Abstract
Background
Species of Microsporidia have been known as opportunistic obligate intracellular parasites particularly in immunocompromised patients. Enterocytozoon bieneusi is one of most prevalent intestinal microsporida parasites in HIV+/AIDS patients. In this study, intestinal microsporidia infection was determined in HIV+/AIDS patients using microscopic and molecular methods.
Methods
Stool samples were collected from HIV+/AIDS patients during 12 months. All of the stool specimens washed with PBS (pH: 7.5). Slim slides were prepared from each sample and were examined using light microscope with 1000X magnification. DNA extraction carried out in microscopic positive samples. DNA amplification and genus/species identification also performed by Nested-PCR and sequencing techniques.
Results
From 81 stool samples, 25 were infected with microsporidia species and E. bieneusi were identified in all of positive samples. No Encephalitozoon spp. was identified in 81 collected samples using specific primers.
Conclusion
E. bieneusi is the most prevalent intestinal microsporidia in immunocompromised patients of Iran. On the other hand, Nested-PCR using specific primers for ssu rRNA gene is an appropriate molecular method for identification of E. bieneusi.
Keywords: HIV+/AIDS patients, Iran, Enterocytozoon bieneusi, Encephalitozoon spp, Nested-PCR
Introduction
Species of microsporidia are obligate intracellular protozoans that have been recognized among broad spectrum of invertebrate and vertebrate hosts (1, 2). Almost 1200 species of microsporidia belong to more than 140 genera were described, so far (1, 3, 4). Since the first human microsporidiosis was identified in 1959, reports of cases of immunocompromised patients who suffer from different form of microsporidia infection have been increased (5-7).
Chronic diarrhea is the most common clinical manifestation in HIV+/AIDS patients with intestinal microsporidiosis, especially in individuals with less than 100 CD 4 + T cell per μl of peripheral blood (8-10). Prevalence of intestinal microsporidia infection has been reported from 2 to 50% and even higher depending on methods of diagnosis, geographical area or hygiene and immunity condition of study population (1, 11, 12). Although the most prevalence of intestinal microsporidiosis could be seen in immunocompromised patients with chronic diarrhea but the infection have also been reported in immunodeficient or even immunecompetent individuals with or without diarrhea, frequently (13, 14). Increasing the number of immunocompromised patients is the main reason that subtle diagnosis of opportunistic parasite such as Encephalitozoon spp. and E. bieneusi in stool has been considered for researchers. Spores of human microsporidia are very small and the size of them is varying from 1μm to 4μm (8, 13).
Many staining and serological methods have been used for diagnosis of microsporidia infection, but molecular techniques based on rRNA gene using Polymerase chain reaction (PCR) and more recently Real - time PCR have been showed 100% validity in detection and species identification of the parasite (15-19).
To date, we did not have any precise information of intestinal microsporidia infection in immunocompromised patients in Iran except rare studies that carried out by Agholi et al. (20, 21). This study aimed to determine the intestinal microsporidia infection in HIV+/AIDS patients in Iran.
Materials & Methods
Sampling
This study were carried out on 81 stool samples which were collected from HIV+/AIDS patients who referred to Imam Khomeini Hospital during 2012-2013. HIV infection was confirmed by Western blotting and AIDS phase also confirmed with CD 4 + T cell count less than 200 per μl of peripheral blood and receiving Anti-Retroviral Treatment (ART). Samples were collected actively from patients who referred to laboratory due to periodic checkup or intestinal disorders. Based on appearance of stool, each of samples was considered in one of grades consisted: formed, diarrhea and watery diarrhea. From 81 stool specimens, 58 and 23 stool samples were collected from men and women, respectively.
Parasitological study
All of stool samples suspended in PBS pH 7.5, and then the suspension filtered with sterile gases for debris exclusion. Final suspension washed three times by sterile PBS and finally supernatant removed and remained pellet divided to 2 parts and each portion re-suspended in alcohol 80% and formalin – PBS 5% for molecular and parasitological assessments, respectively.
Thin slide for all of isolates was provided and staining was carried out using Ryan blue method that described elsewhere (22), previously. Screening carried out under oil immersion lens and small (1.2- 2μm), ovoid, pinkish spores containing median belt considered as microsporidia. DNA extraction performed on positive specimens that proved with light microscopic examination.
