Abstract
CorA is a primary Mg2+ transporter for Bacteria and Archaea. The C-terminal domain of ∼80 amino acids forms three transmembrane (TM) segments, which suggests that CorA is a homo-oligomer. A Cys residue was added to the cytoplasmic C terminus (C317) of Salmonella enterica serovar Typhimurium CorA with or without mutation of the single periplasmic Cys191 to Ser; each mutant retained function. Oxidation of the Cys191Ser Cys317 CorA gave a dimer. Oxidation of Cys317 CorA showed a dimer plus an additional band, apparently cross-linked via both Cys317 and C191. To determine oligomer order, intact cells or purified membranes were treated with formaldehyde or carbon disulfide. Higher-molecular-mass bands formed, consistent with the presence of a tetramer. Cross-linking of the Bacillus subtilis CorA expressed in Salmonella serovar Typhimurium similarly indicated a tetramer. CorA periplasmic soluble domains from both Salmonella serovar Typhimurium and the archaeon Methanococcus jannaschii were purified and shown to retain structure. Formaldehyde treatment showed formation of a tetramer. Finally, previous mutagenesis of the CorA membrane domain identified six intramembrane residues forming an apparent pore that interacts with Mg2+ during transport. Each was mutated to Cys. In mutants carrying a single intramembrane Cys residue, spontaneous disulfide bond formation that was enhanced by oxidation with Cu(II)-1,10-phenanthroline was observed between monomers, indicating that these Mg2+-interacting residues within the membrane are very close to their cognate residue on another monomer. Thus, CorA appears to be a homotetramer with a TM segment of one monomer physically close to the same TM segment of another monomer.
Salmonella enterica serovar Typhimurium has three transport systems mediating influx of Mg2+: CorA, MgtA, and MgtB (6, 7, 22, 24). The CorA system is encoded by the corA gene that constitutively expresses a 37-kDa integral membrane protein (19). The MgtA and MgtB Mg2+ uptake systems are only expressed significantly under conditions of Mg2+ deprivation (4, 25, 26, 31). Phylogenetic studies have shown that CorA homologs are widespread and form the principal Mg2+ uptake system of most Bacteria and Archaea (9, 19, 22).
The secondary structure of CorA is unusual, with a relatively large N-terminal periplasmic domain (CorA-PPD) of about 235 amino acids followed by a compact C-terminal domain of 80 amino acids forming three transmembrane (TM) segments (19). Since three TM domains of a single polypeptide seem too few to form a channel or pore through the membrane, the functional CorA transporter is likely to be an oligomer. Further, since no other protein is apparently required for Mg2+ transport via CorA (6, 7, 22), CorA is presumably a homo-oligomer. To determine the order of the presumed homo-oligomer, chemical cross-linking of CorA in intact cells and membranes and of purified CorA-PPD coupled with site-directed mutagenesis were used. The data indicate that CorA is a homotetramer. Further, within the membrane, a TM segment of one monomer is physically close to its cognate TM in another monomer.
MATERIALS AND METHODS
All media were obtained from Difco (Detroit, Mich.). All other reagents were from Sigma unless otherwise specified. Oligonucleotides were purchased from Oligos, Etc. (Wilsonville, Oreg.) or Genosys (The Woodlands, Tex.). All cells were grown in Luria-Bertani (LB) broth with 0.4% glucose. MgSO4 was used for supplementation of media with Mg2+. Buffers A, B, and C consisted of 20 mM Tris-Cl pH 7, 500 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 0.1% Tween 20, with 1, 40, or 80 mM imidazole-HCl, respectively. The buffers were purged with N2 before use to minimize oxidation catalyzed by the Ni2+-nitrilotriacetic acid (NTA) column.
Cloning of the Bacillus subtilis corA.
