Abstract
Activation of muscle progenitor cell myogenesis and endothelial cell angiogenesis is critical for the recovery of skeletal muscle from injury. Angiopoietin-1 (Ang-1), a ligand of Tie-2 receptors, enhances angiogenesis and skeletal muscle satellite cell survival; however, its role in skeletal muscle regeneration after injury is unknown. We assessed the effects of Ang-1 on fiber regeneration, myogenesis, and angiogenesis in injured skeletal muscle (tibialis anterior, TA) in mice. We also assessed endogenous Ang-1 levels and localization in intact and injured TA muscles. TA fiber injury was triggered by cardiotoxin injection. Endogenous Ang-1 mRNA levels immediately decreased in response to cardiotoxin then increased during the 2 wk. Ang-1 protein was expressed in satellite cells, both in noninjured and recovering TA muscles. Positive Ang-1 staining was present in blood vessels but not in nerve fibers. Four days after the initiation of injury, injection of adenoviral Ang-1 into injured muscles resulted in significant increases in in situ TA muscle contractility, muscle fiber regeneration, and capillary density. In cultured human skeletal myoblasts, recombinant Ang-1 protein increased survival, proliferation, migration, and differentiation into myotubes. The latter effect was associated with significant upregulation of the expression of the myogenic regulatory factors MyoD and Myogenin and certain genes involved in cell cycle regulation. We conclude that Ang-1 strongly enhances skeletal muscle regeneration in response to fiber injury and that this effect is mediated through induction of the myogenesis program in muscle progenitor cells and the angiogenesis program in endothelial cells.
Keywords: angiopoietin-1, angiogenesis, myogenesis, injury, regeneration, myoblasts, skeletal muscle
skeletal muscle exhibits an exceptional capacity to recover from injury. Coordinated processes of vascular growth and maintenance, extracellular matrix deposition, and nerve and muscle fiber regeneration contribute to its recuperative plasticity (9, 48). Most pharmacological therapies that target patients with injured limb muscles are inefficient in that they are largely directed at individual cell types or specific signaling pathways (30, 31). It has become increasingly clear, however, that the muscle vasculature plays an important role in myogenesis, regulation of metabolism, and protein composition of skeletal muscle. In turn, muscle cells secrete angiogenic factors into blood vessels in response to changes in metabolic demand or stress (13, 36).
A skeletal muscle's capacity for tissue repair is conferred by satellite cells located between the basal lamina and the sarcolemma of mature myofibers. Satellite cells are quiescent myoblasts that play critical roles in muscle hypertrophy, recovery from disuse, and regeneration after injury (21). After muscle injury, these satellite cells reenter the cell cycle, proliferate to repopulate the satellite cell pool, and then give rise to a large number of myogenic precursor daughter cells before they fuse to existing myotubes or contribute to the regeneration of necrotic myofibers (39). Strong evidence suggests that rapid activation of satellite cells in response to fiber injury is accomplished through the release of hepatocyte growth factor (HGF) and products of neuronal nitric oxide synthase (nNOS) (3, 38, 43). In addition, satellite cells reside in close proximity to myofiber capillaries and receive support from endothelial cells in the form of various growth factors, including basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) (21). Satellite cells then produce endothelial growth factors that stimulate angiogenesis, thus providing the new tissue with oxygen and nutrients (10). The extent to which traditional “vascular” growth factors such as the angiopoietins also play a role in the determination of skeletal myocyte survival, recovery from insult, and the regulation of cellular metabolic pathways has not yet been determined.
Among the genes expressed early after initiation of skeletal muscle injury are several angiogenic growth factors, including angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), and Tie-2 (the cognate receptor of Ang-1 and Ang-2) (46). Ang-1 is the principal ligand of Tie-2 and it promotes vascular development in embryos and enhances endothelial survival, migration, and differentiation (7). Recent studies indicate that Ang-1 also promotes survival of skeletal and cardiac myocytes and enhances proliferation and differentiation of satellite cells in vitro (12, 25). In addition, Tie-2 receptors have been detected in a subset of skeletal muscle satellite cells that are required for regeneration (2). Collectively, these findings suggest that, in addition to its pro-angiogenic properties, Ang-1 may also possess some myogenic properties and that it may play a critical beneficial role in in vivo regeneration of injured skeletal muscle fibers.
In this study, we hypothesized that overexpression of Ang-1 inside muscle fibers during the early phase of regeneration from necrotic fiber injury results in acceleration of fiber regeneration and, consequently, improvement of muscle contractile performance. We also hypothesize that these effects are due to enhanced regenerative capacity of skeletal muscle precursor (satellite) cells in response to induction of myogenesis and angiogenesis. To identify signaling pathways and mechanisms through which Ang-1 regulates the myogenesis program, we conducted in vitro experiments on skeletal muscle precursor cells and in vivo experiments on cardiotoxin injection-induced fiber-injured tibialis anterior (TA) muscles of mice that had been administered adenovirus-delivered Ang-1 shortly after the onset of fiber injury.
METHODS
This study was carried out in strict accordance with standards established by the Canadian Council of Animal Care and the guidelines and policies of McGill University. All human donors were informed of the procedures and signed consent forms. All animal experiments were approved by the McGill University Animal Ethics Committee and the Human Research Ethics Office of the McGill University Health Centre. The following experiments were performed.
Regulation of Angiopoietin Expression During Muscle Injury and Regeneration in Mice
Adult male C57/Bl6 mice (6–8 wk old) were anesthetized with ketamine (130 mg/kg ip) and xylazine (20 mg/kg ip). Cardiotoxin (10 μM) was injected into one TA muscle to induce in vivo skeletal muscle injury, as previously described (23), and 40 μl of phosphate-buffered saline (PBS) was injected into the contralateral muscle to serve as a control (noninjured). Mice were euthanized by anesthetic overdose at 1, 3, 7, and 14 days postinjury (n = 6 for each). To assess changes in Ang-1 and Ang-2 expression during muscle injury and regeneration, TA muscles were excised immediately after euthanasia and prepared for real-time PCR (qPCR) and immunohistochemistry.
Cell culture.
To detect Ang-1 and Ang-2 expression in pure skeletal muscle precursors and to compare angiopoietin expression in human and murine muscle precursors, primary human and murine skeletal myoblasts were isolated from human vastus lateralis biopsies or dissected TA muscles of adult (8 wk) male C57/Bl6 mice, as previously described (37). To obtain human myoblasts, biopsies were obtained from two male healthy donors (26 and 23 years old). Briefly, muscle samples were subjected to collagenase digestion (0.2% collagenase at 37°C for 60 min) followed by trituration with Pasteur pipettes of decreasing bore size to liberate muscle fibers. Fibers were washed in Dulbecco's Modified Eagle Medium (DMEM), then transferred onto Matrigel-coated culture plates and incubated with DMEM supplemented with 10% horse serum (HS) and 0.5% chick embryo extract (CEE). After 4 days of incubation, myoblasts attached to the substratum were expanded in growth medium [DMEM supplemented with 20% fetal bovine serum (FBS), 10% HS and 1% CEE]. Primary human skeletal myoblasts from the two donors were pooled. Myoblasts were subcultured until passage 6.
Real-time PCR (qPCR).
Total RNA (2 or 5 μg) was extracted from frozen muscle samples or cultured myoblasts using a GenElut Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, Oakville, ON). Total RNA (2 or 5 μg) was reverse transcribed using Superscript II Reverse Transcriptase and random primers, as previously described (19). Expression of murine Ang-1 and Ang-2, human Ang-1, Ang-2, VEGF, and 18S (endogenous control) mRNA was measured using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA). qPCR was performed using a 7500 Real-Time PCR System (Applied Biosystems). All qPCR experiments were performed in triplicate. To determine absolute copy numbers of mRNA expression, standard curves that relate their cycle threshold (CT) values to copy numbers were established as described (33). Copy numbers were then normalized per 104 copies of 18S.
Immunohistochemistry.
Excised TA muscles were fixed overnight in 10% buffered Formalin, dehydrated, and paraffin embedded. Paraffin sections (5 μm) taken from the upper, middle, and lower regions of the muscle were deparaffinized and rehydrated. Rehydrated sections underwent an antigen retrieval protocol (exposure to sodium citrate buffer at 95–100°C for 20 min). Sections were then blocked with Ultra V Block and incubated overnight at 4°C with monoclonal primary antibodies selective to Ang-1 and Ang-2 at a dilution of 1:200 (R&D Systems, Minneapolis, MN). Sections were rewashed and then incubated with Primary Antibody Enhancer (20 min) and Value AP Polymer anti-mouse/rabbit secondary antibodies (dilution of 1:500). Immunohistochemistry was performed using an UltraVision LP Detection System (AP Polymer/Fast Red Chromogen) (Thermo Scientific, Fremont, CA). Tissues were positively stained with Fast Red and counterstained with hematoxylin.
Immunofluorescence.
Frozen TA muscle samples were cut into 10-μm sections. Sections were fixed in 2% paraformaldehyde, permeabilized in 0.2% Triton, blocked in PBS blocking solution (with 2% BSA, 0.2% Triton, and 0.05% Tween), and incubated overnight at 4°C with the following primary antibodies: angiopoietin-1 (EB10272, at a dilution of 1:50) (Everest Biotech, Ramona, CA), angiopoietin-2 (ab8452, at a dilution of 1:200) (Abcam, Toronto, ON), laminin (L9393, at a dilution of 1:750) (Sigma-Aldrich), von Willebrand Factor (ab6994, at a dilution of 1:50) (Abcam), and Pax7 (at a dilution of 1:10) (The Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). Sections were washed with PBS and incubated for 30 min at room temperature with the following secondary antibodies: donkey anti-goat Alexa Fluor 488 (A11055), goat anti-rabbit Alexa Fluor 568 (A11036), and goat anti-rabbit Alexa Fluor 488 (A11008), all supplied by Life Technologies, Burlington, ON. After three washes, sections were counterstained with PBS containing 4′,6-diamino-2-phenylindole (DAPI) nuclear stain, rinsed twice, and mounted with an aqueous-based mounting medium. For Ang-1/laminin and Ang-2/laminin double immunofluorescence, the laminin antibody was directly labeled according to the manufacturer instructions with a Sigma-Aldrich Mix-n-Stain CF Antibody labeling kit (MX568S20). Slides were visualized using a Ziess LSM 780 confocal microscope. Images were processed using Zen software (Zeiss Canada, Toronto, ON).
