Abstract
Background
Clinical manifestations of Strongyloides stercoralis are variable from asymptomatic to hyperinfection and devastating disseminated infections. Hereby, clinical characteristics of a large series of Iranian strongyloidiasis indigenous cases are described.
Methods
The records of people referred to the Helminthological Diagnostic Laboratory of School of Public Health, Tehran University of Medical Sciences and School of Medicine, Gilan University of Medical Sciences, during 2009-2013 were reviewed. For those patients that were infected with S. stercoralis and their clinical manifestations and demographic data were available (70 cases) a checklist was prepared and data analyzed.
Results
Forty-three patients (61.4%) were male and 27 (38.6%) female. Gastrointestinal, cutaneous and pulmonary symptoms were present in 71.4%, 25.7%, and 15.7% of patients, respectively. None of them had larva currens eruption. Eosinophilia was the most prevalent reason for suspicious on S. stercoralis, but the mean was lower in elderly patients. Hyperinfection were recorded in 8 patients (11.4%), and 2 cases had disseminated infection.
Conclusion
Eosinophilia is common both in asymptomatic and symptomatic cases of strongyloidiasis, but the mean tend to lower with increase in age.
Keywords: Strongyloides stercoralis, Clinical characteristics, Hyperinfection, Eosinophilia
Introduction
Strongyloidiasis is one of the soil-transmitted helminth infections caused by Strongyloides stercoralis. It is estimated that about 30 to 100 million people in endemic areas of the world are infected, especially in tropical and subtropical countries (1). It has variable manifestations from asymptomatic to hyperinfection, or disseminated infection (2). Most cases are completely asymptomatic; some patients have mild gastrointestinal, cutaneous, or pulmonary symptoms with or without fever (1). Gastrointestinal and pulmonary symptoms are common in acute strongyloidiasis (3). Presence of eosinophilia is the most important laboratory finding in strongyloidiasis patients (4). Chronic strongyloidiasis causes mild clinical manifestations, but in patients receiving corticosteroid treatment and immunosuppressive therapy or having diseases such as diabetes and hematologic malignancies the disease may change to severe complicated strongyloidiasis (5); and larvae outside the usual migration pattern invade virtually every organ (6, 7).
Strongyloidiasis is still endemic in some provinces of Iran. In 2007, 4.9% of people in rural areas of Mazandaran Province were reported infected with this parasite (8). In a recent study in Gilan Province 42% of a population with eosinophilia were found positive for infectivity with S. stercoralis (4). In a mentally retarded institution of southern Iran 17.3% of the residents were found infected with this nematode (9). S. stercoralis hyperinfection cases have been documented in immunocompromised patients (10-13), and even leading to the death of the patient (11). Nevertheless, in spite of recent frequent reference of suspected patients to diagnostic referral centers, analytical description of indigenous patients is lacking. Therefore, this paper aims to analyze demographic and clinical characteristics of a large series of strongyloidiasis patients from endemic areas of Iran.
Materials & Methods
Patients
Helminthological laboratories of School of Public Health (SPH), Tehran University of Medical Sciences (TUMS) and School of Medicine, Gilan University of Medical Sciences (GUMS) are two referral centers for diagnosis of helminth infections in Iran. Most admitted patients either coming by themselves or are referred by physicians from private clinics, health care units or hospitals due to the presence of eosinophilia in peripheral blood, clinical symptoms and immunocompromised conditions.
In this retrospective descriptive study, the medical records of patients attended these centers during 2009 to 2013 were reviewed. Patients who were registered positive for S. stercoralis were identified; among them anyone whose clinical characteristics and demographic data were completely available was included in this analytical study. There were also some S. stercoralis infected cases with incomplete information; most of them related to patients who could not refer to the centers by themselves due to long distance, elderliness, hospitalization and so on. Therefore, only their stool samples were sent to the laboratories for examination. These patients were not enrolled in this study.
Stool examinations
Identification of S. stercoralis infection in these centers was based on coprological examinations and detection of rhabditiform or filariform larvae by either normal saline direct smear, formalin-ether concentration technique (FECT) or by nutrient-agar plate culture.