The full-length B. subtilis corA gene was cloned from genomic DNA using as forward primer 5′-ACTGGATCCAAGGAGATATACCAATGATCAACATTACCGCAATCAC, which has a BamHI and a ribosomal binding site at the 5′ end, and as reverse primer 5′-ACTGAATTCTTTAAAAATATTGAACCATCCTTTATGTACAAACC, containing an EcoRI site at the 5′ end for cloning into pUC19 to determine complementation. A reverse primer of 5′-ACTCTCGAGTTTAATTCAGATCCTCTTCTGAGATGAGTTTTTGTTCTTTAAAAATATTGAACCATCCTTTATG was used for cloning into expression vector pET-24(+) and contains a XhoI site at the 5′ end and a sequence placing a c-myc epitope at the C terminus. The insert was fully sequenced to ensure the absence of sequence errors. MM281 is a Salmonella serovar Typhimurium strain in which all three endogenous Mg2+ transporters have been deleted. It requires 10 to 100 mM Mg2+ for growth (6, 24). Expression of the c-myc-tagged B. subtilis corA electroporated into MM281 (MM2902) allowed growth without supplemental Mg2+, thus demonstrating that the cloned gene was a functional Mg2+ transporter.
Expression and purification of CorA-PPDs.
The coding regions for Salmonella serovar Typhimurium CorA-PPD (19) and Methanococcus jannaschii CorA-PPD (20) were amplified by PCR with Pfu DNA polymerase (Stratagene, La Jolla, Calif.). The Salmonella serovar Typhimurium CorA-PPD consisted of residues 1 to 231. The forward and reverse primers were 5′-ACTGGCATGCTGAGCGCATTTCAACTGGAAAA-3′ and 5′-ACTAGATCTATCCTTATCGTCATCGTCTTCATTGTGCGGCAGCAGAG-3′, respectively. The M. jannaschii CorA-PPD consisted of residues 1 to 229. The forward and reverse primers to amplify the M. jannaschii CorA-PPD were 5′-ACTGCCATGGTTACGGTAATTGCTATAGCTAAAG- and 5′-ACTAGATCTATCCTTATCGTCATCGTCGTACAGATCAATTAACTGTAAAGTGTCGTAGT-3′. To facilitate cloning, a SphI restriction site and a NcoI restriction site were introduced at the 5′ end of both forward primers, respectively, while a BglII restriction site was introduced at the 5′ ends of the reverse primers. Reverse primers also contained a stretch of sequence encoding an enterokinase cleavage recognition sequence. The resulting PCR products were ligated into pQE-60 (M. jannaschii CorA-PPD) or pQE-70 (Salmonella serovar Typhimurium CorA-PPD) expression vectors (QIAGEN, Valencia, Calif.) and transformed into Escherichia coli DH5α IQ cells (Gibco BRL, Rockville, Md.) to create strains MM2641 and MM2640, respectively. Plasmid inserts were completely sequenced to verify the fidelity of the PCR amplification.
To purify the CorA-PPD fusion proteins, cells were grown in 1 liter of LB broth to an optical density at 600 nm (OD600) of 0.6 to 1. Cultures were induced with 1 mM isopropyl-β-d-thiogalactopyranoside for 4 h at 37°C and harvested by centrifugation. With overexpression of the CorA-PPD, more than 90% of the protein was sequestered in inclusion bodies such that the amount of Tween 20 in buffer A was insufficient for solubilization. The pellets were therefore resuspended in 40 ml of buffer A containing 3% (wt/vol) Empigen (n-dodecyl-N,N-dimethylglycine; Calbiochem, San Diego, Calif.) instead of Tween 20. This resuspension buffer also contained 2 mg of lysozyme/ml, 100 μg of DNase I/ml, and 100 μg of RNase/ml. Empigen solubilized virtually all CorA-PPD present in the cells and consequently gave much higher yields than samples not so treated. However, circular dichroism spectra and cross-linking patterns of CorA-PPD were identical whether or not Empigen was used (data not shown). Resuspended cells were incubated on ice for 30 min, complete lysis was ensured by passage through a French press three times at 8,000 lb/in2, and cell lysates were centrifuged at 17,000 × g at 4°C for 30 min. The supernatants were loaded on 3- by 5-cm columns of Ni2+-NTA agarose that had been charged with NiCl2 and equilibrated with buffer A with 3% (wt/vol) Empigen. The Ni2+-NTA agarose column was sequentially washed with buffer containing 2%, 1%, and no Empigen to remove detergent before elution with imidazole using 100 ml of buffer B followed by 100 ml of buffer C. Ten-milliliter fractions from the buffer C elution were collected; 20 μl of each fraction was removed and analyzed by sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE). Peak fractions were pooled, and the His tag was removed with enterokinase according the manufacturer's directions (Invitrogen, Carlsbad, Calif.). Typical yields were 1 mg/liter for Salmonella serovar Typhimurium CorA-PPD and >3 mg/liter for M. jannaschii CorA-PPD.