Immunoblotting.
Human skeletal muscle myoblasts were washed twice with ice-cold PBS and lysed on ice in a buffer consisting of 50 mM HEPES, 150 mM NaCl, 100 mM NaF, 5 mM EDTA, 0.5% Triton X-100, and protease inhibitors (5 mg/ml aprotinin, 2 mg/ml leupeptin, and 100 mM phenylmethanesulfonyl fluoride). Lysates were centrifuged at 1,000 g for 5 min. Supernatants were boiled for 5 min then loaded onto tris-glycine SDS-polyacrylamide gels. Protein content was precipitated out of culture medium using the acetone precipitation method, reconstituted with SDS sample buffer, and loaded onto tris-glycine SDS-polyacrylamide gels. Proteins were electrophoretically transferred onto polyvinylidene difluoride membranes, blocked for 1 h with 5% nonfat dry milk, incubated overnight at 4°C with primary mouse anti-human Ang-1 or Ang-2 antibodies at a dilution of 1:750 (R&D Systems), and detected with horseradish peroxidase-conjugated anti-mouse secondary antibody (dilution of 1:10,000) and ECL reagents. Equal loading of proteins was confirmed by reprobing with anti-β-Tubulin antibody at a dilution of 1:1,000 (Sigma-Aldrich).
Regulation of Skeletal Muscle Regeneration by Ang-1 and Ang-2
Recombinant adenoviruses expressing human Ang-1 (Ad-Ang-1) or Ang-2 (Ad-Ang-2) were constructed as previously described (45). Adult male C57/Bl6 mice were anesthetized and injected with cardiotoxin or PBS as described above and allowed to recover. Four days later when maximal levels of muscle myoblast proliferation are usually reached in cardiotoxin-injured skeletal muscles (49), mice were anesthetized and injured TA muscles were injected with 30 μl of Ad-Ang-1, Ad-Ang-2, or Ad-GFP (control) (1.5 × 109 virus particles/muscle). Contralateral muscles served as controls (noninjured). Ten days later (14 days postinjury), mice were anesthetized and TA muscles were either left intact for in situ measurements of contractility or excised for measurements of indices of regeneration. Pilot experiments revealed that 14 days postinjury was the optimal time point at which treatment impact on muscle force generation was shown.
In situ TA muscle contractility.
Anesthetized mice were immobilized in the supine position. The distal tendon of the TA was isolated and tied to the lever arm of a dual mode force transducer/length servomotor system mounted on a mobile micrometer stage. The exposed section of the muscle was kept moist with an isotonic saline drip (37°C) and directly stimulated with an electrode placed on the belly of the muscle. Supramaximal stimuli (pulse durations of 2 ms) were delivered using a computer-controlled electrical stimulator (model S44; Grass Instruments, Quincy, MA). Muscle force and length signals were stored on a computer using Labdat/Anadat software (RHT InfoData, Montreal, QC). Force-frequency relationships were determined at muscle optimal length by sequential supramaximal stimulations for 300 ms at 10, 30, 50, 100, and 120 Hz, with a 2-min interval between each stimulation train. Muscle length was measured with a microcaliper and weighed. Muscle force was normalized to tissue cross-sectional area (expressed as Newtons/cm2).
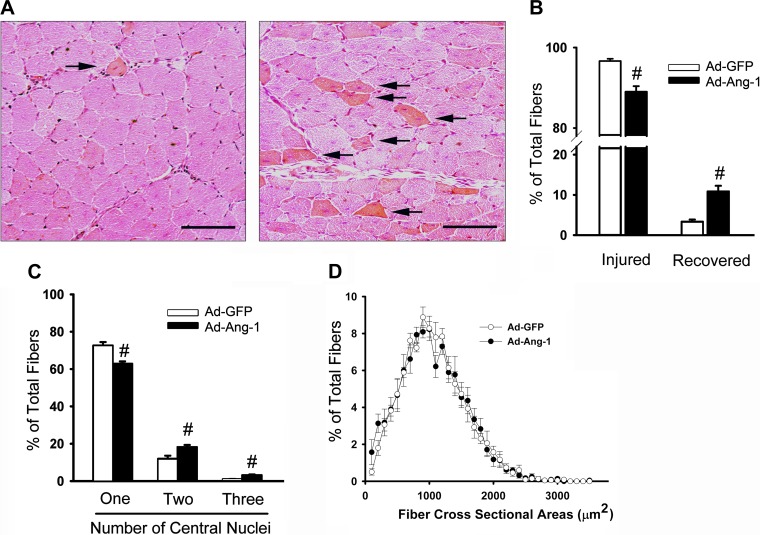
Morphological and molecular indices of regeneration.
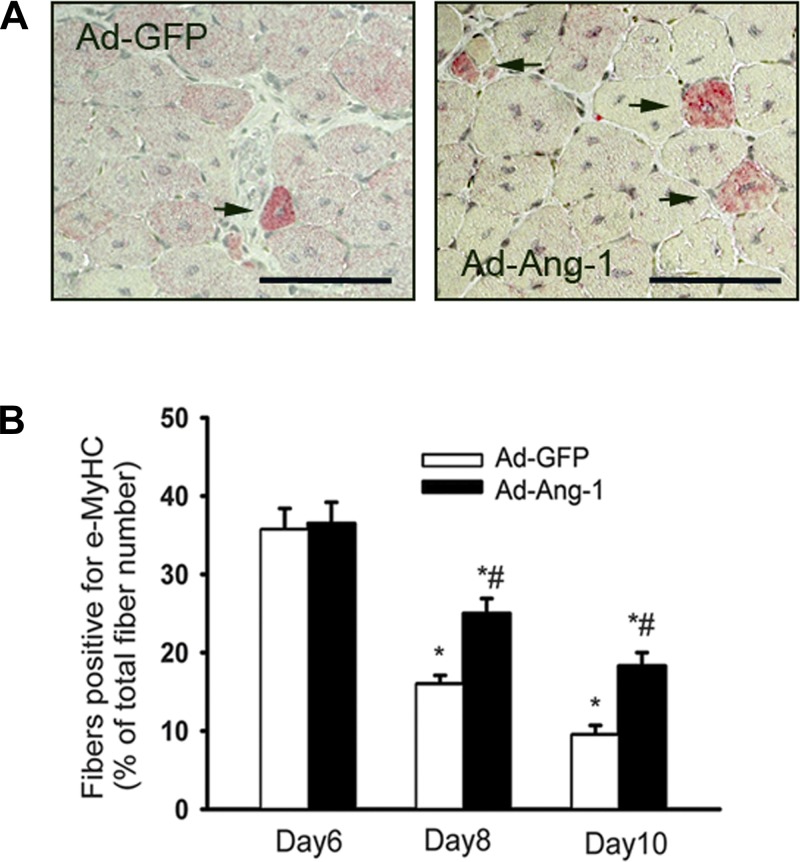
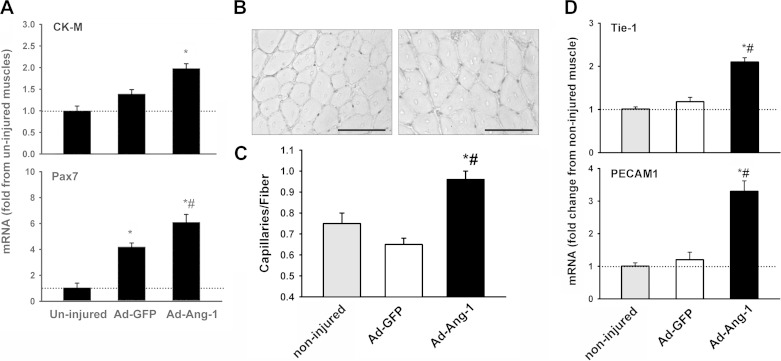
Control and injured TA muscles were excised 10 days postinjury, either fixed overnight in 10% buffered Formalin and paraffin embedded or snap frozen in liquid nitrogen and stored at −80°C for subsequent RNA analyses. Transverse sections (5-μm thick) were cut from upper, middle, and lower regions of each muscle. Sections were deparaffinized, rehydrated, stained with eosin and hematoxylin, and used to detect three indices of muscle regeneration, including ratios of injured and regenerated fibers, the number of nuclei per regenerated fiber, and fiber cross-sectional area (CSA). Images were captured with an inverted microscope and analyzed with Image-Pro Plus (MediaCybernetics, Bethesda, MD). A total of 250 fibers per muscle sample were visualized, and the numbers of injured and recovered fibers were counted and expressed as a percentage of total counted fibers. Differentiation between injured and recovered fibers was based on the intensity of eosin staining. Injured fibers were identified as those with central nuclei and relatively weak eosin staining (increased RNA content stains weakly with eosin), whereas recovered fibers were defined as those with central nuclei and relatively strong eosin staining (18). Primary antibodies selective to eMyHC (Developmental Studies Hybridoma Bank, University of Iowa) at a dilution of 1:10 and von Willebrand Factor (Chemicon International, Temecula, CA) at a dilution of 1:100 were used, respectively, to detect of the ratio of fibers expressing embryonic myosin heavy chain (eMyHC), an index of regeneration, and to quantify the capillary density. Immunohistochemistry was performed as described above. Tissues were positively stained with Fast Red and counterstained with hematoxylin. Muscle capillarization was quantified as capillary-to-fiber ratio, which was obtained by dividing the total number of capillaries by the total number of fibers on 200 randomly selected fibers (4).
