovo could regulate myocardial glycogen utilization and content through GS activation, a process that can cope with an adverse environment. These data are in line with the fact that A1AR activation preserves (10) or increases (7) glycogen level through activation of GS (9) in the ischemic-reperfused rat heart. It is noteworthy that GSK3β is also markedly phosphorylated in the anoxic-reoxygenated embryonic chick heart (34).
Hypoxia Downregulated the Adenosinergic System
Importantly, downregulation of ADA by hypoxia was the most marked and rapid in A relative to V and OT and compared with all the genes investigated. In addition, the level of ADA downregulation throughout hypoxia was significant after 1, 4, and 6 h in the A, V, and OT, respectively, thus displaying a decreasing atrio-ventriculo-conotruncal gradient (Fig. 6). As previously documented (41), ADA basal expression also exhibits a gradient along the heart tube (A = 1 > V = 0.5 > OT = 0.2). In combination with downregulation of ADA the transient upregulation of CD73 expression after 2 h hypoxia could temporarily contribute to interstitial accumulation of ADO, specially in atrial myocardium (Fig. 6), a phenomenon that disappeared after 6 h when both eADA and CD73 are downregulated. Regarding ADO signaling in the developing heart, such a region-specific gene expression and regulation indicates that A copes with adverse conditions the most rapidly compared with V and OT.
HIF-1α was strongly expressed as in the embryonic mouse heart (20), and its expression at the mRNA and protein level unexpectedly decreased in A, V, and OT after 6 h of hypoxia. Such a HIF-1α downregulation is also observed in the heart of the Tibet chicken embryo developing under hypoxia (13% O2) (63) and in various cell types (23, 52). The transcriptional control of A2BAR, CD73, ENT1, and AdK is known to involve HIF-1α (6), which is in line with our findings. Nevertheless, whether transcription of A1AR, A3AR, ENT3, ENT4, CNT3, CD39, and ADA involves HIF-1α remains unknown. The mechanism by which HIF-1α is negatively regulated by prolonged hypoxia is beyond the scope of this study.
In atria the functionally predominant receptor A1AR (40, 43) was the most downregulated by hypoxia while expression of the other receptors was unchanged (Fig. 5). The bradycardic and arrhythmogenic effects of ADO on both N and H6 hearts (Fig. 2) could be explained by the fact that A1AR shows the highest ligand activity and is the main receptor implicated in ADO-induced pacemaking and conduction disturbances (40). Furthermore, downregulation of A1AR in A and V might be critical since this receptor plays an important role in protection of the embryonic heart against prolonged hypoxia (49, 58). In line with this observation we found that the duration of electrical activity of A and V under anoxia was shorter in H6 hearts (in which A1AR is downregulated) than in N hearts (Fig. 8).
All enzymes and transporters (except ENT1 in A) were significantly less expressed in H than in N hearts. In particular, the reduced expression of the transporters could contribute to extracellular accumulation of ADO during hypoxia. However, the fact that the ADO level only doubled and INO level remained unchanged after 6 h hypoxia (Fig. 2B) could be partly due to the global downregulation of the expression of enzymes and transporters. This phenomenon might be considered as a process limiting an excessive and potentially arrhythmogenic accumulation of ADO during hypoxia. Indeed, by comparison, the only pharmacological inactivation of ADA and ENTs in N hearts, with no functional alteration of other components, rapidly increases ADO level eightfold within only 2 h and provokes arrhythmias (41).