DNA extraction, PCR and Sequencing
For DNA extraction 250 μl from stool suspended in sterile PBS was transferred to 1.5 ml tube. After centrifuging in 5000 rpm for 10 minutes, supernatant was removed and then 400 μl of lysis buffer (100 mM Tric, 10 mM EDTA, 2% SDS, (final pH = 8)), 20 μg/μl Proteinase K and acide washed Glass beads size 450 – 600 μm were added to remained pellet. Samples were vortexed for 2 minutes vigorously and incubated at 60°C for 3 hours. Samples vortexing was repeated for 30 sec every 30 minutes. Finally, after centrifuging in 3000 rpm for 5 min, supernatant transferred to Bioneer stool DNA extraction kit (Bioneer Corporation, Daejeon, Korea). Purified DNA was stored at -20 °C until use.
Nested – PCR was performed using genus specific primers, which were designed, based on ssu rRNA gene using online software (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). The first pair primers, PMicF (5′- GGTTGATTCTGCCTGACG - 3′) and PMicR (5′ - CTTGCGAGC(G/A)TACTATCC - 3′), amplified 779 bp of ssu rRNA gene of Encephalitozoon spp and E. bieneusi. The second PCR employed primers EnbF (5′- GGTAATTTGGTCTCTGTGTG - 3′) and EnbR (5′- CTACACTCCCTATCCGTTC -3′) to amplify 440 bp and also EncepF (5′- AGTACGATGATTTGGTTG- 3′) and EncepR (5′- ACAACACTATATAGTCCCGTC- 3′) to amplify 629 bp fragments for E. bieneusi and Encephalitozoon spp., respectively.
First PCR reaction was performed in final volume 25 μl containing 2.5 μl of 10X PCR buffer, 2mM MgCl2, 200μM dNTP, 1.5 unit of Taq polymerase (Fermentase, Thermo Fisher Scientific, Lithuania) and 10 ρM of each primers. Amplifications were carried out in PeqLab thermocycler (PEQLAB Biotechnologie GmbH, Germany) under condition, 95 °C for 5 min followed by 35 cycles of 94 °C for 40 sec, 55 °C for 45 sec and 72 °C for 45 sec and final extension of 72 °C for 4 min. The second PCR condition consisted 95 °C for 5 min followed by 25 cycles of 94 °C for 35 sec, 57°C for 35 sec, 72 °C for 40 sec and 72 °C for 3 min as a final extension. 5μl of PCR products were electrophoresed on 1.5% of agarose gel and were visualized after ethidium bromide staining. PCR products of positive samples were sequenced using ABI 3130 (California, USA) and the results were compared using BLAST software in GenBank database.
Statistical test
Data analyzing were performed by chi-square (X2) test, using SPSS software (version 18, SPSS Inc., Chicago, IL). A P- value <0.05 was considered statistically significant.
Results
From eighty one stool samples which were collected from HIV+/AIDS patients, twenty five (30.86%) samples were positive for intestinal microsporidia infection, microscopically (Fig. 1) and all of them confirmed with Nested-PCR. Positive cases were observed in 8 (34.8%) and 17(29.31%) of women and men, respectively. No statistically significant difference was found in the both groups (P = 0.631). Chronic watery or moderate diarrhea were existed in 13 (52%) of positive cases. Positive cases were also seen in 12 (48%) patients with history of diarrhea. No statistically significant difference was found in the both groups (P = 0.986).
Fig. 1.