To detect changes in markers of satellite cell number (Myogenin, Pax7), muscle-specific proteins (Creatine Kinase M isoform), and endothelial cell abundance (PECAM1, Tie-1) in control and injured TA muscles, qPCR using specific primers (see online Supplemental Table S1) was performed as described above. β-Actin was used as an endogenous control. Relative quantifications of specific gene expression were compared with levels detected in noninjured muscles using the comparative threshold cycle (ΔΔCT) method.
Mechanisms of Ang-1-Induced Skeletal Muscle Regeneration
We performed several experiments using primary human skeletal muscle myoblasts.
Cell culture.
Isolated human and murine skeletal muscle precursor cells (myoblasts) derived from either human vastus lateralis biopsies or dissected TA muscles of adult (8 wk) male C57/Bl6 mice were established in culture as previously described (32).
Myoblast survival and apoptosis.
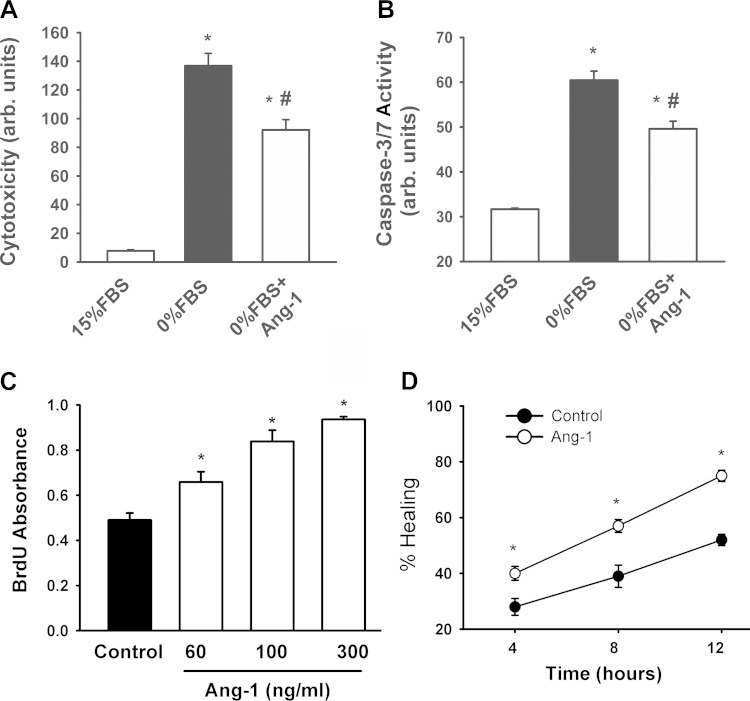
Effects of Ang-1 on myoblast cell survival and apoptosis were measured using CytoTox-Fluor Cytotoxicity and Caspase-Glo 3/7 assays, respectively (Promega, Madison, WI). Human primary skeletal muscle myoblasts (25 × 103 cells) were seeded into 96-well plates and maintained for 12 h in skeletal muscle basal medium (SkBM) containing 15% FBS. Culture medium was then replaced with SkBM (0% FBS) containing either PBS or 300 ng/ml of recombinant human Ang-1. Cell cytotoxicity and caspase-3/7 activity were measured 36 h later, according to the manufacturer's instructions.
Myoblast proliferation.
Effects of Ang-1 on myoblast cell proliferation were measured using a BrdU incorporation assay (Roche Applied Science, Laval, QC). Human primary skeletal muscle myoblasts were seeded into 96-well plates (1 × 104 cells) and maintained for 12 h in SkBM containing 15% FBS. PBS (control) or Ang-1 (60, 100, or 300 ng/ml) was added to culture medium. Thirty minutes later, BrdU reagent (10 μM) was added to each well and cells were incubated for 24 h, fixed, and labeled according to the manufacturer's instructions (Roche Applied Science, Laval, QC). Absorbance was measured using a microplate reader at 370 nm and corrected for background signals.
Myoblast migration.
Effects of Ang-1 on myoblast migration were measured using a scratch wound healing assay. Human primary skeletal muscle myoblasts (1 × 105 cells) were seeded into six-well culture plates and maintained for 24 h in SkBM containing 15% FBS. Cells were then carefully wounded using a 200-μl pipette tip (1). Cellular debris was removed by washing with PBS. After wounding was completed, culture medium was replaced with SkBM containing 5% FBS and either PBS (control) or 300 ng/ml recombinant human Ang-1. Wounds were photographed immediately after wounding (time = 0) and 4, 8, and 12 h later. Migration was measured as reduction in wound diameter following migration of cells into the cell-free zone (1). For each parameter, three wells of a given six-well plate were used, and the procedure was performed in triplicate.
Myoblast differentiation.
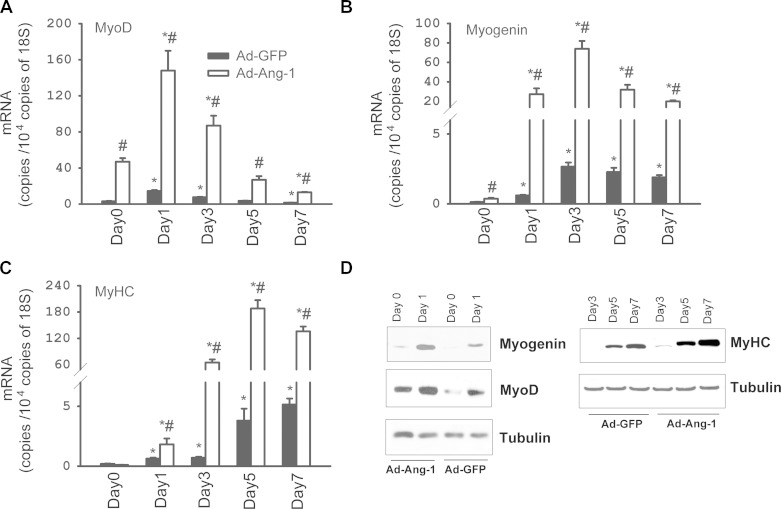
Subconfluent human primary skeletal muscle myoblasts were maintained for 6 h in SkBM containing 250 multiplicity of infection (MOI) of Ad-Ang-1 or Ad-GFP (control cells). Cells were then washed and maintained for 48 h in SkBM containing 15% FBS to reach full confluence. Cells were collected (day 0 = myoblast phase) for RNA extraction and protein expression measurements or differentiated into myotubes by replacing medium with DMEM supplemented with 2% HS. Differentiating cells were collected at 1, 3, 5, and 7 days of incubation in the differentiation medium. Total RNA (2 μg) was extracted and reverse transcribed as described above. Expression of the myogenic transcription factors MyoD and Myogenin, muscle-specific MyHC, and 18S (control) was measured during differentiation using qPCR as described above (see online Supplemental Table S1). Protein immunoblotting with primary antibodies specific to MyoD at a dilution of 1:750 (Santa Cruz Biotechnology, Dallas, TX), Myogenin and MyHC at a dilution of 1:20 (both from the Developmental Studies Hybridoma Bank, University of Iowa) were used to determine protein levels. Ang-1 overexpression in cells infected with Ad-Ang-1 was detected by measuring Ang-1 protein in myoblast lysates using primary Ang-1 antibody (Mofarrahi M and Hussain SN; unpublished observations).
Regulation of myogenin expression.
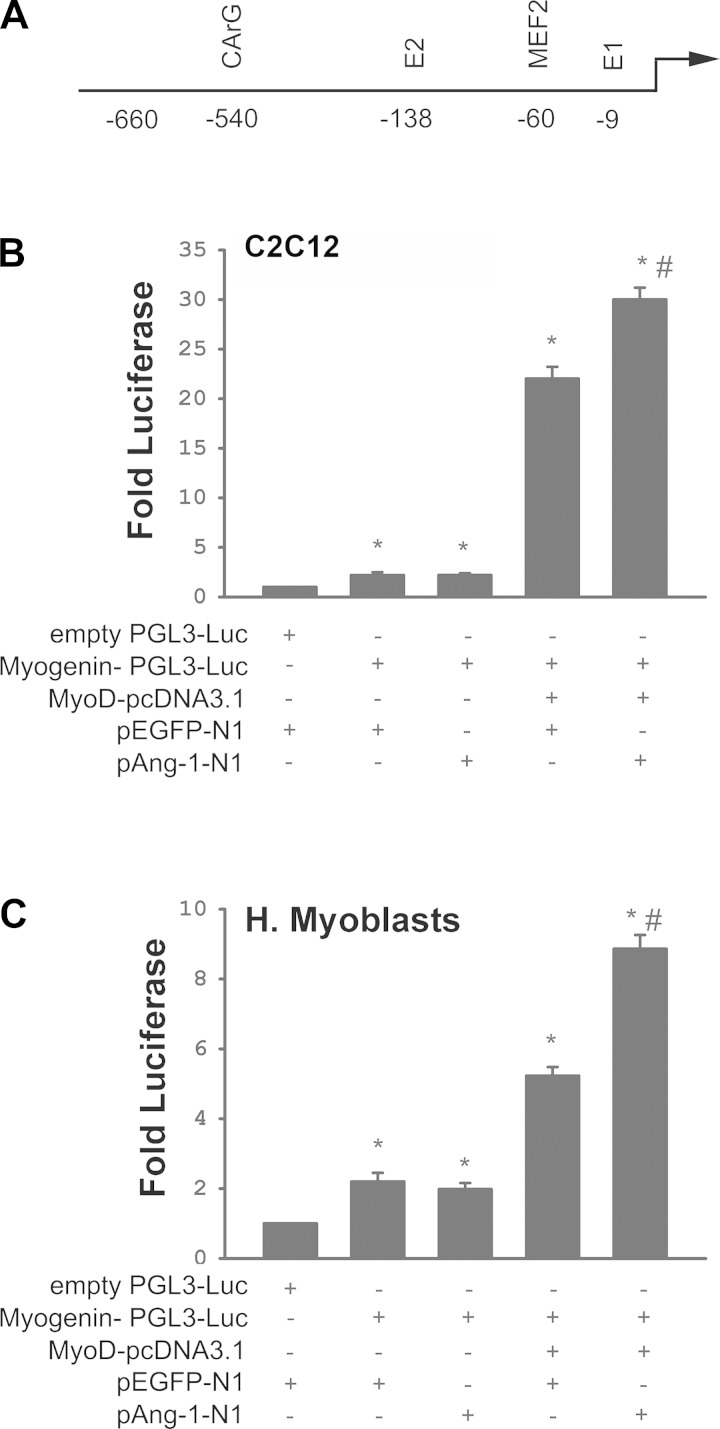
A firefly luciferase reporter plasmid driven by a Myogenin promoter (Myogenin-pGL3-Luc, 660 base pair fragment) was PCR cloned from mouse genomic DNA as previously described (29). A MyoD-pcDNA3.1 expression vector was generated as previously described (11), and an expression vector expressing human Ang-1 (pAng-1-N1) was generated by cloning human Ang-1 cDNA into a pEGFP-N1 vector (Clontech, Mountain View, CA). An empty pEGFP-N1 vector was used as a control. Human skeletal muscle myoblasts or C2C12 murine myoblasts were transiently transfected with empty PGL3-Luc or Myogenin-pGL3-Luc vectors in the presence or absence of MyoD-pcDNA3.1, pAng-1-N1, or pEGFP-N1 vectors using Lipofectamine 2000 according to the manufacturer's instructions (Life Technologies). Cells were lysed 48 h later, and firefly luciferase activity was measured using a Dual Luciferase Assay Kit (Promega) and normalized to relative firefly luciferase activity.