For nutrient-agar plate culture, about 3g of fresh stool sample was placed in the center of the dish, incubating at 26-30 °C for 48-72 h. Then, the plates were examined by stereomicroscope and in case of the presence of larvae or their tracks the identification of S. stercoralis and differentiation from other possible nematodes were performed as explained by Moghaddassani et al. (14). The other most encountering nematodes were Trichostrongylus spp. and Rhabditis spp.
Parasitic load of S. stercoralis infected patients were categorized in three ranks: -Low infection: direct smear and FECT negative, 1-4 larvae counted on agar plate surface; -Moderate infection: direct smear negative, FECT positive, 5-10 larvae counted on agar plate surface; -High infection: direct smear and FECT positive, more than 10 larvae counted on agar plate surface. Among third group, those cases with enormous number of larvae in their stool examinations (Fig.1), and presence of severe pulmonary symptoms were also recorded as hyperinfection.
Fig. 1.

Presence of numerous Strogyloides stercoralis larvae in microscopic surface view of agar plate culture of a patient with hyperinfection syndrome (Original)
Data analysis
A checklist was prepared and patients demographic information (including sex, age, place of birth, current place of residency, travel history, clinical manifestations (gastrointestinal, skin and pulmonary symptoms), relative eosinophil and risk factors were entered. Statistical analysis was performed by SPSS software (version 18). Data was analyzed by Chi-square test (X2) or Fisher’s exact test. T-test was used to compare average of eosinophil counts in asymptomatic and symptomatic patients. Mann-Whitny U test was used to compare parasitic loads in asymptomatic and symptomatic patients. Pearson coefficient of correlation was computed between age and eosinophil counts.
Results
Overall, 70 parasitologically positive indigenous cases of S. stercoralis were subjected in this descriptive analytical study. Twenty eight patients were referred to the School of Medicine (GUMS), during 2010-2013; all of them being both native and resident of Gilan Province. The other 42 patients were referred to the School of Public Health (TUMS), during 2009-2013. The distribution of these people according to their place of birth is illustrated in Fig 2. Not all these people were still residents of their native provinces, but rather 16 individuals (38.1%) being immigrants to other provinces, majority (14 individuals) to Tehran.
Fig. 2.
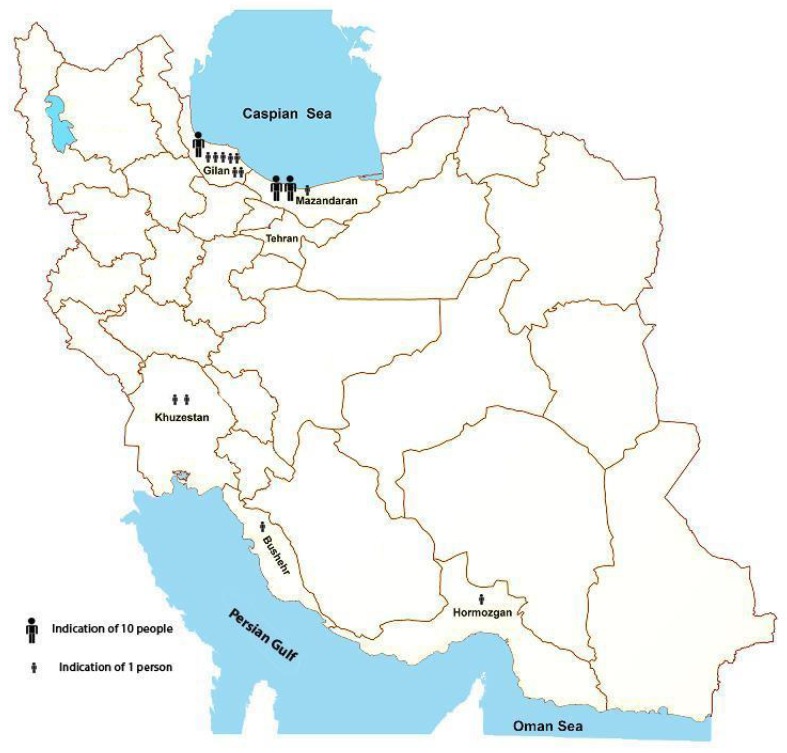
Map of Iran showing native Province of strongyloidiasis patients (n = 42) referring to the School of Public Health, Tehran University of Medical Sciences during 2009-2013
Table 1 Shows distribution of the patients based on the sex, age groups, and clinical manifestations. Among all 70 patients described in this study, 43 people (61.4%) were male and 27 people (38.6%) female. Clinical symptoms were not found significant between male and female patients. The age of the patients varied from 29 years to 83 years of old, with the mean of 60.4 (±SD) years. A total of 11 patients (15.7%) were asymptomatic; rest of them (59; 84.3%) reported manifestation. The most complain was gastrointestinal symptoms, so that 50 patients (71.4%) reported epigastria pain, abdominal discomfort, borborygmus, intermittent diarrhea and constipation, bloating, and nausea. Although 18 patients (25.7%) complained of cutaneous symptoms including itching and rash, but no larva currens eruption was recorded, even in cases of overwhelming infections. Pulmonary symptoms including dyspnea, cough and sputum discharge were recorded in 11 patients (15.7%).