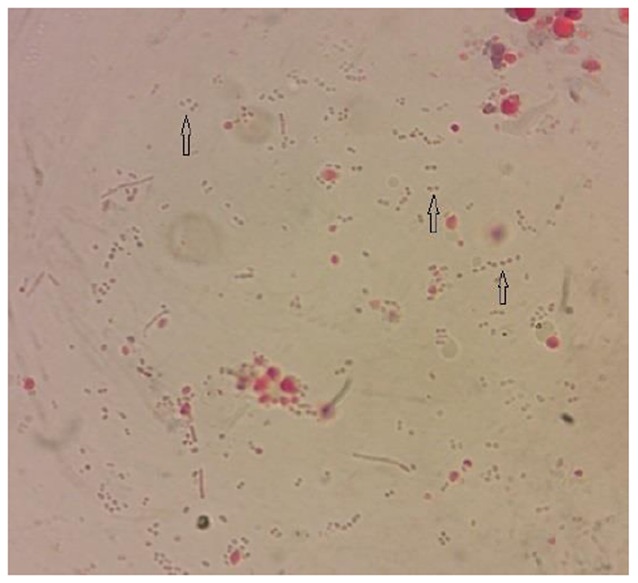
Enterocytozoon bieneusi spores (arrows) isolated from stool of HIV+/AIDS patient. 1000X Magnification (Original)
All the positive samples showed 440 bp fragment of E. bieneusi (Fig. 2). DNA amplification of Encephalitozoon spp. did not found by specific primers belonging to that. Sequencing results of five cases, which were selected randomly from positive samples, were compared in GenBank and consequently all of PCR results were proved. The accession numbers of sequenced samples including of KF875441, KF875442, KF875443, KF875444 and KF875445. Results of study are summarized in Table 1.
Fig. 2.
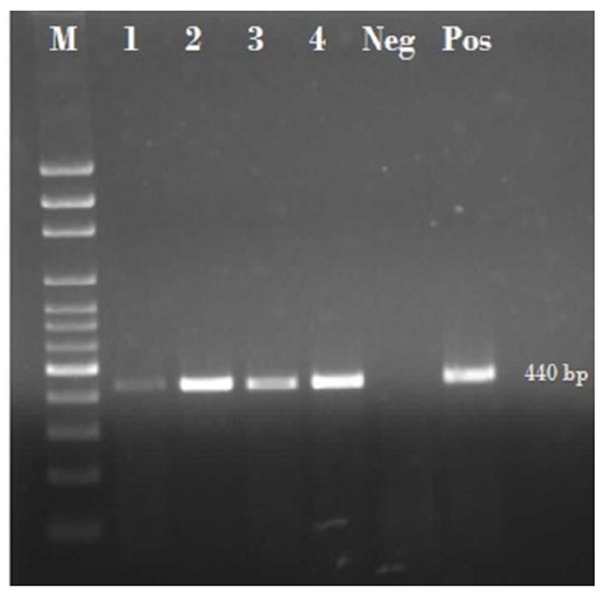
Gel electrophoresis of 440 bp fragment of E. bieneusi using Nested- PCR, M: 100bp marker, 1 to 4: samples of E. bieneusi, Neg: negative control, Pos: positive control
Table 1.
General characteristic of 81 HIV+/AIDS patients co-infected with Enterocytozoon bieneusi
| No. of collected samples | No. (%) of infected samples | ||
|---|---|---|---|
| Gender | Male | 58 | 17(29.31) |
| Female | 23 | 8(34.8) | |
| Age(yr) | 20- 35 | 41 | 16(39.02) |
| 36-50 | 33 | 6(18.18) | |
| 51-65 | 7 | 3(42.85) | |
| Stool appearance | Formed | 39 | 12(30.77) |
| Diarrhea | 24 | 7(29.16) | |
| Watery diarrhea | 18 | 6(33.33) | |
Discussion
In our study, E. bieneusi obtained from 30.86% of HIV+/AIDS patients. The percent of infected patients in both genders was approximately equal and chronic sever or moderate diarrhea was seen in more than half of samples.
Although, more recently Agholi et al. (21) described microsporidiosis in some HIV+/AIDS patients (356/8) but there are not any precise information about microsporidia infection and also genus or species that are involve in transmission cycle of infection in those patients in Iran. Our findings clearly show that the microsporidiosis could be seen in more HIV+/AIDS patients than that was mentioned in previous study (21). Differentiation in findings likely related to manners of stool preparation or DNA extraction. As most researchers have been declared, DNA extraction from microsporidia spores in stool has many complexities. E. bieneusi and Encephalitozoon spp. spores have very small size, rigid double layer wall and also low counts in stool samples. As a result, stool preparation and strong DNA extraction methods can impress the quality and quantity of molecular results (17, 23, 24).
Our results also show that E. bieneusi is the most important agent of intestinal microsporidia infection in HIV+/AIDS patients in Iran. The finding is in agreement with other studies from different areas of the world. It is interest to mentioned that E. bieneusi is one of most prevalent microsporidia parasites which were reported from immunocompromised individuals such as HIV+/AIDS patients in most countries (11) and probably play an important role in intestinal involvement and subsequently chronic diarrhea in HIV+/AIDS patients (21, 25-27).