Mechanisms of Ang-1 action.
We compared mRNA expression profiles of myoblasts in which human Ang-1 was overexpressed using adenoviruses to those infected with control adenoviruses (Ad-GFP). Human primary skeletal muscle myoblasts were infected with Ad-Ang-1 (n = 3 wells) or Ad-GFP (control, n = 3 wells) viruses as described above. Cells were maintained for 48 h in complete culture medium, and then medium was changed to SkBM containing 5% FBS. Cells were collected 12 h later and total RNA was extracted as described above. Total RNA (100 ng) was amplified using an Illumina RNA Amplification kit (Illumina, San Diego, CA) and labeled by incorporating biotin-16-UTP. Samples were hybridized to Illumina Gene Expression Sentrix BeadChip Human Ref-8_V2 as recommended by the manufacturer. Data analysis was performed using R software (version 2.15.0) and Bioconductor array packages (http://www.bioconductor.org). Preprocessing of data expression was performed using a lumi package (16). Quality control of each chip was performed based on sample correlation, probe ratio of hybridization, and distribution of intensity and variance. A filtering step was applied based on P values, which represent the probability of the observed signal being significantly different from the background signal. Probes with a detection probability of P < 0.05 in at least 50% of replicates in each experimental group were retained. After background correction, a variance-stabilizing transformation (VST) algorithm was applied to stabilize the variance in raw expression (28) and a robust spline normalization (RSN) algorithm was applied for normalization. Differential analyses between Ad-Ang-1 and Ad-GFP groups were performed with a moderated t-test (limma package) to determine fold changes and P values (40). Based on the false discovery rate (FDR) approach, a gene list with a probability of P < 0.05 controlling the false-positive rate was selected. After annotation of selected genes with a lumiHumanAll.db package (version 1.18.0), functional analysis of the gene list was accomplished in two steps: gene ontology (GO) analysis using a GOstat package (17) followed by KEGG pathway analysis for gene expression profile. An mfuzz clustering algorithm was applied to identify different expression profiles.
Statistical Analysis
Statistical analysis was performed using one-way and two-way analyses of variance (ANOVA) for comparisons between multiple groups followed by a Tukey post hoc test for comparisons between individual groups. P < 0.05 (two-tailed) was considered statistically significant.
RESULTS
Regulation of Endogenous Angiopoietin Expression During Muscle Injury and Regeneration
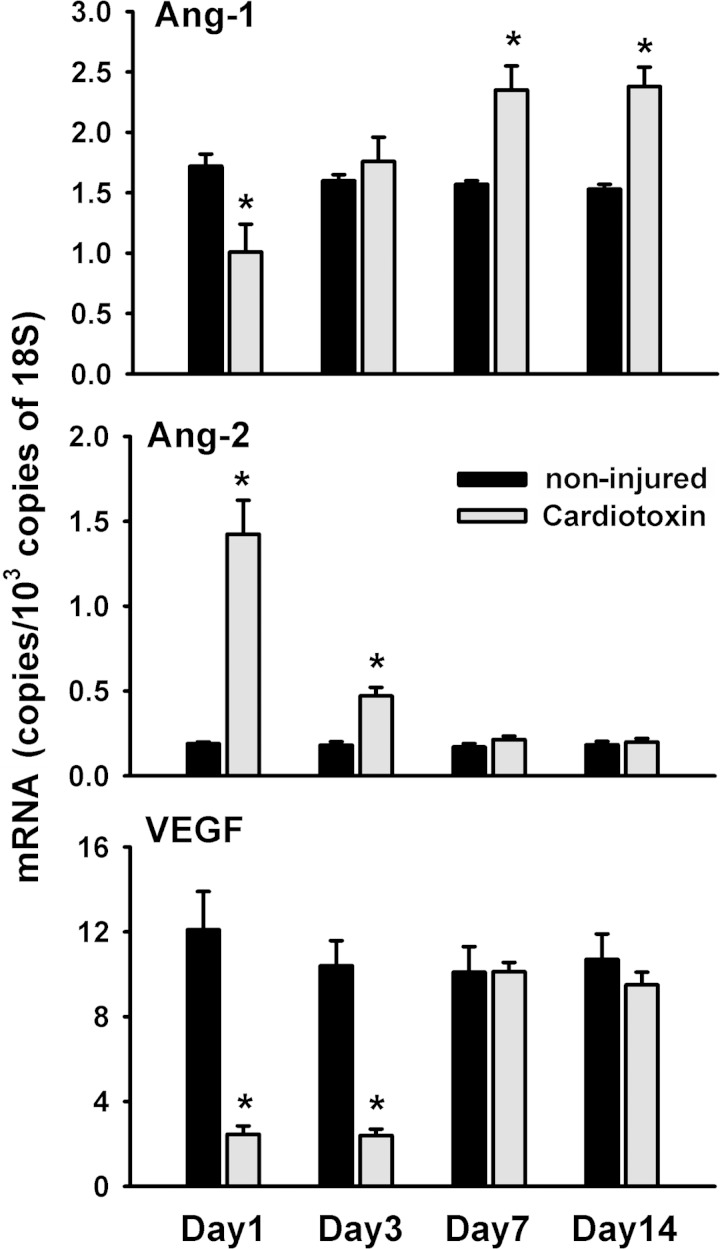
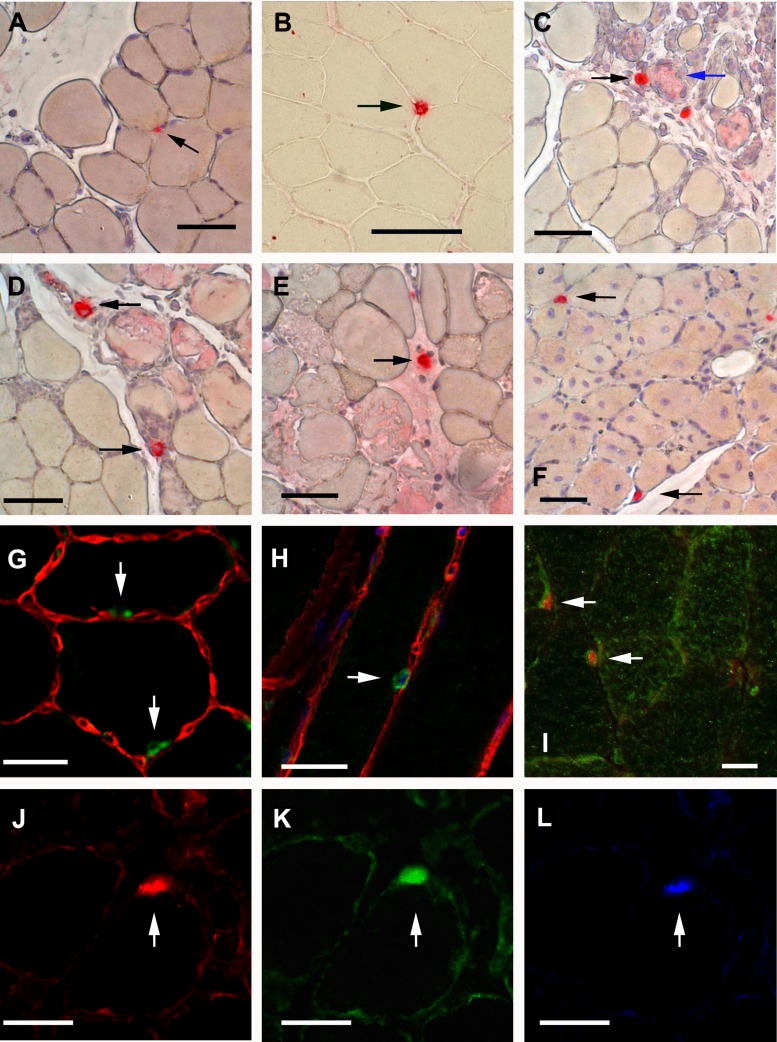
In injured TA muscle, Ang-1 and VEGF mRNA levels decreased significantly by day 1 postinjury (Fig. 1). Ang-1 mRNA levels then increased significantly on days 7 and 14 postinjury compared with noninjured muscle (Fig. 1). Ang-2 mRNA levels increased significantly only on days 1 and 3 postinjury (Fig. 1). Immunostaining revealed that Ang-1 protein was expressed in mononuclear cells residing between muscle fibers both in noninjured and recovering TA muscles (black arrows in Fig. 2, A–F). Positive Ang-1 staining was also detected in blood vessels but not in nerve fibers (not shown). To confirm that Ang-1-positive mononuclear cells are skeletal muscle satellite cells, we first performed double immunofluorescence for Ang-1 and laminin (a marker of basal lamina) in noninjured and recovering TA muscles. Ang-1-positive cells (green) appeared to lie within basal lamina (laminin stains red) of skeletal muscle fibers (Fig. 2, G and H). To confirm that Ang-1-positive cells were indeed satellite cells, we performed double immunofluorescence for Ang-1 and Pax7 (a marker of satellite cells) proteins. Ang-1-positive mononuclear cells (green) also stained positively for Pax7 protein (red), confirming that these cells are skeletal muscle satellite cells (Fig. 2, I–L). It should be noted that not all Pax7-positive cells stained positively for Ang-1 protein, suggesting that not all muscle satellite cells abundantly express Ang-1 protein.