Adenosine Was Cardioprotective During Anoxia-Reoxygenation
Although the embryonic/fetal heart develops and functions normally in an intrauterine environment characterized by a low oxygen level, it reacts rapidly to abrupt oxygen shortage and reoxygenation (19, 31, 36, 44). Such a stress was used in the context of ADO signaling to reveal possible functional differences between N and H6 hearts, which are not detectable otherwise. In the embryonic chick heart, the functional disturbances (i.e., chrono-, dromo- and inotropic effects) and the ultrastructural modifications (e.g., mitochondrial and nuclear swelling) induced by 30-min anoxia followed by 60-min reoxygenation are reversible (46). However, our present data show that the development of the 4-day-old chick embryo under hypoxia (6 h) led to an increased vulnerability of the heart subsequently exposed to anoxia/reoxygenation, even though ADO prolonged activity and improved A-V conduction during anoxia and normalized ventricular activation (QTp) under preanoxia and reoxygenation. For the same heart rate, QTp interval was shorter in H6 hearts than in N hearts, and this difference was abolished by ADO during preanoxia and troughout reoxygenation (Fig. 9). A possible interpretation is that such a QTp normalization in H6 hearts by ADO could be associated with the subtle increase of calcium entry in cardiomyocytes through L-type Ca2+ and/or TRPCs channels via A1AR activation as we recently demonstrated in the same experimental model (40, 43).
ADO may specially protect atrial pacemaking against a postanoxic stress as the bradycardia in the N hearts upon reoxygenation was suppressed by ADO. The fact that this effect was not observed in H6 hearts indicates that 6 h of hypoxia at high ADO level could improve the resistance against reoxygenation disturbances, alike in ADO-mediated preconditioning of ventricular myocytes of 14-day-old chick embryo (24) and in chronically hypoxic cardiomyocytes from rat heart (4).
We previously showed that reoxygenation of the embryonic heart after 30 min anoxia produces a burst of reactive oxygen species (ROS) in A, V, and OT (37). Furthermore, in chick embryonic cardiomyocytes (10-day-old embryo) the peak of ROS at reoxygenation is reduced by ADO through A1AR and A2AR (60). Also in adult cardiomyocytes, pretreatment with ADO reduces the reoxygenation-induced ROS production (30) and has antioxidant properties (15, 54, 61). The fact that H6 hearts displayed the lowest tolerance to 30 min anoxia and the most delayed recovery at reoxygenation relative to N hearts could be partly explained by glycogen depletion (30%). Indeed, we previously showed that glycogen depletion augments the reoxygenation-induced ROS production and/or weaken the defense against oxyradicals (37). Such observations and our present findings suggest that ADO-induced preservation of glycogen can afford cardioprotection against anoxia/reoxygenation following hypoxia.
Perspectives and Significance
The heart is the first organ to become fully functional early during embryogenesis and rapidly assures a proper blood flow to provide oxygen and nutrients to the other growing organs. Thus any disturbances of its activity by a stress could have detrimental consequences during embryonic or fetal life. The intrauterine hypoxia represents a clinically relevant stress at the level of the developing organism and exacerbates the ATP-derived ADO production. ADO is a major regulator of the function of the developing cardiovascular system, therefore, its metabolism and signaling under adverse conditions deserve to be investigated. Together, our data show for the first time that a reduction in ambient oxygen availability during early cardiogenesis rapidly decreases expression of enzymes, transporters, and specific receptors involved in the adenosinergic system (see Fig. 1B), which disturbs the electromechanical activity of the heart. However, such a downregulation of the components of the adenosinergic system can be regarded as a coping response to prolonged hypoxia, limiting the potentially detrimental accumulation of ADO in the myocardium without interfering with ADO signaling. Our experimental model can help to better decipher the cellular and molecular mechanisms underlying embryonic and fetal cardiac dysfunction induced by ADO accumulation when oxygenation is deficient.
GRANTS
This work was supported by the Swiss National Science Foundation (310030-27633).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E. Robin and E. Raddatz conception and design of research; E. Robin, F. Marcillac, and E. Raddatz performed experiments; E. Robin, F. Marcillac, and E. Raddatz analyzed data; E. Robin and E. Raddatz interpreted results of experiments; E. Robin and E. Raddatz prepared figures; E. Robin and E. Raddatz drafted manuscript; E. Robin and E. Raddatz edited and revised manuscript; E. Robin, F. Marcillac, and E. Raddatz approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Hadi Khalil for the determination of caspase-3 activity, Anne-Catherine Thomas for skillful technical assistance, André Singy for the construction of the hypoxic chamber, and Dominique Grandjean from the Swiss Federal Institute of Technology (Lausanne) for adenosine and inosine assay by HPLC.