Table 1.
Distribution of the strongyloidiasis patients according to the sex, age groups and clinical manifestations
| Men (n = 43) | Women (n = 27) | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age groups | Gastrointestinal symptoms No. (%) | Pulmonary symptoms No. (%) | Cutaneous symptoms No. (%) | Fever No. (%) | Age groups | Gastrointestinal symptoms No. (%) | Pulmonary symptoms No. (%) | Cutaneous symptoms No. (%) | Fever No. (%) | No. (%) |
| 40≤ | 1 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 40≤ | 2 (9.0) | 0 (0.0) | 1 (14.2) | 1 (33.3) | 6 (8.6) |
| 41-60 | 11 (39.2) | 1 (25.0) | 6 (54.5) | 1 (25.0) | 41-60 | 8 (36.3) | 4 (57.1) | 2 (28.5) | 1 (33.3) | 26 (37.1) |
| 61≥ | 16 (57.1) | 3 (75.0) | 5 (45.4) | 3 (75.0) | 61≥ | 12 (54.5) | 3 (42.8) | 4 (57.1) | 1 (33.3) | 38 (54.3) |
| Total | 28 (65.1) | 4 (9.3) | 11 (25.5) | 4 (9.3) | total | 22 (81.4) | 7 (25.9) | 7 (25.9) | 3 (11.1) | 70 (100) |
Determination of relative eosinophil count could be possible for 52 patients; of them 51 patients (98.1%) showed peripheral eosinophilia, ranging from 6% to 66%, with the mean of 22.7%. The patient without elevated eosinophils was a 63 old man with gastrointestinal disorders, suffering from colitis whose parasite load was high. The mean of relative eosinophilia among asymptomatic and symptomatic patients were 36.28 and 20.63, respectively; and this difference was significant (P = 0.001). There was statistically significant correlation between age and eosinophilia, so that its rate reduced with ageing (r = -324, P = 0.019).
In 22 patients (31.4%) one or more predisposing conditions were known including diabetes (7, 10%), chronic liver diseases (7, 10%), chronic renal failure (5, 7.1%), cancer (3, 4.3%), heart valve replacement (2, 2.8%), rheumatoid (2, 2.8%), colitis (1, 1.4%), bone marrow transplant (1, 1.4%), and liver transplant (1, 1.4%).
Respect to the parasitic load, 22.9%, 31.4%, and 45.7% of patients (n = 70) had low, moderate and high infection rate, respectively. Five patients were hospitalized, among them a 64 years old lady with pulmonary distress and heart failure was recorded having disseminated infection. She improved after taking ivermectine, and her subsequent examinations for strongyloidiasis, over 13 month after medication were negative. Among patients with high infection rate, 8 cases (11.4%) showed hyperinfection syndrome (6 male, 2 female), as well. One of them recorded as disseminated hyperinfection. This patient was 60 years old male with hematologic malignancy, being under prolonged corticosteroid therapy. These conditions led to death of the patient. His infectivity with S. stercoralis was not noticed till the last day of his life. Presence of clinical manifestations had positive association both with parasitic loads (P = 0.007; Mann-Whitny U test), and immunocompromised conditions (P = 0.015; Fisher’s exact test). No significant difference was found between parasitic load either with gender or age groups.