Fig. 1.
Angiopoietin (Ang)-1 (top), Ang-2 (middle), and VEGF (bottom) mRNA expression in cardiotoxin-injured and regenerating tibialis anterior (TA) muscle. Ang-1, Ang-2, and VEGF in TA muscle during cardiotoxin-induced injury and regeneration, expressed as fold change relative to noninjured muscle. Values are means ± SE (n = 7 per group). *P < 0.05 compared with noninjured muscle.
Fig. 2.
Localization of Ang-1 and Pax7 proteins in cardiotoxin-injured and regenerating TA muscle. A–F: immunostaining for Ang-1 protein in noninjured (A, B) and injured TA muscles on day 1 (C), day 3 (D), day 7 (E), and day 14 (F) postinjury. Positive staining is indicated by bright red color (black arrows). False positive staining is indicated by pink staining (blue arrows). Black scale bars, 50 μm. G and H: merged images of immunofluorescence staining for laminin (red) and Ang-1 (green) in noninjured TA muscle. White arrows point to Ang-1-positive cells. White scale bars, 25 μm. I: merged image of immunofluorescence staining for Pax7 (red) and Ang-1 (green) proteins in noninjured TA muscle. White arrows point to Ang-1-positive Pax7-positive cells. White scale bar, 25 μm. J–L: immunofluorescence staining for Pax7 (red, J), Ang-1 (green, K) and DAPI (blue, L) in recovering (14 days postinjury) TA muscle section. White arrows point to Ang-1-positive Pax7-positive cell. White scale bars, 25 μm.
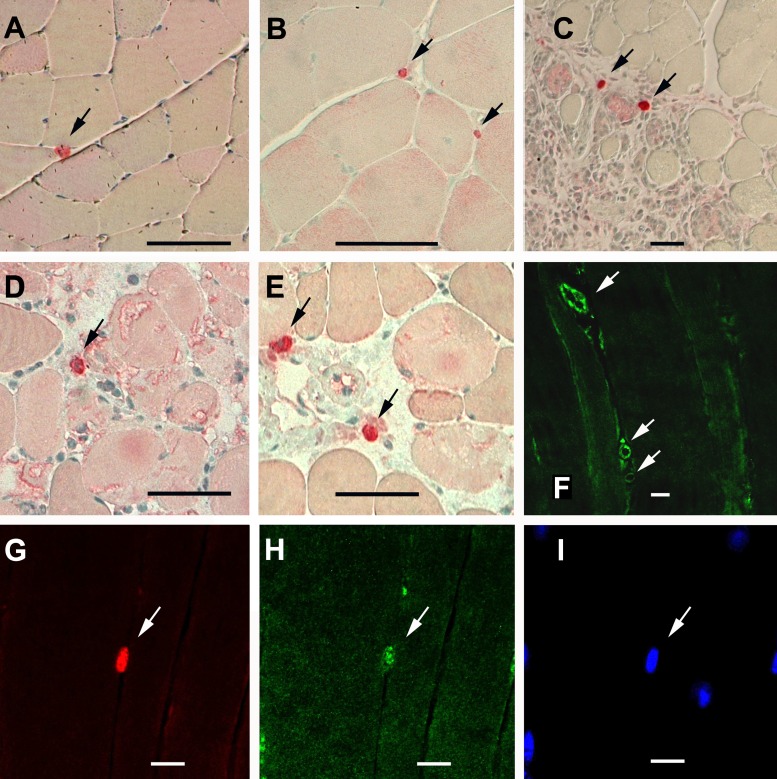
Ang-2 protein was expressed in mononuclear cells residing between muscle fibers and appears to lie within the basal lamina (as indicated by laminin staining) in both noninjured and recovering TA muscles (black arrows in Fig. 3, A–E). Positive Ang-2 staining was also detected in blood vessels (Fig. 3E). Double immunofluorescence for Ang-2 and Pax7 proteins demonstrated that Ang-2-positive mononuclear cells also stained positively for Pax7, thereby confirming that they were skeletal muscle satellite cells (Fig. 3, F–H). As in the case of Ang-1, not all Pax7-positive cells stained positively for Ang-2 protein, suggesting that not all muscle satellite cells abundantly express Ang-2.
Fig. 3.
Localization of Ang-2 and Pax7 proteins in cardiotoxin-injured and regenerating TA muscle. A–E: immunostaining for Ang-2 protein in noninjured (A, B) and injured TA muscles on day 1 (C, D), and day 14 (E) postinjury. Positive staining is indicated by bright red color (black arrows). False positive staining is indicated by pink staining. F: immunofluorescence staining for Ang-2 protein in blood vessels of noninjured TA muscle (white arrows). Black scale bars, 50 μm. G–I: immunofluorescence staining for Pax7 (red, G), Ang-2 (green, H), and DAPI (blue, I) in recovering (14 days postinjury) TA muscle section. White arrows point to Ang-2-positive Pax7-positive cell. White scale bars, 25 μm.
To verify that skeletal muscle satellite cells do indeed express Ang-1 and Ang-2, we used qPCR and immunoblotting on primary human and murine skeletal muscle myoblast samples (see methods). qPCR analysis revealed that Ang-1 expression was relatively higher than that of Ang-2 in human and murine myoblasts. Immunoblotting confirmed the presence of Ang-1 proteins in myoblast lysate and culture medium, indicating that skeletal muscle myoblasts actively secrete Ang-1 protein.
Regulation of Skeletal Muscle Regeneration by Ang-1 and Ang-2
In situ TA contractility.
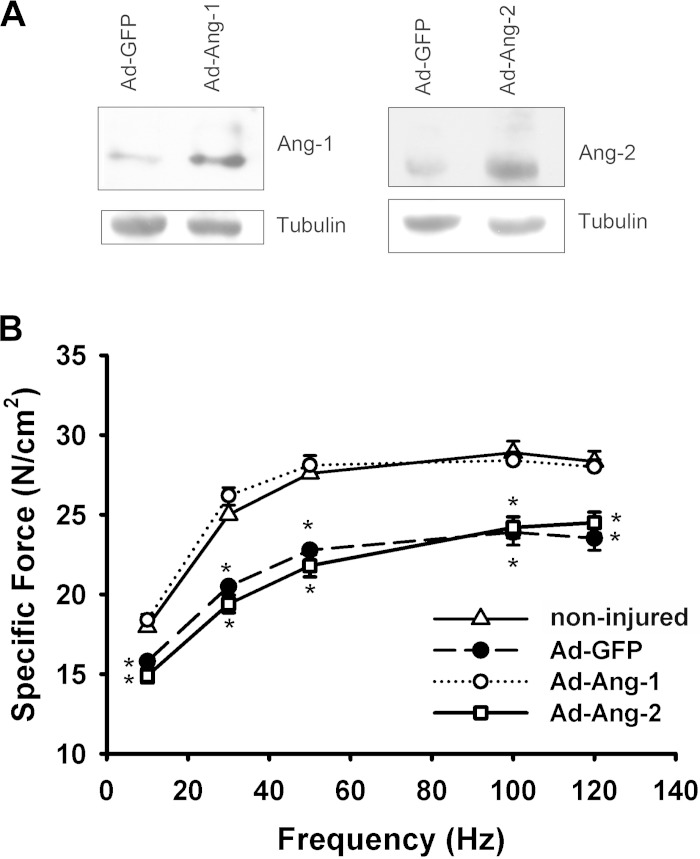
Immunoblotting of TA samples collected at the end of the experimental period (14 days postinjury) confirmed that injection of Ad-Ang-1 and Ad-Ang-2 significantly increased muscle Ang-1 and Ang-2 protein levels, respectively (Fig. 4A). Submaximal and maximal force generation in injured TA muscles infected with Ad-Ang-2 or Ad-GFP viruses were significantly lower than in noninjured muscles (Fig. 4B). Submaximal and maximal force generations in injured TA muscles infected with Ad-Ang-1 viruses were not different compared with noninjured muscles (Fig. 4B). It can be concluded, therefore, that Ang-1 enhances TA contractile recovery, whereas Ang-2 exerts no effect.
Fig. 4.
Ang-1 improves contractility of cardiotoxin-injured TA muscle. A: representative immunoblots for Ang-1, Ang-2, and β-tubulin proteins in TA muscles injected with Ad-GFP, Ad-Ang-1, or Ad-Ang-2 viruses on day 4 postinjury. Muscle samples were collected 14 days after injury. B: in situ force frequency of TA muscles 14 days postinjury. Data of noninjured muscles were also shown. All injured muscles received a single injection of Ad-GFP, Ad-Ang-1, or Ad-Ang-2 virus on day 4 postinjury. Values are means ± SE, n = 6 per group. *P < 0.05 compared with noninjured.