REFERENCES
- 1.Abd-Elfattah AS, Aly H, Hanan S, Wechsler AS. Myocardial protection in beating heart cardiac surgery: I: pre- or postconditioning with inhibition of es-ENT1 nucleoside transporter and adenosine deaminase attenuates post-MI reperfusion-mediated ventricular fibrillation and regional contractile dysfunction. J Thorac Cardiovasc Surg 144: 250–255, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbosky L, Lawrence DK, Karunamuni G, Wikenheiser JC, Doughman YQ, Visconti RP, Burch JB, Watanabe M. Apoptosis in the developing mouse heart. Dev Dyn 235: 2592–2602, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J 9: 359–365, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Borchert GH, Yang C, Kolar F. Mitochondrial BKCa channels contribute to protection of cardiomyocytes isolated from chronically hypoxic rats. Am J Physiol Heart Circ Physiol 300: H507–H513, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen F, De Diego C, Chang MG, McHarg JL, John S, Klitzner TS, Weiss JN. Atrioventricular conduction and arrhythmias at the initiation of beating in embryonic mouse hearts. Dev Dyn 239: 1941–1949, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Ann Rev Physiol 74: 153–175, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jonge R, de Jong JW, Giacometti D, Bradamante S. Role of adenosine and glycogen in ischemic preconditioning of rat hearts. Eur J Pharmacol 414: 55–62, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology 21: 29–37, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Fraser H, Lopaschuk GD, Clanachan AS. Alteration of glycogen and glucose metabolism in ischaemic and post-ischaemic working rat hearts by adenosine A1 receptor stimulation. Br J Pharmacol 128: 197–205, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan XT, Cook MA, Moffat MP, Karmazyn M. Transient ischemia in the presence of an adenosine deaminase plus a nucleoside transport inhibitor confers protection against contractile depression produced by hydrogen peroxide. Possible role of glycogen. J Mol Cell Cardiol 28: 1165–1176, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Gardier S, Pedretti S, Sarre A, Raddatz E. Transient anoxia and oxyradicals induce a region-specific activation of MAPKs in the embryonic heart. Mol Cell Biochem 340: 239–247, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Ghatpande SK, Billington CJ Jr, Rivkees SA, Wendler CC. Hypoxia induces cardiac malformations via A1 adenosine receptor activation in chicken embryos. Birth defects research Part A. Clin Mol Teratol 82: 121–130, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giussani DA, Salinas CE, Villena M, Blanco CE. The role of oxygen in prenatal growth: studies in the chick embryo. J Physiol 585: 911–917, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez-Correa J, Hod M, Passoneau JV, Freinkel N. Glycogen and enzymes of glycogen metabolism in rat embryos and fetal organs. Biol Neonate 59: 294–302, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Hack B, Witting PK, Rayner BS, Stocker R, Headrick JP. Oxidant stress and damage in post-ischemic mouse hearts: effects of adenosine. Mol Cell Biochem 287: 165–175, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 88: 49–92, 1951. [PubMed] [Google Scholar]
- 17.Han M, Trotta P, Coleman C, Linask KK. MCT-4, A511/Basigin and EF5 expression patterns during early chick cardiomyogenesis indicate cardiac cell differentiation occurs in a hypoxic environment. Dev Dyn 235: 124–131, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Headrick JP, Ashton KJ, Rose'meyer RB, Peart JN. Cardiovascular adenosine receptors: expression, actions and interactions. Pharmacol Therap 140: 92–111, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Jensen A, Garnier Y, Berger R. Dynamics of fetal circulatory responses to hypoxia and asphyxia. Eur J Obstet Gynecol Reprod Biol 84: 155–172, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan J, Ahuja P, Bodenmann S, Knapik D, Perriard E, Krek W, Perriard JC. Essential role of developmentally activated hypoxia-inducible factor 1alpha for cardiac morphogenesis and function. Circ Res 103: 1139–1146, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn 220: 175–186, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Leone RJ Jr, Merrill GF. Inhibition of adenosine deaminase and