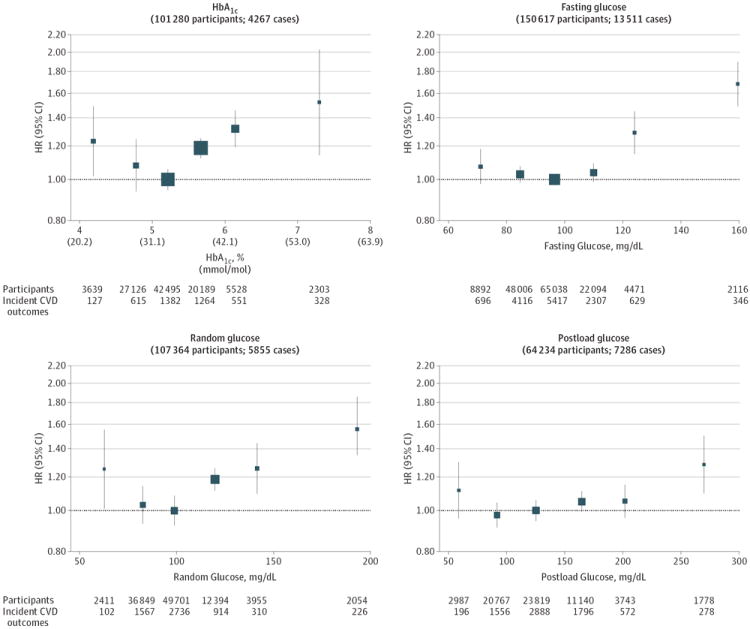
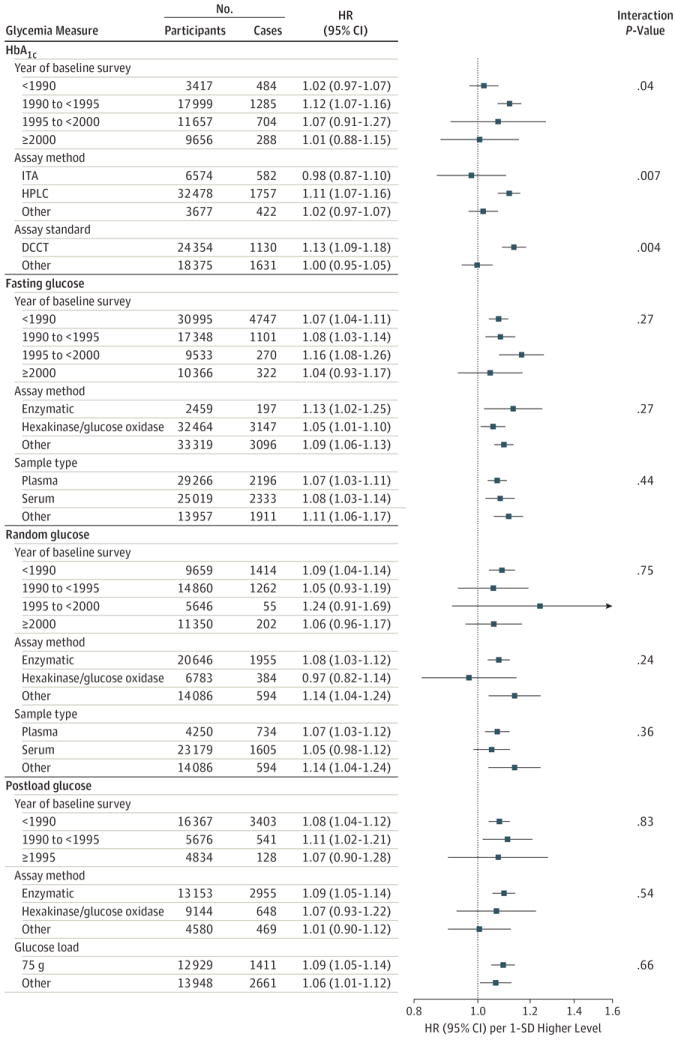
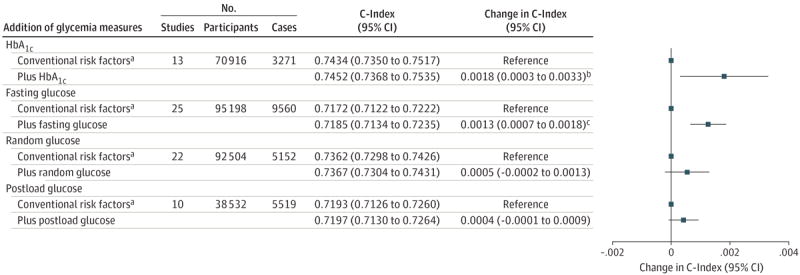
The Emerging Risk Factors Collaboration1,
Emanuele Di Angelantonio
Emanuele Di Angelantonio, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Pei Gao
Pei Gao, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Hassan Khan
Hassan Khan, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Adam S Butterworth
Adam S Butterworth, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
David Wormser
David Wormser, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Stephen Kaptoge
Stephen Kaptoge, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Sreenivasa Rao Kondapally Seshasai
Sreenivasa Rao Kondapally Seshasai, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Alex Thompson
Alex Thompson, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Nadeem Sarwar
Nadeem Sarwar, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Peter Willeit
Peter Willeit, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Paul M Ridker
Paul M Ridker, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Elizabeth LM Barr
Elizabeth LM Barr, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Kay-Tee Khaw
Kay-Tee Khaw, FMedSci
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Bruce M Psaty
Bruce M Psaty, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Hermann Brenner
Hermann Brenner, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Beverley Balkau
Beverley Balkau, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Jacqueline M Dekker
Jacqueline M Dekker, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Debbie A Lawlor
Debbie A Lawlor, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Makoto Daimon
Makoto Daimon, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Johann Willeit
Johann Willeit, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Inger Njølstad
Inger Njølstad, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Aulikki Nissinen
Aulikki Nissinen, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Eric J Brunner
Eric J Brunner, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Lewis H Kuller
Lewis H Kuller, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Jackie F Price
Jackie F Price, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Johan Sundström
Johan Sundström, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Matthew W Knuiman
Matthew W Knuiman, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Edith J M Feskens
Edith J M Feskens, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
W M M Verschuren
W M M Verschuren, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Nicholas Wald
Nicholas Wald, FRS
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Stephan J L Bakker
Stephan J L Bakker, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Peter H Whincup
Peter H Whincup, FRCP
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Ian Ford
Ian Ford, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Uri Goldbourt
Uri Goldbourt, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Agustín Gómez-de-la-Cámara
Agustín Gómez-de-la-Cámara, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
John Gallacher
John Gallacher, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Leon A Simons
Leon A Simons, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Annika Rosengren
Annika Rosengren, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Susan E Sutherland