In our study E. bieneusi was detected in 12 (48%) of those patients who had not any severe or moderate diarrhea indication, at the sampling time. Although no statistical relationship between diarrhea and microsporidiosis were seen in this study but it is interest to mention that according to recent research, intestinal microsporidiosis could be seen in individuals with intermittent diarrhea or rarely without history of acute or chronic diarrhea (13). However, all of HIV+/AIDS patients in our study, received Anti-Retroviral Treatment (ART) and consequently their CD 4 + T cell count improved. On the other hand, some patients received different drug for signing treatment of diarrhea that probably both of reasons could be efficacious in diarrhea remission at the sampling time.
So far, prevalence of different genus of Microsporidia has not been known completely in Iran and probably the cases of involvement with various forms of microsporidiosis are more than our expectancy. Furthermore, it is need to assessment the infection get noticed in susceptible patients such as transplant recipients and cancer patients who are under immunosuppressing treatment and chemotherapy, respectively, or patients with other congenital or acquired immune system failures as at risk individuals.
Conclusion
E. bieneusi is probably the most prevalent intestinal microsporidia genus particularly in HIV+/AIDS in Iran. Strong stool preparation and molecular methods such as Nested-PCR could be useful for detection of intestinal microsporidia infection.
Acknowledgments
The authors would like to appreciate Mrs. Fatemeh Tarighi, Dr. khadijeh Khanaliha and Mrs. Zeinab Askari for laboratory cooperation. This study is a part of PhD thesis and supported by Tehran University of Medical Sciences, Iran (grant No: 91-01-160-17172). The authors declare that they have no conflicts of interest.
References
- 1.Canning EU, Lom J, Dykova I. The microsporidia of vertebrates. Academic Press; 1986. [Google Scholar]
- 2.Sprague V, Becnel JJ, Hazard EI. Taxonomy of phylum microspora. Crit Rev Microbiol. 1992;18(5-6):285–395. doi: 10.3109/10408419209113519. [DOI] [PubMed] [Google Scholar]
- 3.Voronin VN. Macrotaxonomy of the Microsporidia phylum. Parazitologiia. 2001;35(1):35–44. [PubMed] [Google Scholar]
- 4.Gannon J. A survey of Encephalitozoon cuniculi in laboratory animals colonies in the United Kingdom. Lab Anim. 1980;14:91–4. doi: 10.1258/002367780780942917. [DOI] [PubMed] [Google Scholar]
- 5.Schottelius J, da Costa SC. Microsporidia and acquired immunodeficiency syndrome. Mem Inst Oswaldo Cruz. 2000;95(Suppl 1):133–9. doi: 10.1590/s0074-02762000000700022. [DOI] [PubMed] [Google Scholar]
- 6.Didier ES, Didier PJ, Snowden KF, Shadduck JA. Microsporidiosis in mammals. Microbes Infect. 2000;2(6):709–20. doi: 10.1016/s1286-4579(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 7.Matsubayashi H, Koike I, Mikata I, Takei H, Hagiwara S. A case of Encephalitozoon-like infection in man. Arch Pathol. 1959;67:181–7. [PubMed] [Google Scholar]
- 8.Didier ES, Weiss LM. Microsporidiosis: current status. Curr Opin Infect Dis. 2006;19(5):485–92. doi: 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morpeth SC, Thielman NM. Diarrhea in patients with AIDS. Curr Treat Options Gastroenterol. 2006;9(1):23–37. doi: 10.1007/s11938-006-0021-8. [DOI] [PubMed] [Google Scholar]
- 10.Lewthwaite P, Gill GV, Hart CA, Beeching NJ. Gastrointestinal parasites in the immunocompromised. Curr Opin Infect Dis. 2005;18(5):427–35. doi: 10.1097/01.qco.0000182104.40128.18. [DOI] [PubMed] [Google Scholar]
- 11.Weber R, Bryan RT, Schwartz DA, Owen RL. Human Microsporidial infection. Clin Microbiol Rev. 1994;7:421–6. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyle CM, Wittner M, Kotler DP, Noyer C, Orenstein JM, Tanowitz HB, et al. Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determfination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clin Infect Dis. 1996;23(5):1002–6. doi: 10.1093/clinids/23.5.1002. [DOI] [PubMed] [Google Scholar]