Discussion
S. stercoralis is considered as the most neglected among soil-transmitted helminth infections (15). In the reports pertaining to previous decades, in temperate and subtropical areas of Iran it was coexisted with hookworms, with lower prevalences (16). With the sharp decrease in prevalences of most soil-transmitted helminthes during recent two decades in Iran (17), contrary to the past strongyloidiasis is now more prevalent than hookworms. The actual prevalence of S. stercoralis in the country is unclear but the recent rare records (4, 8, 9) indicate that strongyloidiasis is still prevalent in the same previous known endemic areas, but with higher prevalences than hookworms. This issue is due to the ability of S. stercoralis for the establishment of autoinfection cycle in infected individuals for several decades, even whole life. For this reason S. stercoralis has age cumulative distribution, and is more prevalent in older population. Complicated cases of strongyloidiasis, as a consequence of immunocompromised conditions, have also been reported recently (10-13). Additionally, increase of corticosteroid and other immunosuppressive therapy and predisposing risk factors has led to frequent reference of suspected people to referral centers for differential diagnosis. Therefore, this descriptive analytical study deals with the largest series of indigenous Iranian strongyloidiasis patients. Considering the original provinces of the patients (n = 70) in descending order of magnitude, Gilan, Mazandaran, Khuzestan, Boushehr, and Hormozgan Provinces comprised 64.3%, 30%, 2.9%, 1.4%, and 1.4% of patients, correspondingly. The first two provinces are bordering to Caspian Sea and the others to Persian Gulf; therefore, suitable moist environment, especially heavy rainfall and temperate areas of the north, is in favor of S. stercoralis lifecycle.
Higher infection of males than females (61.4% v.s. 38.6%) which is compatible with other studies (18) is indication of greater exposure of male gender with source of infection as a result of working in farm, rice and tea field, outdoors activities, gardening and so on.
The two most initiation reasons to refer the patients for diagnosis of strongyloidiasis to the above mentioned referral centers were peripheral eosinophilia and gastrointestinal symptoms. Similar results have been stated in other studies (3, 19). All the cases in the present study were selective and referred by themselves or by their physicians because of eosinophilia, clinical symptoms or predisposing factors. Although 25.7% of patients complained of cutaneous symptoms, but similar to other studies in northern Italy (20), imported cases to Spain (3), and hyperinfection cases in Iran (10-12), none of them had larva currens eruption, even cases of overwhelming or disseminated infections. But, in a study on imported strongyloidiasis, it has been found mostly in white patients who acquired their infection in Southeast Asia (21). Absence of this characteristics sign has negative effect on diagnosis of S. stercoralis. Future comparative molecular studies using isolates from patients with and without larva currens and from different geographical areas of the world will clarify the probable effect of different strains on this cutaneous symptom.
Based on the parasitic load, patients could be divided in three groups with low, moderate and high infection rate. Statistically significant difference was found between presence of clinical manifestations and parasitic load (P = 0.007; Mann-Whitny U test). Greater clinical manifestations were coincident with increasing in parasitic load, therefore, leading to easier detection of infections.
Peripheral eosinophilia usually occurs in allergic disease and parasitic helminth infections (22). It could be considered as an important laboratory finding in patients with strongyloidiasis (4, 23-25). Different studies reported various rates of peripheral eosinophilia for strongyloidiasis patients including 22.5% (3), 63.6% (19), 82.6% (21) and 90% (25).
In this study mean of relative eosinophilia was 22.7%, but the trends changed according to the clinical manifestations and age of the patients. The mean of relative eosinophilia was statistically higher in asymptomatic patients (36.3%) than that of symptomatic (20.3%) (P = 0.001). Moreover, rate of eosinophilia reduced with the increase in age (r = -324, P = 0.019). Although eosinophilia is a marked indication of S. stercoralis infection but clinicians should bear in mind that in areas with endemicity of other diseases associated with eosinophilia, like fascioliasis, hydatidosis, toxocariasis, trichinellosis, the presence of this clue should be accompanied with the outcomes of other measures in order to discriminate etiological agents. Immunosuppressive conditions were recorded for 31.4% of patients (22 individuals) in the current study. These risk factor conditions included of diabetes, chronic liver diseases, chronic renal failure, cancer, heart valve replacement, rheumatoid, colitis, bone marrow transplant, and liver transplant. Among these immunosuppressed patients, 5 cases were hospitalized, the fate of one of them, who had hematological malignancy, ended with death; making the mortality rate of 20% for strongyloidiasis associated hospitalized patients. This rate in a study on immigrant population estimated to be 16.7% (26). In another study, half of the cancer patients with strongyloidiasis had solid organ malignancy and the remaining had hematological malignancy (27). The previously reported fatal case of strongyloidiasis in Iran had chronic lymphocytic leukemia (11). Therefore, chemotherapy, especially steroid therapy, in such patients with background diseases is life threatening.