Markers of myogenesis and angiogenesis.
Ten days after injury, the proportion of recovered fibers significantly increased in TA muscles infected with Ad-Ang-1 viruses compared with those infected with Ad-GFP viruses (Fig. 5). No differences were observed in terms of fiber cross-sectional area (Fig. 5). The proportion of fibers expressing eMyHC (Fig. 6) and the mRNA levels of Pax7 (marker of satellite cells) and CK-M (marker of regenerated muscle fibers) (Fig. 7) significantly increased in injured TA muscles injected with Ad-Ang-1 viruses compared with those injected with Ad-GFP viruses. Vascular regeneration in injured muscles was measured by quantifying capillary density [indicated by positive von Willebrand factor staining and the expression of two selective endothelial cell markers (PECAM1, Tie-1)]. Capillary density and expression of Tie-1 and PECAM1 significantly increased in injured TA muscles infected with Ad-Ang-1 viruses compared with those infected with Ad-GFP viruses (Fig. 7). It can be concluded, therefore, that Ang-1 increases both myogenesis and angiogenesis in injured TA muscles.
Fig. 5.
Ang-1 enhances muscle fiber regeneration. A: eosin and hematoxylin staining of TA muscles 10 days postinjury. All injured muscles received a single injection of Ad-GFP (left) or Ad-Ang-1 (right) virus on day 4 postinjury. Black arrows point to recovered muscle fibers. Black scale bars, 100 μm. B: number of injured and recovered fibers expressed as percentage of total counted fibers (250 fibers). C: number of fibers with one to three central nuclei expressed as percentage of total counted fibers (250 fibers). D: fiber cross-sectional areas of TA muscles measured on day 10 postinjury. Values are means ± SE, n = 7 per group. #P < 0.05 compared with Ad-GFP.
Fig. 6.
Ang-1 enhances muscle fiber regeneration in cardiotoxin-injured TA muscle. A: representative cross-sections of injured TA muscles. All injured muscles received a single injection of Ad-GFP or Ad-Ang-1 viruses on day 4 postinjury and then examined 10 days postinjury for embryonic myosin heavy chain (MyHC) (red staining, indicated by black arrows). Black scale bars, 100 μm. B: number of fibers expressing embryonic MyHC in tibialis anterior muscles measured on days 6, 8, and 10 postinjury. Values are means ± SE, n = 6 per group. *P < 0.05 compared with day 6. #P < 0.05 compared with Ad-GFP.
Fig. 7.
Ang-1 enhances in vivo myogenesis and angiogenesis in cardiotoxin-injured TA muscle. A: mRNA expression of creatine kinase M isoform (CK-M) and Pax7 measured using qPCR in noninjured and injured TA muscles. All injured muscles received a single injection of Ad-GFP or Ad-Ang-1 virus on day 4 postinjury. n = 6 per group. *P < 0.05 compared with noninjured TA muscles. #P < 0.05 compared with injured TA injected with Ad-GFP viruses. B: immunostaining for von Willebrand factor in injured TA muscles. All injured muscles received a single injection of Ad-GFP (left) or Ad-Ang-1 (right) virus on day 4 postinjury. Positive staining is indicated by black staining (capillary endothelial cells). Black scale bars, 100 μm. C and D: capillary density and Tie-2 and PECAM 1 mRNA levels in injured TA muscles. All injured muscles received a single injection of Ad-GFP or Ad-Ang-1 virus on day 4 postinjury. Values are means ± SE, n = 6 per group. *P < 0.05 compared with noninjured. #P < 0.05 compared with Ad-GFP.
Mechanisms of Ang-1-Induced Skeletal Muscle Regeneration
In vitro myogenesis.
Cytotoxicity and caspase-3/7 activity of human skeletal muscle myoblasts significantly increased upon removal of FBS from the culture medium (Fig. 8, A and B). Ang-1 significantly attenuated these responses (Fig. 8, A and B). Ang-1 significantly increased myoblast BrdU incorporation and migration 4, 8, and 12 h after being added to culture medium (Fig. 8, C and D). It can be concluded, therefore, that Ang-1 promotes myoblast survival, proliferation, and migration. At any given time in the process of myoblast differentiation into myotubes, Ad-Ang-1 virus-induced overexpression of Ang-1 in human skeletal muscle myoblasts significantly increased Ang-1 protein levels and mRNA and protein levels of MyoD, Myogenin, MyHC (Fig. 9), and CK-M (not shown). It can be concluded, therefore, that Ang-1 promotes myoblast differentiation. To assess the possibility that Ang-1-induced myogenin expression is dependent on the level of MyoD expression, myogenin promoter activity was measured in murine (C2C12) and human skeletal muscle myoblasts transfected with an Ang-1 plasmid in the presence and absence of a MyoD expression plasmid. C2C12 myoblasts were used instead of primary murine myoblasts because technical difficulties were encountered in trying to achieve acceptable levels of transfection efficiency in the latter. In the presence of a MyoD expression plasmid, myogenin promoter activity was relatively low, albeit significantly higher than activity measured in the absence of MyoD expression plasmid (Fig. 10), indicating that overexpression of MyoD exerts an effect on myogenin promoter activity. In the absence of a MyoD expression plasmid, overexpression of Ang-1 exerted no influence on myogenin promoter activity. Conversely, it significantly increased in the presence of a MyoD expression plasmid (Fig. 10). It can be concluded, therefore, that Ang-1 induction of myogenin in skeletal myoblasts requires the presence of relatively high MyoD expression levels.
Fig. 8.
Ang-1 enhances myoblast survival, proliferation, and migration. A and B: cytotoxicity and caspase 3/7 activity of human muscle myoblasts incubated for 36 h in media containing 15% FBS, 0% FBS, or 0% FBS + recombinant Ang-1 protein. Values are means ± SE, n = 6 per group. *P < 0.05 compared with 15% FBS. #P < 0.05 compared with 0% FBS. C: effects of Ang-1 on BrdU incorporation in human muscle myoblasts. Values are means ± SE, n = 6 per group. *P < 0.05 compared with controls. D: effects of Ang-1 on human skeletal myoblast migration as measured by wound healing assays. Values expressed as percent healing, n = 6 per group. *P < 0.05 compared with controls.
Fig. 9.
Ang-1 enhances myoblast differentiation. A and C: mRNA expression of MyoD, myogenin, and myosin heavy chain (MyHC) in human skeletal myoblasts infected with Ad-GFP or Ad-Ang-1 viruses during differentiation into myotubes. Day 0 refers to myoblast phase. Values are means ± SE, n = 8 per group. *P < 0.05 compared with day 0. #P < 0.05 compared with Ad-GFP. D: representative immunoblots of myogenin, MyoD, and MyHC proteins in human skeletal myoblasts infected with Ad-GFP or Ad-Ang-1 viruses during differentiation into myotubes.
Fig. 10.
Ang-1 promotes myogenin activity. A: diagram of myogenin reporter construct with predicted transcription factor binding sites. B and C: luciferase activity of empty reporter plasmid (PGL3-Luc) or myogenin reporter plasmid cotransfected with MyoD, Ang-1, or EGFP (control) expression plasmids in C2C12 myoblasts (B) and human skeletal myoblasts (C). n = 6 per group. *P < 0.05 compared with empty PGL3-Luc plasmid. #P < 0.0 compared with other treatments.
Ang-1-mediated regulation of gene expression.
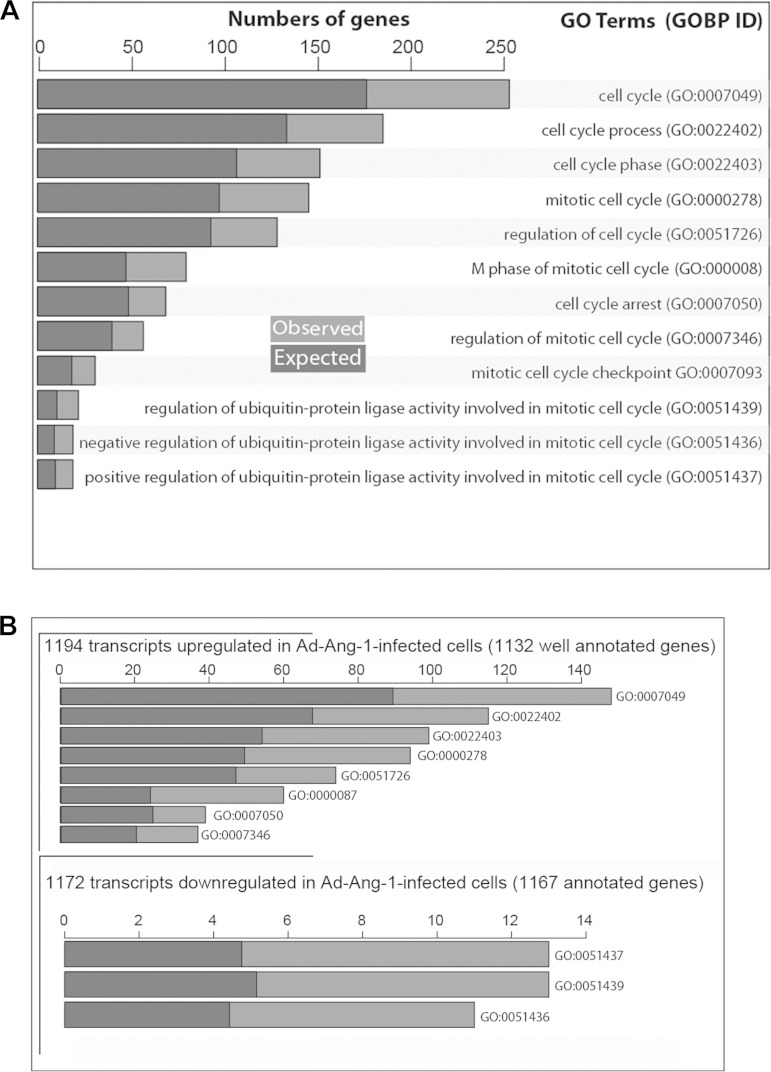
Transcriptomes of human skeletal myoblasts infected with Ad-Ang-1 and Ad-GFP viruses were compared using Illumina microarrays. The array platform is composed of 22,177 probe sets targeting genes and all known alternatively spliced variants from the NCBI Reference Sequence Database, Release 17. Differential gene expression of 2,366 transcripts was detected in myoblasts infected with Ad-Ang-1 viruses compared with those infected with Ad-GFP viruses. MyoD, myogenin, p21, and PGC1α were among the transcripts that were significantly upregulated in cells infected with Ad-Ang-1. To define the biological processes modulated by Ang-1 in skeletal myoblasts, a Gene Ontology (GO) term analysis was performed using all significantly modulated transcripts and the following arbitrary thresholds: odd ratio >1.5, P < 0.005, and FDR <0.08 (Fig. 11). The analysis detected 177 biological processes that were significantly altered by Ang-1, including kinase activity, protein folding, proteasome catabolism, and protein ubiquitination (see online Supplemental Table S2). Among these, 12 are associated with cell cycle regulation, reflecting the influence of Ang-1 on skeletal myoblast proliferation (see online Supplemental Table S2). To further investigate how Ang-1 affects the myoblast cell cycle, a pathway analysis was performed on all 2,366 transcripts. With the use of a threshold of P < 0.01, 22 upregulated genes were detected in the cell cycle pathway, including CCND2, CCNB2, CCNA2, CDC2, CDK6, and CDC25B (KEGG ID4110) (see online Supplemental Table S3). It can be concluded, therefore, that Ang-1 exerts selective effects on several regulators of cell cycle processes in skeletal myoblasts.