Susan E Sutherland, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Cecilia Björkelund
Cecilia Björkelund, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Dan G Blazer
Dan G Blazer, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Sylvia Wassertheil-Smoller
Sylvia Wassertheil-Smoller, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Altan Onat
Altan Onat, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Alejandro Marín Ibañez
Alejandro Marín Ibañez, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Edoardo Casiglia
Edoardo Casiglia, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
J Wouter Jukema
J Wouter Jukema, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Lara M Simpson
Lara M Simpson, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Simona Giampaoli
Simona Giampaoli, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Børge G Nordestgaard
Børge G Nordestgaard, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Randi Selmer
Randi Selmer, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Patrik Wennberg
Patrik Wennberg, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Jussi Kauhanen
Jussi Kauhanen, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Jukka T Salonen
Jukka T Salonen, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Rachel Dankner
Rachel Dankner, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Elizabeth Barrett-Connor
Elizabeth Barrett-Connor, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Maryam Kavousi
Maryam Kavousi, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Vilmundur Gudnason
Vilmundur Gudnason, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Denis Evans
Denis Evans, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Robert B Wallace
Robert B Wallace, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Mary Cushman
Mary Cushman, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Ralph B D’Agostino Sr
Ralph B D’Agostino Sr, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Jason G Umans
Jason G Umans, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Yutaka Kiyohara
Yutaka Kiyohara, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Hidaeki Nakagawa
Hidaeki Nakagawa, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Shinichi Sato
Shinichi Sato, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Richard F Gillum
Richard F Gillum, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Aaron R Folsom
Aaron R Folsom, MD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Yvonne T van der Schouw
Yvonne T van der Schouw, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Karel G Moons
Karel G Moons, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Simon J Griffin
Simon J Griffin, DM
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Naveed Sattar
Naveed Sattar, FRCP
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Nicholas J Wareham
Nicholas J Wareham, FRCP
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Elizabeth Selvin
Elizabeth Selvin, PhD
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
Simon G Thompson
Simon G Thompson, FMedSci
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1,
John Danesh
John Danesh, FRCP