Cys mutants.
Cys substitution mutations within TM2 and TM3 of Salmonella serovar Typhimurium CorA have been previously described (23, 30). Cys mutants in intramembrane residues generally retained some transport capacity, and all appeared to insert properly in the membrane (30). To create additional cysteine mutants, pMAS29 (30) carrying a functional Salmonella serovar Typhimurium corA gene was mutagenized using a QuikChange mutagenesis system (Stratagene). All mutations were verified by sequencing the entire coding region of the membrane domain. Initial mutagenesis was performed in E. coli DH5α IQ. The plasmid was passaged through Salmonella serovar Typhimurium JR501 for modification and then transformed into the Salmonella serovar Typhimurium MM281 strain to determine functionality. Insertion of a Cys at the C terminus (Cys317 CorA) gave Salmonella serovar Typhimurium strain MM1322, mutation of the single Cys residue in CorA to Ser gave Salmonella serovar Typhimurium strain MM1318 (Cys191Ser CorA), and mutation of both residues gave Salmonella serovar Typhimurium strain MM1324 (Cys191Ser Cys317 CorA).
Membrane sample preparation.
Single colonies of the appropriate strains were used to inoculate 25 ml of LB broth containing appropriate antibiotics (30) and 100 mM MgSO4 and incubated by shaking overnight. The cells were pelleted, resuspended in 1 ml of ice-cold 10 mM Tris-Cl and 150 mM NaCl, pH 7.5, and lysed by two passages through a French press at 12,000 lb/in2. Cellular debris were removed by centrifugation at 16,000 × g for 15 min. Membranes were pelleted by centrifugation at 100,000 × g for 1 h, resuspended on ice in 0.5 ml of the same buffer, and dispersed by repeated pipetting until no clumps were visible. Protein concentration was determined using a Pierce bicinchoninic acid assay (Rockford, Ill.) with bovine serum albumin as the standard. Protein samples were precipitated with an equal volume of ice-cold 20% (wt/vol) trichloroacetic acid and washed once with ice-cold 70% ethanol before addition of bicinchoninic acid reagent to avoid lipid interference.
Sodium tetrathionate and Cu(II)-1,10-phenanthroline oxidation.
For cross-linking with sodium tetrathionate, aliquots of a freshly made 100 mM stock solution were added to membrane fractions of each strain to final concentrations of 5 μM up to 10 mM. Samples were incubated for 30 to 60 min at ambient temperature. Reactions were stopped by precipitation with an equal volume of 20% (wt/vol) trichloroacetic acid and incubated on ice for 20 min. Cross-linking with Cu(II)-1,10-phenanthroline was done according to the method of Falke and Koshland (3, 15), by incubating membrane preparations of each strain with final concentrations of 1.5, 0.75, or 0.15 mM cross-linker for 1 h. Reactions were stopped by the addition of SDS-PAGE loading buffer. Proteins were analyzed by SDS-PAGE and Western blotting. Approximately 10 or 50 μg of total protein was added for minigels or 20-cm gels, respectively.
Formaldehyde cross-linking.
The protocol for formaldehyde cross-linking was adapted from protocols of Prossnitz et al. and Skare et al. (16, 18). Approximately 15 ml of E. coli DH5α IQ, Salmonella serovar Typhimurium LT2 (MM1442), MM1947 (MM281/pMAS29), or the cysteine mutants (MM1318, MM1322, MM1324) or other strains as noted were grown to an OD600 of 0.5, pelleted, and resuspended in 100 mM sodium phosphate, pH 6.8, to an OD600 of 0.5. For each strain, 12-ml aliquots of cells were incubated with 324 μl of 37% formaldehyde (1% final concentration). After various times of incubation, 4-ml cells were pelleted and washed with the same buffer. The pellet was resuspended in SDS-PAGE buffer and heated to 60°C prior to gel loading for electrophoresis. Cells with an OD600 of approximately 1 were loaded onto a 20-cm SDS-10% PAGE gel.