Fig. 11.
Ang-1 regulates the expression of cell cycle-related genes. A and B: expected and observed number of significantly regulated genes in human primary skeletal myoblasts infected with Ad-Ang-1 according to their roles in cell cycle regulation. A gene ontology (GO) analysis using GO terms was performed on each cluster after clustering of expression data.
DISCUSSION
We demonstrate for the first time that Ang-1 plays a role in the regeneration of skeletal muscle from necrotic injury and that this role is mediated through induction of the myogenic program in muscle progenitor cells in concert with the angiogenesis program in endothelial cells. In the differentiation process, for example, Ang-1 induces myogenin, which is essential to the development of functional skeletal muscle.
Skeletal muscle regeneration following injury is regulated by a highly coordinated gene expression program that drives muscle precursor cells to be activated very rapidly followed by proliferation and differentiation of these cells (6, 49). In the cardiotoxin injury model, activation of satellite cell proliferation begins as early as 6 h after injury and transitions to a sustained differentiation program after 5–6 days, which coincides with reconstitution of the fascicular fiber architecture and the appearance of muscle fibers with differentiating centralized nuclei (21). The myogenic transcriptional program relies heavily on basic helix-loop-helix (bHLH) transcription factors, such as MyoD, Myf5, MRF4, and myogenin, and p21, a cyclin-dependent kinase inhibitor (21). Notably, similar transcriptional programs drive in vivo skeletal muscle satellite cell proliferation/differentiation and in vitro maturation of isolated myoblasts.
Our in vitro studies clearly indicate that Ang-1 enhances myogenin transcription in a MyoD-dependent fashion and that Ang-1 drives the differentiation process by enhancing expression of MyHC. In our in vivo studies, it is possible that the Ang-1 effect on injured TA muscle myogenesis was due to paracrine signaling coming from Ang-1-stimulated endothelial cells, a result of Tie-2 being predominantly expressed on these cells. However, because we observed a similar Ang-1 effect on the myogenic differentiation program in isolated muscle cells, an observation that was also noted by Lee et al. (25), it is likely that Ang-1 enhances muscle regeneration by targeting both muscle satellite cell differentiation and Tie-2-mediated endothelial neovascularization. It is also possible that Ang-1 promotes muscle regeneration by inducing other types of cells that invade injured muscles to differentiate toward myogenic lineage. One such type is the vascular pericyte, which possesses myogenic potential that is distinct from satellite cells (15). One recent study indicates that vascular pericytes resident in small vessels of skeletal muscles contribute to the normal development of muscle fibers, and this contribution significantly increases in acutely injured and chronically regenerating fibers (14).
Little is known about the cellular origins of angiopoietin expression in normal and injured skeletal muscles. In this study, we report for the first time that Ang-1 is expressed in satellite cells in vivo and in isolated primary skeletal myoblasts in vitro. In an earlier study, we demonstrated that primary skeletal myoblasts express Ang-2 (32). Skeletal myoblasts, then, express both Ang-1 and Ang-2. It should be emphasized, however, that the relative abundance of Ang-1 mRNA is greater than that of Ang-2 mRNA, making it the principal angiopoietin that is produced by skeletal myoblasts and myotubes. Furthermore, unlike Ang-2, which exerts no effect on tibialis muscle regeneration in vivo, Ang-1 does indeed enhance regeneration following necrotic fiber injury. This difference is consistent with the observations that Ang-2 exerts a relatively weak and context-dependent effect on angiogenesis (7, 20) and no effect on the myogenesis program in myoblasts (32).
We described a bimodal pattern of Ang-1 expression in injured TA muscle, with a decrease immediately after necrotic injury is initiated, followed by an increase on days 7 and 14 postinjury. Ang-2 expression appears to be the mirror image of that of Ang-1, with an immediate increase followed by return to baseline values after 7 days postinjury. The time course of Ang-2 expression in injured TA muscle in this study is similar to that described by Wagatsuma (46) in freeze-injured gastrocnemius muscle. However, the initial decline in Ang-1 mRNA expression observed on day 1 postinjury in this study differs from that described by Wagatsuma who observed an initial rise in Ang-1 mRNA levels. Reasons behind this difference in Ang-1 time course are not clear. We speculate that the type of muscle (gastrocnemius vs. tibialis anterior) and injury model (freeze vs. cardiotoxin-induced) may influence the degree to which endogenous Ang-1 expression is altered.
Mechanisms underlying enhanced Ang-1 expression during the recovery phase of injured TA muscle remain unclear. The hedgehog signaling pathway recently emerged as an important regulator of myogenesis and angiogenesis programs in ischemia-induced muscle injury; enhanced angiogenesis by this pathway may be mediated through upregulation of Ang-1 expression (26, 34, 41). Furthermore, it has been recently reported that the hedgehog transcription factor Gli3 has promotes myoblast differentiation and angiogenesis in ischemia-induced muscle injury, and Gli3-induced angiogenesis is due to upregulation of pro-angiogenesis factors, particularly thymidine phosphorylase and Ang-1 (35). These results suggest that the hedgehog signaling pathway is an important regulator of Ang-1 production. Further studies are required to elucidate the functional role of this pathway in cardiotoxin-induced injury.
The timing of Ang-1 in the regeneration of skeletal muscle and development of myotubes suggest that it works after myoblast fusion, a time frame characterized by sarcomeric assembly in the mature myofiber. In our in vivo experiments, Ang-1 was overexpressed on day 4 postinjury. Myosin, M-line proteins, and serum response factor (SRF)-regulated striated α-actin expression occurs during this period of muscle differentiation (27, 47), indicating functional organization of myotube sarcomeric structure. At sequentially later stages of muscle maturation, such as proliferation and fusion, SRF is necessary for skeletal muscle growth/maturation in vivo and in vitro and is believed to function in concert with myocardin-related transcription factors (MRTFs) and KLF3 at CArG motifs (22, 27, 47). In the late stages of maturation, a specific role has been established in vitro for SRF in β-integrin-mediated RhoA signaling and regulation of α-actin expression (47).
Since Ang-1 is known to signal through β1 integrins in both skeletal muscle and endothelial cells (8, 12), it is possible that it functions as a complementary regulatory pathway of SRF transcriptional activity and sarcomeric organization in the latter stages of myocyte differentiation. However, it is not yet known whether or not Ang-1 is required for MEF2C- and SRF-induced transcriptional expression of myofibrillar proteins and subsequent organization of the mature sarcomere. If it were required, it would firmly establish Ang-1 as a multipotent anabolic signaling molecule in muscle biology.
In cultured endothelial cells, Ang-1 promotes survival, proliferation, migration, and differentiation and inhibits apoptosis. Deletion of Ang-1 during early phases of vasculogenesis leads to major impairment of vascular formation and embryonic lethality (42). Jeansson et al. (24) reported that deletion of Ang-1 after day E13.5 in adult mice produced no immediate vascular phenotype. However, when combined with injury or microvascular stress, Ang-1 deletion triggered major organ damage and fibrosis (24). These results suggest that Ang-1 is dispensable in quiescent vessels but plays important roles in modulating the muscular response to injury.
Ang-1 exerts its vascular effects mainly through activation of Tie-2 receptors, which colocalize with αvβ3 integrins upon ligation, whereas Ang-2 recruits integrin subunits and triggers their internalization and degradation (44). In this study, Ang-1 increased skeletal myoblast survival, proliferation, migration, and differentiation (Figs. 8 and 9). The presence of Tie-2 receptors in skeletal myoblasts and the involvement of these receptors in the effects of Ang-1 on these cells remain to be determined. Dallabrida et al. (12) failed to detect Tie-2 receptors in primary skeletal myoblasts, whereas Abou-Khalil et al. (2) did in a subset of skeletal muscle satellite cells (reserve cells) and showed that their activation by Ang-1 promotes quiescence. In support of this finding, Ang-1 has also been shown to regulate hematopoietic stem cell (HSC) quiescence by promoting adhesion of HSCs to endosteal osteoblasts (5).