1University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Gao, Khan, Butterworth, Wormser, Kaptoge, A. Thompson, Sarwar, P. Willeit, Khaw, Griffin, Wareham, S. G. Thompson, Danesh); St George’s University of London, London, United Kingdom (Kondapally Seshasai, Whincup); Brigham and Women’s Hospital, Boston, Massachusetts (Ridker); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); University of Washington, Seattle (Psaty); Group Health Research Institute, Seattle, Washington (Psaty); German Cancer Research Center, Heidelberg, Germany (Brenner); Inserm, Villejuif, France (Balkau); University Paris-Sud, Villejuif, France (Balkau); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Dekker); University of Bristol, Bristol, United Kingdom (Lawlor); Yamagata University, Japan (Daimon); Medical University Innsbruck, Austria (J. Willeit); University of Tromsø, Tromsø, Norway (Njølstad); National Institute of Health and Welfare, Helsinki, Finland (Nissinen); University College London, London, United Kingdom (Brunner); University of Pittsburgh (Kuller); University of Edinburgh, Edinburgh, United Kingdom (Price); Uppsala University, Uppsala, Sweden (Sundström); University of Western Australia, Perth, Australia (Knuiman);Wageningen University, Wageningen, the Netherlands (Feskens); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Verschuren); Wolfson Institute of Preventive Medicine, London, United Kingdom (Wald); University of Groningen, University Medical Center Groningen, the Netherlands (Bakker); University of Glasgow, Glasgow, United Kingdom (Ford, Sattar); Sheba Medical Center, Tel Hashomer, Israel (Goldbourt); Hospital 12 de Octubre, Madrid, Spain (Gómez-de-la-Cámara); Cardiff University, Cardiff, United Kingdom (Gallacher); University of New South Wales, Kensington, Australia (Simons); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); Medical University of South Carolina, Charleston (Sutherland); University of Gothenburg, Gothenburg, Sweden (Björkelund); Duke University Medical Center, Durham, North Carolina (Blazer); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of Istanbul, Istanbul, Turkey (Onat); San Jose Norte Health Centre, Zaragoza, Spain (Marín Ibañez); University of Padova, Padova, Italy (Casiglia); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Texas School of Public Health, Houston (Simpson); Istituto Superiore di Sanità, Rome, Italy (Giampaoli); Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Umeå University, Umeå, Sweden (Wennberg); University of Eastern Finland, Kuopio, Finland (Kauhanen); University of Helsinki, Helsinki, Finland (Salonen); The Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel (Dankner); Tel Aviv University, Tel Aviv, Israel (Dankner); The Feinstein Institute for Medical Research, New York, New York (Dankner); University of California, San Diego (Barrett-Connor); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi); Icelandic Heart Association, Reyjavik, Iceland (Gudnason); University of Iceland, Reykjavik, Iceland (Gudnason); Rush University Medical Center, Chicago, Illinois (Evans); University of Iowa College of Public Health, Iowa City (Wallace); University of Vermont, Burlington (Cushman); Boston University, Boston, Massachusetts (D’Agostino); Georgetown University Medical Centre, Washington, DC (Umans); Kyushu University, Kyushu, Japan (Kiyohara); Kanazawa Medical University, Ishikawa, Japan (Nakagawa); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Osaka, Japan (Sato); Howard University, Washington, DC (Gillum); University of Minnesota, Minneapolis (Folsom); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands (van der Schouw, Moons); Johns Hopkins University, Baltimore, Maryland (Selvin)
1