Western blot analysis.
Proteins were electrophoresed on 10% polyacrylamide gels and transferred onto nitrocellulose (Schleicher & Schuell, Keene, N.H.). An antibody to the N-terminal 16 amino acids of Salmonella serovar Typhimurium CorA (23) was used at a dilution of 1:10,000 to 1:20,000. Donkey anti-rabbit horseradish peroxidase-linked secondary antibody (Amersham) was used at 1:10,000 dilution. Proteins were visualized by enhanced chemiluminescence (Amersham). The resulting films were scanned into Canvas version 8 or 9 (ACD Systems of America, Miami, Fla.). Other than cropping, no alterations were made except some adjustment of brightness and contrast.
Circular dichroism.
Spectra were collected on a Jasco 600 spectropolarimeter. The pathlength was usually 0.1 cm, and spectra were collected at several protein concentrations between 0.1 and 5 mg/ml. Ellipticity was measured at 1-nm intervals. Deconvolution of the spectrum was performed with multiple computer algorithms (17), all of which indicated an α-helical content of >70% with no detectable β-sheet component. This is in agreement with computer analysis of 68% α-helix by the Chou-Fasman (2) and 85% α-helix by the Garnier-Robson (5) algorithms.
RESULTS
Formation of CorA dimers by cysteine cross-linking.
The location of the C terminus of CorA within the cytoplasm is well defined. In most homologs, it consists of only six amino acids, the first three of which are invariably Arg or Lys. These positive charges serve to anchor TM3 in the membrane and at the same time define which residues are within the lipid bilayer and which are in the cytosol. We reasoned that in a homo-oligomer of CorA, the C-terminal segment of one monomer might be sufficiently close to that of other monomers to interact despite possible charge repulsion from the anchoring Arg and Lys residues. Salmonella serovar Typhimurium CorA contains a single Cys residue at position 191 which was mutated to Ser (Cys191Ser CorA), with retention of about 55% of wild-type transport activity and no change in apparent Mg2+ or Ni2+ affinity (data not shown). Addition of a Cys residue to the C terminus (Cys317 CorA) or the construction of a Cys191Ser Cys317 CorA also had no effect on apparent cation affinity and gave 85 and 25% of wild-type transport activity, respectively. All mutants were present in the cell membrane at normal abundance and migrated identically to wild type (reference 30 and data not shown).
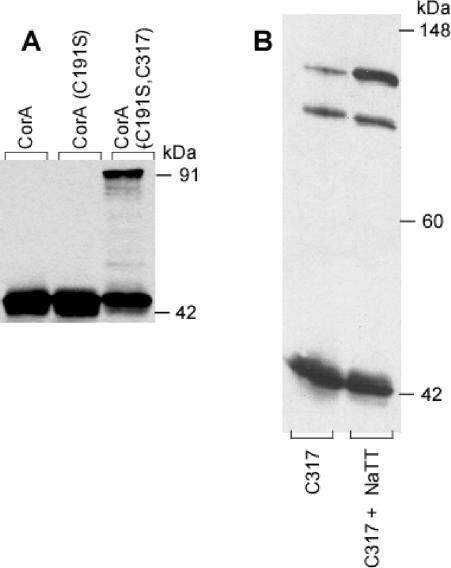
Sodium tetrathionate treatment of membrane vesicles was used to oxidize Cys residues in CorA. The CorA monomer from Salmonella serovar Typhimurium has an expected molecular mass of 37 kDa but migrates on both reducing or nonreducing SDS-PAGE gels at about 42 kDa. The Cys191Ser CorA did not exhibit cross-linking with or without tetrathionate, showing only a monomer band at about 42 to 44 kDa (Fig. 1A). Wild-type CorA also did not exhibit significant cross-linking (Fig. 1A), although an occasional minor band at an apparent dimer position was evident on nonreducing gels (data not shown). In contrast, the Cys191Ser Cys317 CorA containing only a C-terminal Cys residue showed a second band at 91 kDa in the presence of tetrathionate, consistent with formation of a dimer. Cross-linking was significant but not complete in any sample. The C317 CorA, containing cysteines at both Cys191 and Cys317, showed spontaneous formation of the 91-kDa dimer band plus an additional band at 104 kDa (Fig. 1B). Tetrathionate oxidation enhanced formation of the 104-kDa band. Presumably, formation of a disulfide bond between the Cys317 residues of two monomers can force Cys191 residues sufficiently close together to allow formation of a second disulfide bond within the dimer. The alternative possibility, that the 104-kDa band is a trimer formed by Cys191-Cys191 cross-links between two monomers and a Cys317-Cys317 cross-link between one of those monomers and a third monomer, seems unlikely both because of the relatively low molecular mass of the third band and because the Cys191 residues do not appear to interact significantly in the wild-type protein.