In this study, transcriptome analyses of human myoblasts infected with Ad-Ang-1 viruses demonstrated alterations in the expression of 2,366 transcripts that are involved in various biological processes, including cell cycle regulation. Upregulated genes such as CYCLIN D2 (CCND2), CYCLIN B2 (CCNB2), CYCLIN A2 (CCNA2), and CYCLIN-DEPENDENT KINASE 1 (CDC2) are critical regulators of cell cycle progress, and their upregulation may explain the increased myoblast proliferation that was seen in response to Ang-1 exposure in vitro. This explanation is in agreement with that of Lee et al. (25) but contradicts the study of Abou-Khalil et al. that used reserve cells (2), for reasons that are as yet unclear. We speculate that skeletal progenitor cell subtypes exhibit different responses to Ang-1 depending on such factors as the presence or absence of Tie-2 receptors and the types of signaling pathways that are activated.
Clearly, additional studies are required to elucidate the exact contributions of Tie-2 receptors and integrins to the signaling pathways and biological effects of Ang-1 on skeletal muscle progenitors. It should also be emphasized that this study does not rule out the possibility that improved contractile performance is related to factors beyond Ang-1-induced myogenesis and angiogenesis, as Ang-1 also exerts direct effects on muscle contractility and the regulation of Ca2+ inside regenerating muscle fibers. This view is supported by the work of Lee et al. (25), who described increases in basal and caffeine- and KCl-induced Ca2+ flux in primary skeletal myotubes exposed to recombinant Ang-1 protein. They also showed that Ang-1 upregulates the expression of several proteins involved in Ca2+ flux, a response that is likely to lead to improved muscle force generation capacity.
Perspectives and Significance
Ang-1 has been studied primarily in the vasculature and hematopoietic system. Our current study demonstrates that in addition to promoting quiescence in specific population of myoblasts, Ang-1 clearly enhances the recovery of contractile performance and muscle fiber regeneration in injured skeletal muscles in vivo and stimulates survival, migration, proliferation, and differentiation of skeletal muscle progenitors in vitro. Taken together, these results demonstrate for the first time that a single ligand (Ang-1) functions in both the maintenance of the endothelium and the regeneration of myofibers in injured skeletal muscles. Because current pharmacological therapies targeting patients with pathological limb disease or injury are inefficient and largely directed toward individual cell types or specific signaling pathways, our current findings demonstrate the plausibility of developing singular therapeutic strategies to enhance recovery in multiple cell types, improving overall clinical outcomes particularly in patients with Duchenne Muscular Dystrophy. The success of this strategy will be dependent on future research dedicated to the generation of stable and protease-resistant form of Ang-1 than can be delivered to skeletal muscle fibers.
GRANTS
This work was funded by the Canadian Institutes of Health Research and the National Heart, Lung, and Blood Institute. J. M. McClung is the recipient of a NIH/NHLBA grant (K99HL103797-01). M. Mofarrahi is the recipient of a Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. S. N. A. Hussain is the recipient of a James McGill Professorship, McGill University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.M., J.M.M., C.D.K., G.D., and S.N.A.H. conception and design of research; M.M., J.M.M., C.D.K., E.C.D., B.T., N.M., A.E.P., L.H., S.H., and G.D. performed experiments; M.M., J.M.M., C.D.K., E.C.D., B.T., N.M., A.E.P., L.H., S.H., and G.D. analyzed data; M.M., J.M.M., C.D.K., E.C.D., B.T., N.M., A.E.P., L.H., S.H., G.D., and S.N.A.H. interpreted results of experiments; M.M., J.M.M., C.D.K., G.D., and S.N.A.H. edited and revised manuscript; M.M., J.M.M., C.D.K., E.C.D., B.T., N.M., A.E.P., L.H., S.H., G.D., and S.N.A.H. approved final version of manuscript; C.D.K. and S.N.A.H. drafted manuscript; E.C.D. and S.N.A.H. prepared figures.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to D. Mayaki and Min Fu for their technical assistance and A. Gatensby for editing the manuscript.
REFERENCES
- 1.Abdel-Malak NA, Srikant CB, Kristof AS, Magder SA, Di Battista JA, Hussain SNA. Angiopoietin-1 promotes endothelial proliferation and migration through AP-1 dependent autocrine production of interleukin-8. Blood 111: 4145–4154, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Abou-Khalil R, Le GF, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, Lafuste P, Montarras D, Chazaud B. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell 5: 298–309, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165: 307–312, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand 95: 203–205, 1975. [DOI] [PubMed] [Google Scholar]
- 5.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118: 149–161, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bentzinger CF, von Maltzhahn J, Rudnicki MA. Extrinsic regulation of satellite cell specification. Stem Cell Res Ther 1: 27, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 98: 1014–1023, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between α5β1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol 170: 993–1004, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Christov C, Chretien F, bou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18: 1397–1409, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creemers EE, Sutherland LB, Oh J, Barbosa AC, Olson EN. Coactivation of MEF2 by the SAP domain proteins myocardin and MASTR. Mol Cell 23: 83–96, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res 96: e8–24, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Deasy BM, Feduska JM, Payne TR, Li Y, Ambrosio F, Huard J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther 17: 1788–1798, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2: 499, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9: 255–267, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics 24: 1547–1548, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics 23: 257–258, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Gayraud-Morel B, Chretien F, Flamant P, Gomes D, Zammit PS, Tajbakhsh S. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev Biol 312: 13–28, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Gosker HR, Schols AM, Kapchinsky S, Bourbeau J, Sandri M, Jagoe RT, Debigare R, Maltais F, Taivassalo T, Hussain SN. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 188: 1313–1320, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Harfouche R, Hussain SNA. Signaling and regulation of endothelial cell survival by angiopoietin-2. Am J Physiol Heart Circ Physiol 291: H1635–H1645, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534–551, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Himeda CL, Ranish JA, Pearson RC, Crossley M, Hauschka SD. KLF3 regulates muscle-specific gene expression and synergizes with serum response factor on KLF binding sites. Mol Cell Biol 30: 3430–3443, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata A, Masuda S, Tamura T, Kai K, Ojima K, Fukase A, Motoyoshi K, Kamakura K, Miyagoe-Suzuki Y, Takeda S. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin. Am J Pathol 163: 203–215, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121: 2278–2289, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee EH, Woo JS, Hwang JH, Park JH, Cho CH. Angiopoietin 1 enhances the proliferation and differentiation of skeletal myoblasts. J Cell Physiol 228: 1038–1044, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Lee SW, Moskowitz MA, Sims JR. Sonic hedgehog inversely regulates the expression of angiopoietin-1 and angiopoietin-2 in fibroblasts. Int J Mol Med 19: 445–451, 2007. [PubMed] [Google Scholar]
- 27.Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164: 1007–1019, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SM, Du P, Huber W, Kibbe WA. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res 36: e11, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long X, Creemers EE, Wang DZ, Olson EN, Miano JM. Myocardin is a bifunctional switch for smooth versus skeletal muscle differentiation. Proc Natl Acad Sci USA 104: 16570–16575, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller KJ, Thaloor D, Matteson S, Pavlath GK. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Physiol Cell Physiol 278: C174–C181, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell CA, McGeachie JK, Grounds MD. The exogenous administration of basic fibroblast growth factor to regenerating skeletal muscle in mice does not enhance the process of regeneration. Growth Factors 13: 37–55, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Mofarrahi M, Hussain SN. Expression and functional roles of angiopoietin-2 in skeletal muscles. PLos One 6: e22882, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mofarrahi M, Nouh T, Qureshi S, Guillot L, Mayaki D, Hussain SNA. Regulation of angiopoietin expression by bactrial lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol 294: L955–L963, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake PR, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 7: 706–711, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Renault MA, Vandierdonck S, Chapouly C, Yu Y, Qin G, Metras A, Couffinhal T, Losordo DW, Yao Q, Reynaud A, Jaspard-Vinassa B, Belloc I, Desgranges C, Gadeau AP. Gli3 regulation of myogenesis is necessary for ischemia-induced angiogenesis. Circ Res 113: 1148–1158, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM, Hoying JB, Allen RE. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol 296: C1321–C1328, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim 31: 773–779, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve 23: 239–245, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Smith CK, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol 159: 379–385, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Smyth GK. Limma: linear models of microarray data. In: Bioinformatics and Computional Biology Solutions using R and Bioconductors, edited by Gentleman R, Carey V, Dudoit S, Irizarry R and Huber W. New York: Springer, 2005, p. 397–420. [Google Scholar]
- 41.Straface G, Aprahamian T, Flex A, Gaetani E, Biscetti F, Smith RC, Pecorini G, Pola E, Angelini F, Stigliano E, Castellot JJ Jr, Losordo DW, Pola R. Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J Cell Mol Med 13: 2424–2435, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suri C, Jone PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato T, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE-2 receptor, during embryonic angiogenesis. Cell 87: 1171–1180, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 194: 114–128, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Thomas M, Felcht M, Kruse K, Kretschmer S, Deppermann C, Biesdorf A, Rohr K, Benest AV, Fiedler U, Augustin HG. Angiopoietin-2 stimulation of endothelial cells induces αvβ3 integrin internalization and degradation. J Biol Chem 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nature Med 6: 460–463, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Wagatsuma A. Endogenous expression of angiogenesis-related factors in response to muscle injury. Mol Cell Biochem 298: 151–159, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Wei L, Zhou W, Croissant JD, Johansen FE, Prywes R, Balasubramanyam A, Schwartz RJ. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J Biol Chem 273: 30287–30294, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Winkler T, von Roth P, Matziolis G, Schumann MR, Hahn S, Strube P, Stoltenburg-Didinger G, Perka C, Duda GN, Tohtz SV. Time course of skeletal muscle regeneration after severe trauma. Acta Orthop 82: 102–111, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 278: 8826–8836, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.