- 13.Franzen C, Muller A. Microsporidiosis: human diseases and diagnosis. Microbes Infect. 2001;3:389–400. doi: 10.1016/s1286-4579(01)01395-8. [DOI] [PubMed] [Google Scholar]
- 14.Hautvast JL, Tolboom JJ, Derks TJ, Beckers P, Sauerwein RW. Asymptomatic intestinal microsporidiosis in a human immun-odeficiency virus-seronegative, immunoco-mpetent Zambian child. Pediatr Infect Dis J. 1997;16(4):415–6. doi: 10.1097/00006454-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates The Enteric Opportunistic Infections Working Group. N Engl J Med. 1992;326(3):161–6. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 16.Awadalla HN, el Naga IF, el-Temsahi MM, Negm AY. Detection of Microsporidia by different staining techniques. J Egypt Soc Parasitol. 1998;28(3):729–38. [PubMed] [Google Scholar]
- 17.Franzen C, Muller A. Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia. Clin Microbiol Rev. 1999;12(2):243–85. doi: 10.1128/cmr.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franzen C, Muller A, Hartmann P, Hegener P, Schrappe M, Diehl V, et al. Polymerase chain reaction for diagnosis and species differentiat-ion of microsporidia. Folia Parasitol (Praha) 1998;45(2):140–8. [PubMed] [Google Scholar]
- 19.Menotti J, Cassinat B, Sarfati C, Liguory O, Derouin F, Molina JM. Development of a real-time PCR assay for quantitative detection of Encephalitozoon intestinalis DNA. J Clin Microbiol. 2003;41(4):1410–3. doi: 10.1128/JCM.41.4.1410-1413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agholi M, Hatam GR, Motazedian MH. Microsporidia and coccidia as causes of persistence diarrhea among liver transplant children: incidence rate and species/genotypes. Pediatr Infect Dis. J. 2013;32(2):185–7. doi: 10.1097/INF.0b013e318273d95f. [DOI] [PubMed] [Google Scholar]
- 21.Agholi M, Hatam GR, Motazedian MH. HIV/AIDS-associated opportunistic protozoal diarrhea. AIDS Res Hum Retroviruses. 2013;29(1):35–41. doi: 10.1089/aid.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan NJ, Sutherland G, Coughlan K, Globan M, Doultree J, Marshall J, et al. A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol. 1993;31(12):3264–9. doi: 10.1128/jcm.31.12.3264-3269.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gool T, Canning EU, Dankert J. An improved practical and sensitive technique for the detection of microsporidian spores in stool samples. Trans R Soc Trop Med Hyg. 1994;88(2):189–190. doi: 10.1016/0035-9203(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 24.Subrungruang I, Mungthin M, Chavalitshe-winkoon-Petmitr P, Rangsin R, Naaglor T, Leelayoova S. Evaluation of DNA extraction and PCR methods for detection of Enterocytozoon bienuesi in stool specimens. J Clin Microbiol. 2004;42(8):3490–4. doi: 10.1128/JCM.42.8.3490-3494.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samie A, Obi CL, Tzipori S, Weiss LM, Guerrant RL. Microsporidiosis in South Africa: PCR detection in stool samples of HIV-positive and HIV-negative individuals and school children in Vhembe distric, Limpopo Provincet. Trans R Soc Trop Med Hyg. 2007;101(6):547–54. doi: 10.1016/j.trstmh.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobo ML, Xiao L, Antunes F, Matos O. Microsporidia as emerging pathogens and the implication for public health: A 10-year study on HIV-positive and -negative patients. Int J Parasitol. 2012;42(2):197–205. doi: 10.1016/j.ijpara.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Ojuromi OT, Izquierdo F, Fenoy S, Fagbenro-Beyioku A, Oyibo W, Akanmu A et al. Identification and characterization of microsporidia from fecal samples of HIV-positive patients from Lagos Nigeria. PLoS One. 2012;7(4):e35239. doi: 10.1371/journal.pone.0035239. [DOI] [PMC free article] [PubMed] [Google Scholar]