FIG. 1.
Addition of a Cys residue at the C terminus of CorA allows dimer formation. Salmonella serovar Typhimurium strains MM1318 (Cys191Ser), MM1322 (Cys317), and MM1324 (Cys191Ser Cys317) and a wild-type strain (MM1442) were grown in LB broth to an OD600 of 0.5 to 1.0, and membrane fractions were incubated with or without 10 mM tetrathionate. The samples were then run on nondenaturing PAGE before Western blotting with anti-CorA antibody and scanning into Canvas. No editing was performed except adjustment of brightness and contrast. Panel A shows the wild-type, the Cys− mutant, and the double mutant strains treated with tetrathionate. Some preparations showed a small amount of the 91-kDa band before tetrathionate oxidation (see panel B); the amount of the 91-kDa species was markedly increased by oxidation with tetrathionate. Panel B shows the formation of a third band at 110 kDa in the Cys 317 mutant containing both a cytoplasmic (Cys317) and a periplasmic (Cys191) cysteine. The band was enhanced by tetrathionate oxidation, in addition to the 91- and 44-kDa bands.
Formaldehyde and carbon disulfide cross-linking of membrane-bound CorA.
To determine whether CorA could form a higher order oligomer, we investigated a variety of cross-linking agents, both hydrophilic and hydrophobic, for cross-linking CorA in intact cells, spheroplasts, and membrane vesicles. Hydrophobic agents included carbon disulfide (CS2), disuccinimidyl suberate, dimethylpimelimidate, and others with different lengths of spacer arms between reactive groups. Relatively hydrophilic cross-linking agents included 3,3′-dithiobis[sulfosuccinimidylpropionate], glutaraldehyde, and formaldehyde among others. Only formaldehyde and CS2 were able to cross-link CorA.
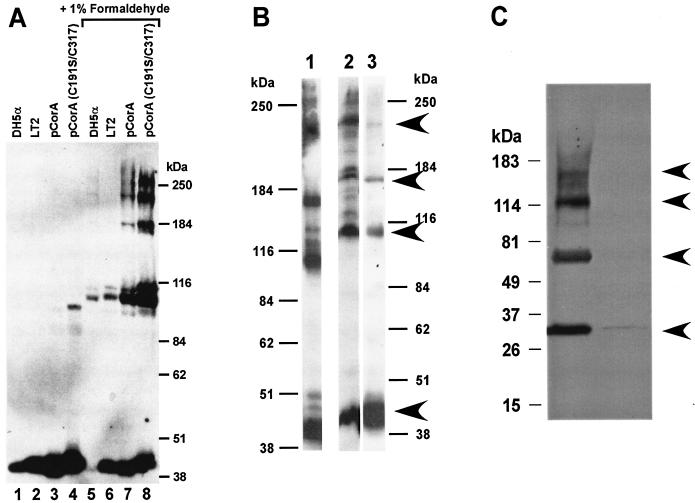
For formaldehyde cross-linking, the Mg2+ transport-deficient strain MM281 (6) was used as a negative control and showed no bands reactive with the anti-CorA antibody under any conditions tested as previously determined (19, 21). E. coli DH5α was tested since the Salmonella serovar Typhimurium antibody cross-reacts well with the highly homologous E. coli CorA. Formaldehyde treatment of E. coli, wild-type Salmonella serovar Typhimurium, or Salmonella serovar Typhimurium strains carrying high-copy plasmids overexpressing wild type or various Cys mutants of CorA resulted in the formation of additional higher-molecular-mass bands detected with the anti-CorA antibody. In both whole cells and membrane preparations, bands consistent with a tetramer were formed at approximately 42, 105 and 110, 180, and 225 kDa (Fig. 2A), consistent with formation of a tetramer. A similar tetrameric pattern of cross-linking was observed in membrane preparations (Fig. 2B, lane 1) or in whole cells expressing CorA from a low-copy-number vector (Fig. 2B, lane 2). In cells overexpressing the C317 CorA, a higher-molecular-mass band at about 260 kDa was also seen (Fig. 2A, lane 8). This band was not always well separated from the stacking gel and did not appear in any strains that were not markedly overexpressing CorA. CS2 treatment of intact cells (Fig. 2B, lane 3) or membranes (data not shown) also suggested a tetramer and did not show any bands of higher mass than the apparent tetramer even in cells overexpressing the C317 CorA. Finally, cross-linking of a c-myc-tagged CorA of the gram-positive B. subtilis expressed in MM281 also gave bands consistent with the presence of a tetramer (Fig. 2C).