administration of adenosine increase hypoxia induced ventricular ectopy. Basic Res Cardiol 90: 234–239, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Li QF, Wang XR, Yang YW, Lin H. Hypoxia upregulates hypoxia inducible factor (HIF)-3alpha expression in lung epithelial cells: characterization and comparison with HIF-1alpha. Cell Res 16: 548–558, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Liang BT. Protein kinase C-dependent activation of KATP channel enhances adenosine-induced cardioprotection. Biochem J 336: 337–343, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallet ML. Proarrhythmic effects of adenosine: a review of the literature. Emergency Med J 21: 408–410, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancini L, Bertossi M, Ribatti D, Bartoli F, Nico B, Lozupone E, Roncali L. The effects of long-term hypoxia on epicardium and myocardium in developing chick embryo hearts. Intl J Microcirc Clin Exper 10: 359–371, 1991. [PubMed] [Google Scholar]
- 27.Meghji P, Rubio R, Berne RM. Intracellular adenosine formation and its carrier-mediated release in cultured embryonic chick heart cells. Life Sci 43: 1851–1859, 1988. [DOI] [PubMed] [Google Scholar]
- 28.Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handbook Exper Pharmacol 161–188, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahorski SR, Rogers KJ. An enzymic fluorometric micro method for determination of glycoen. Analytic Biochem 49: 492–497, 1972. [DOI] [PubMed] [Google Scholar]
- 30.Narayan P, Mentzer RM Jr, Lasley RD. Adenosine A1 receptor activation reduces reactive oxygen species and attenuates stunning in ventricular myocytes. J Mol Cell Cardiol 33: 121–129, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Ostadal B, Ostadalova I, Dhalla NS. Development of cardiac sensitivity to oxygen deficiency: comparative and ontogenetic aspects. Physiol Rev 79: 635–659, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Park J, Gupta RS. Adenosine metabolism. Adenosine kinase, and evolution. In: Adenosine. A Key Link Between Metabolism and Brain Activity, edited by Masino S and Boison D. New York: Springer, 2013, p. 23–54. [Google Scholar]
- 33.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med 10: 653–666, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedretti S, Raddatz E. STAT3alpha interacts with nuclear GSK3beta and cytoplasmic RISK pathway and stabilizes rhythm in the anoxic-reoxygenated embryonic heart. Basic Res Cardiol 106: 355–369, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Porter GA Jr, Rivkees SA. Ontogeny of humoral heart rate regulation in the embryonic mouse. Am J Physiol Regul Integr Comp Physiol 281: R401–R407, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Raddatz E, Gardier S, Sarre A. Physiopathology of the embryonic heart (with special emphasis on hypoxia and reoxygenation). Ann Cardiol Angeiol (Paris) 55: 79–89, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Raddatz E, Thomas AC, Sarre A, Benathan M. Differential contribution of mitochondria, NADPH oxidases, and glycolysis to region-specific oxidant stress in the anoxic-reoxygenated embryonic heart. Am J Physiol Heart Circ Physiol 300: H820–H835, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol 295: R583–R595, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivkees SA, Wendler CC. Regulation of cardiovascular development by adenosine and adenosine-mediated embryo protection. Arterioscler Thromb Vasc Biol 32: 851–855, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robin E, Sabourin J, Benoit R, Pedretti S, Raddatz E. Adenosine A1 receptor activation is arrhythmogenic in the developing heart through NADPH oxidase/ERK- and PLC/PKC-dependent mechanisms. J Mol Cell Cardiol 51: 945–954, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Robin E, Sabourin J, Marcillac F, Raddatz E. Involvement of CD73, equilibrative nucleoside transporters and inosine in rhythm and conduction disturbances mediated by adenosine A1 and A2A receptors in the developing heart. J Mol Cell Cardiol 63: 14–25, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Romano R, Rochat AC, Kucera P, De Ribaupierre Y, Raddatz E. Oxidative and glycogenolytic capacities within the developing chick heart. Pediatr Res 49: 363–372, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Sabourin J, Antigny