Considering that all the patients analyzed in this study, as well as previous reported strongyloidiasis hyperinfection cases in Iran (10, 11, 13) are originated from endemic areas of north and south provinces of the country, the necessity for early diagnosis and proper treatment before initiation of suppressive therapy in peoples with history of residency or travel to these areas is highlighted. Awareness of physicians about predisposing factors and related risks is urgently needed. Establishment of referral diagnostic centers for strongyloidiasis in all endemic provinces of the country is recommended. Population based studies to assess true prevalence in each area, finding infected individuals and treatment and following up is necessary.
Conclusion
Strongyloidiasis may cause life threatening infection. In Iran it is mostly in patients originating from endemic provinces of the country. Detection of infection in asymptomatic patients requires multiple sampling, utilizing sensitive diagnostic techniques and eosinophil counts.
Acknowledgments
The authors would like to thank all people who had contribution in this study, especially Ms Zahra Heidari, Mrs. Zahra Sayyad Talaie and Mrs. Mojgan Aryaeipour from the SPH-TUMS for their kind assistance. This research was partially supported by the Tehran University of Medical Sciences; Project No. 91-01-160-17294. The authors declare that there is no conflict of interests.
References
- 1.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17(1):208–17. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norsarwany M, Abdelrahman Z, Rahmah N, Ariffin N, Norsyahida A, Madihah B, Zeehaida M. Symptomatic chronic strongyloidiasis in children following treatment for solid organ malignancies: Case reports and literature review. Trop Biomed. 2012;29(3):479–88. [PubMed] [Google Scholar]
- 3.Gonzalez A, Gallo M, Valls ME, Munoz J, Puyol L, Pinazo MJ, Mas J, Gascon J. Clinical and epidemiological features of 33 imported Strongyloides stercoralis infections. Trans R Soc Trop Med Hyg. 2010;104(9):613–6. doi: 10.1016/j.trstmh.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Ashrafi K, Tahbaz A, Rahmati B. Strongyloides stercoralis: The most prevalent parasitic cause of eosinophilia in Gilan Province, northern Iran. Iran J Parasitol. 2010;5(3):40–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Marcos LA, Terashima A, Canales M, Gotuzzo E. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Re. 2011;13(1):35–46. doi: 10.1007/s11908-010-0150-z. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040–7. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 7.Igra-Siegman Y, Kapila R, Sen P, Kaminski ZC, Louria DB. Syndrome of hyperinfection with Strongyloides stercoralis. Rev Infect Dis. 1981;3(3):397–407. doi: 10.1093/clinids/3.3.397. [DOI] [PubMed] [Google Scholar]
- 8.Kia EB, Mahmoudi M, Zahabiun F, Meamar AR. An evaluation on the efficacy of agar plate culture for detection of Strongyloides stercoralis. Iran J Parasitol. 2007;2(1):29–34. [Google Scholar]
- 9.Shokri A, Sarasiabi KS, Teshnizi SH, Mahmoodi H. Prevalence of Strongyloides stercoralis and other intestinal parasitic infections among mentally retarded residents in central institution of southern Iran. Asian Pac J Trop Biomed. 2012;2(2):88–91. doi: 10.1016/S2221-1691(11)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesheli HM, Moghaddam TG, Zahedpasha Y, Norouzi AR. Acute lymphoblastic leukemia with eosinophilia and Strongyloides stercoralis hyperinfection. Iran J Pediatr. 2011;21(4):549–52. [PMC free article] [PubMed] [Google Scholar]