FIG. 2.
Formaldehyde cross-linking indicates that CorA is a tetramer. Intact cells of E. coli DH5α, wild-type Salmonella serovar Typhimurium LT2 (MM1442), Salmonella serovar Typhimurium MM281 carrying pMAS29, a high-copy plasmid expressing CorA (MM1927), or Salmonella serovar Typhimurium MM1324, carrying the same plasmid vector expressing the Cys191Ser Cys317 CorA, were grown and treated as described in Materials and Methods. The resulting Western blot was scanned into Adobe Photoshop 5.0 and transferred into Canvas. No editing was done except some alteration of brightness and contrast. Gels from several different experiments were exposed for various times before development. Note that virtually all E. coli CorA was cross-linked by formaldehyde, while in the other three strains formaldehyde converted a large amount of monomer to higher-molecular-weight bands.
The apparent molecular masses of the bands do not correspond exactly to multiples of the CorA monomer. However, this is to be expected for at least two reasons. First, the CorA monomer migrates somewhat anomalously, running at 42 to 43 kDa rather than the expected 36 to 37 kDa, and thus its higher order forms might also be expected to migrate anomalously. Second, cross-linking generates a polypeptide that is not linear, thus altering its migration on gels.
Additional bands of lesser intensity were evident at various molecular masses in some experiments with formaldehyde (Fig. 2A and B), a result that is not unexpected since formaldehyde is highly reactive and would cross-link two adjacent proteins whatever their origin. Some but not all of these bands were eliminated if an affinity-purified CorA antibody was used for Western blotting (data not shown). These bands are generally not evident in CS2 cross-linked samples or in B. subtilis samples visualized with a c-myc antibody (Fig. 2B and C), suggesting that they are nonspecific. In addition, even with short exposures to formaldehyde, a significant amount of protein remained at the interface with the stacking gel. Similar material at the stacking gel interface was not seen in experiments using CS2 to cross-link CorA, probably because bond formation is far slower with CS2 than with formaldehyde (hours versus seconds). It is possible that the presence of very-high-molecular-mass bands could indicate a higher order oligomer (e.g., Fig. 2A, lane 8). However, multiple experiments with different times of exposure or protein and formaldehyde concentrations suggest that these additional minor bands are most likely due to adventitious cross-linking of other proteins to CorA under conditions of relatively high protein and formaldehyde concentrations and/or long exposure times.
The apparent dimer band appeared as a doublet in most formaldehyde cross-linking experiments. This is likely due to some degree of disulfide cross-linking at Cys191 and/or Cys317 in these experiments which in turn allows enhanced formaldehyde cross-linking. Consistent with this interpretation, the 110-kDa band is greatly increased in experiments with the Cys317 CorA expressed either in E. coli (data not shown) or Salmonella serovar Typhimurium (Fig. 2A, lane 8). Electrophoresis of formaldehyde cross-linked cells in the presence of 3 mM dithiothreitol diminished formation of the 110-kDa band although it was never entirely eliminated (data not shown). This is similar to observations with the Tar receptor, where very harsh conditions were necessary to eliminate some disulfide cross-links (13).