F, Robin E, Frieden M, Raddatz E. Activation of transient receptor potential canonical 3 (TRPC3)-mediated Ca2+ entry by A1 adenosine receptor in cardiomyocytes disturbs atrioventricular conduction. J Biol Chem 287: 26688–26701, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarre A, Maury P, Kucera P, Kappenberger L, Raddatz E. Arrhythmogenesis in the developing heart during anoxia-reoxygenation and hypothermia-rewarming: an in vitro model. J Cardiol 17: 1350–1359, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Sedmera D, Kockova R, Vostarek F, Raddatz E. Arrhythmias in the developing heart. Acta Physiol (Oxf) 213: 303–320, 2014. doi: 10.1111/apha.12418. [DOI] [PubMed] [Google Scholar]
- 46.Sedmera D, Kucera P, Raddatz E. Developmental changes in cardiac recovery from anoxia-reoxygenation. Am J Physiol Regul Integr Comp Physiol 283: R379–R388, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Sharma SK, Lucitti JL, Nordman C, Tinney JP, Tobita K, Keller BB. Impact of hypoxia on early chick embryo growth and cardiovascular function. Pediatr Res 59: 116–120, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Simon MC, Liu L, Barnhart BC, Young RM. Hypoxia-induced signaling in the cardiovascular system. Ann Rev Physiol 70: 51–71, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stambaugh K, Jacobson KA, Jiang JL, Liang BT. A novel cardioprotective function of adenosine A1 and A3 receptors during prolonged simulated ischemia. Am J Physiol Heart Circ Physiol 273: H501–H505, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugishita Y, Watanabe M, Fisher SA. Role of myocardial hypoxia in the remodeling of the embryonic avian cardiac outflow tract. Dev Biol 267: 294–308, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Szentmiklosi AJ, Cseppento A, Harmati G, Nanasi PP. Novel trends in the treatment of cardiovascular disorders: site- and event- selective adenosinergic drugs. Curr Med Chem 18: 1164–1187, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha. J Biol Chem 279: 14871–14878, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Van der Sterren S, Agren P, Zoer B, Kessels L, Blanco CE, Villamor E. Morphological and functional alterations of the ductus arteriosus in a chicken model of hypoxia-induced fetal growth retardation. Pediatr Res 65: 279–284, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell 134: 279–290, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Liang B, Skibsbye L, Olesen SP, Grunnet M, Jespersen T. GIRK channel activation via adenosine or muscarinic receptors has similar effects on rat atrial electrophysiology. J Cardiovasc Pharmacol 62: 192–198, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Webster WS, Abela D. The effect of hypoxia in development. Birth Defects Res C Embryo Today 81: 215–228, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Wendler CC, Amatya S, McClaskey C, Ghatpande S, Fredholm BB, Rivkees SA. A1 adenosine receptors play an essential role in protecting the embryo against hypoxia. Proc Natl Acad Sci USA 104: 9697–9702, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wendler CC, Poulsen RR, Ghatpande S, Greene RW, Rivkees SA. Identification of the heart as the critical site of adenosine mediated embryo protection. BMC Dev Biol 10: 57, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J 20: 1251–1253, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Xu Z, Cohen MV, Downey JM, Vanden Hoek TL, Yao Z. Attenuation of oxidant stress during reoxygenation by AMP 579 in cardiomyocytes. Am J Physiol Heart Circ Physiol 281: H2585–H2589, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Xu Z, Park SS, Mueller RA, Bagnell RC, Patterson C, Boysen PG. Adenosine produces nitric oxide and prevents mitochondrial oxidant damage in rat cardiomyocytes. Cardiovasc Res 65: 803–812, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Zaza A, Rocchetti M, DiFrancesco D. Modulation of the hyperpolarization-activated current (I(f)) by adenosine in rabbit sinoatrial myocytes. Circulation 94: 734–741, 1996. [DOI] [PubMed] [Google Scholar]
- 63.Zhang LF, Lian LS, Zhao CJ, Li JY, Bao HG, Wu C. Expression pattern of HIF1alpha mRNA in brain, heart and liver tissues of Tibet chicken embryos in hypoxia revealed with quantitative real-time PCR. Animal 1: 1467–1471, 2007. [DOI] [PubMed] [Google Scholar]