- 11.Kia EB, Rahimi HR, Mirhendi H, Nilforoushan MR, Talebi A, Zahabiun F, Kazemzadeh H, Meamar AR. A case of fatal strongyloidiasis in a patient with chronic lymphocytic leukemia and molecular characterization of the isolate. Korean J Parasitol. 2008;46(4):261–3. doi: 10.3347/kjp.2008.46.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meamar AR, Rezaian M, Mohraz M, Hadighi R, Kia EB. Strongyloides stercoralis hyper-infection syndrome in HIV+/AIDS patients in Iran. Parasitol Res. 2007;101(3):663–5. doi: 10.1007/s00436-007-0531-x. [DOI] [PubMed] [Google Scholar]
- 13.Tabei SZ, Asadian F, Fakhar M, Safaei A. Gastrointestinal hyper infection due to Strongyloides stercoralis in a patient with behcet’s syndrome. Comp Clin Pathol. 2009;18(1):89–91. [Google Scholar]
- 14.Moghaddassani H, Mirhendi H, Hosseini M, Rokni M, Mowlavi G, Kia E. Molecular diagnosis of Strongyloides stercoralis infection by PCR detection of specific DNA in human stool samples. Iran J Parasitol. 2011;6(2):23–30. [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P. Strongyloidiasis-the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967–72. doi: 10.1016/j.trstmh.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Arfaa F. Medical helminthology. 7. Tehran: Khosravi Press; 2010. [Google Scholar]
- 17.Rokni MB. The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol. 2008;102(4):283–95. doi: 10.1179/136485908X300805. [DOI] [PubMed] [Google Scholar]
- 18.Chordia P, Christopher S, Abraham OC, Muliyil J, Kang G, Ajjampur S. Risk factors for acquiring Strongyloides stercoralis infection among patients attending a tertiary hospital in south India. Indian J Med Microbiol. 2011;29(2):147–51. doi: 10.4103/0255-0857.81797. [DOI] [PubMed] [Google Scholar]
- 19.Valerio L, Roure S, Fernandez-Rivas G, Basile L, Martinez-Cuevas O, Ballesteros AL, Ramos X, Sabria M. Strongyloides stercoralis, the hidden worm. Epidemiological and clinical characteris-tics of 70 cases diagnosed in the north metropolitan area of Barcelona, Spain, 2003-2012. Trans R Soc Trop Med Hyg. 2013;107(8):465–70. doi: 10.1093/trstmh/trt053. [DOI] [PubMed] [Google Scholar]
- 20.Genta RM, Gatti S, Linke MJ, Cevini C, Scaglia M. Endemic strongyloidiasis in northern Italy: Clinical and immunological aspects. Q J Med. 1988;68(257):679–90. [PubMed] [Google Scholar]
- 21.Nuesch R, Zimmerli L, Stockli R, Gyr N, Christoph Hatz FR. Imported strongyloidosis: A longitudinal analysis of 31 cases. J Travel Med. 2005;12(2):80–4. doi: 10.2310/7060.2005.12204. [DOI] [PubMed] [Google Scholar]
- 22.Thomas B, Nutman MD. Evaluation and differential diagnosis of marked, persistent eosinophilia. Immunol Allergy Clin North Am. 2007;27(3):529–49. doi: 10.1016/j.iac.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill GV, Welch E, Bailey JW, Bell DR, Beeching NJ. Chronic Strongyloides stercoralis infection in former British far east prisoners of war. QJM. 2004;97(12):789–95. doi: 10.1093/qjmed/hch133. [DOI] [PubMed] [Google Scholar]
- 24.Roman-Sanchez P, Pastor-Guzman A, Moreno-Guillen S, Igual-Adell R, Suner-Generoso S, Tornero-Estebanez C. High prevalence of Strongyloides stercoralis among farm workers on the mediterranean coast of Spain: Analysis of the predictive factors of infection in developed countries. Am J Trop Med Hyg. 2003;69(3):336–40. [PubMed] [Google Scholar]
- 25.Loutfy MR, Wilson M, Keystone JS, Kain KC. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66(6):749–52. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]
- 26.Muennig P, Pallin D, Sell RL, Chan MS. The cost effectiveness of strategies for the treatment of intestinal parasites in immigrants. N Engl J Med. 1999;340(10):773–9. doi: 10.1056/NEJM199903113401006. [DOI] [PubMed] [Google Scholar]
- 27.Safdar A, Malathum K, Rodriguez SJ, Husni R, Rolston KV. Strongyloidiasis in patients at a comprehensive cancer center in the United States. Cancer. 2004;100(7):1531–6. doi: 10.1002/cncr.20120. [DOI] [PubMed] [Google Scholar]