Cross-linking of the purified PPD of CorA.
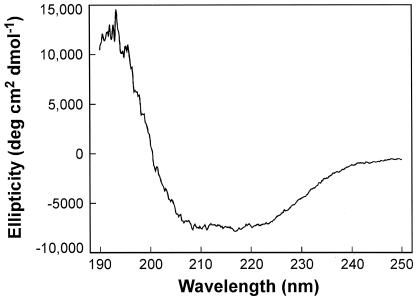
The topology of CorA suggests that it can be treated as a simple two-domain protein, i.e., a soluble N-terminal PPD (CorA-PPD) of about 235 to 240 amino acids and a C-terminal highly hydrophobic membrane domain of about 75 to 80 residues. We reasoned that a truncated CorA consisting only of the CorA-PPD might self-assemble after purification. A C-terminal six-His tag for purification of the CorA-PPD was therefore inserted into the corA genes from both Salmonella serovar Typhimurium and M. jannaschii, after residues 231 and 229, respectively. The archaeal M. jannaschii CorA has previously been shown to express sufficiently well in Salmonella serovar Typhimurium, where it exhibits transport properties virtually identical to those of the Salmonella serovar Typhimurium CorA despite only 12% amino acid identity (20). The constructs also contained an enterokinase cleavage site to allow removal of the His tag. This was necessary since formaldehyde would likely cross-link at the His residues. Although most of the CorA-PPD expressed was found in inclusion bodies, it could be solubilized with Empigen detergent. The CorA-PPD proteins remained soluble after removal of the detergent and apparently folded spontaneously during purification since circular dichroism spectra indicated an α-helical content of >70% (Fig. 3), consistent with predictions from various computer algorithms indicating that the soluble domain is virtually all α-helical in structure (9).
FIG. 3.
Circular dichroism spectrometry shows that purified CorA-PPD retains it structure. The purified Salmonella serovar Typhimurium CorA-PPD was used to obtain a circular dichroic spectrum on a Jasco 600 spectropolarimeter (see Materials and Methods). The negative maximum from about 209 to 220 nm is indicative of α-helical structure. Computer deconvolution of the spectrum indicates that the protein is at least 70% α-helix with no detectable β-sheet structure.
To determine if the purified soluble domain could self-assemble, formaldehyde was used for cross-linking. With both Salmonella serovar Typhimurium (data not shown) and M. jannaschii (Fig. 4) CorA-PPD, higher-molecular-mass bands consistent with cross-linking of a tetramer were seen after formaldehyde treatment. We attempted to enhance oligomer formation by treatment with formaldehyde in the presence of substrate cation (Mg2+) (data not shown) or Co(III)hexaammine (12), a selective inhibitor of CorA (Fig. 4). However, neither Mg2+ nor Co(III)hexaammine had a significant effect on CorA cross-linking. Cross-linking of the Salmonella serovar Typhimurium CorA-PPD was examined at different protein concentrations. Formation of higher order oligomers increased roughly in proportion to the increasing protein concentrations, but there was no evidence of complexes greater in size than the apparent tetramer (data not shown). In addition, boiling of the samples before electrophoresis to break the formaldehyde-induced cross-links broke down the cross-linked complexes and regenerated, as expected, the monomer band (data not shown).
FIG. 4.
Formaldehyde treatment of purified M. jannaschii CorA-PPD indicates a homotetramer. The M. jannaschii CorA-PPD protein was purified and subjected to formaldehyde treatment as described in Materials and Methods and the legend to Fig. 2. The resulting gel was scanned into Canvas. No editing was performed except adjustment of brightness and contrast. Additional experiments at different protein or formaldehyde concentrations or different times of exposure to formaldehyde give similar results. Boiling of the samples before electrophoresis regenerated a significant amount of the monomer band as expected (data not shown). In the experiment shown, formaldehyde treatment was performed in the absence (left lane) or presence (right lane) of the selective CorA inhibitor, Co(III)hexaammine (12).
Cross-linking of Cys residues in TM domains.
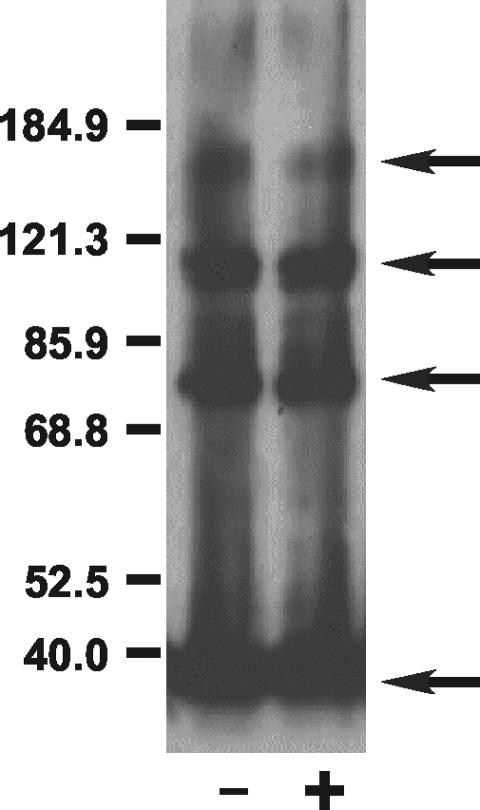
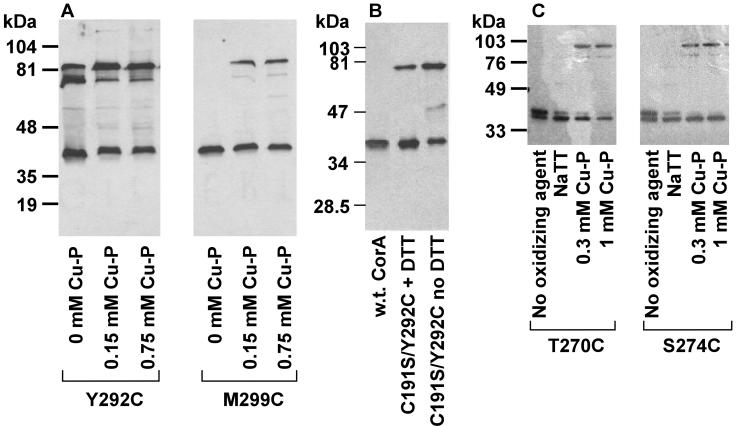
In previous mutagenesis work, several residues within TM2 and TM3 were changed to Cys (23, 30). These included Thr270, Ser274, and Met278 in TM2 and Tyr292 and Met299 in TM3, all of which seem to form part of a Mg2+ permeable “pore” within the membrane (23, 30). During control studies to determine if the mutant CorA proteins were inserted in proper amounts in the membrane, we noticed that strains carrying a Tyr292Cys mutation in TM3 of CorA formed spontaneous cross-links even in the absence of an oxidizing agent (Fig. 5A). Cross-linking was enhanced somewhat by treatment with Cu(II)-1,10-phenanthroline. These results indicated that the Tyr292 residue of one monomer must be very close to a Tyr292 residue in another monomer. Cu(II)-1,10-phenanthroline treatment of strains carrying the Thr270Cys, Ser274Cys, or Met299Cys (Fig. 5B) CorA proteins also induced considerable formation of cross-linked protein, indicating that Thr270, Ser274, and Met299 could all interact with their cognate residues on another monomer. Sodium tetrathionate, which is charged and thus relatively membrane impermeable, did not induce formation of apparent dimers of these CorA proteins carrying Cys residues within the membrane (Fig. 5B).
FIG. 5.
Intramembrane cysteine residues can cross-link to their cognate residue on another monomer. Strains were grown and membranes fractionated when treated with Cu(II)-1,10-phenanthroline as indicated in Materials and Methods. The gel was scanned into Canvas. No editing was performed except adjustment of brightness and contrast. Panel A shows cross-linking of the Tyr292Cys and Met299Cys mutants in TM3 of CorA. The Tyr292Cys CorA mutant cross-links spontaneously, but cross-linking is enhanced by Cu(II)-1,10-phenanthroline treatment. Panel B shows cross-linking of the Cys191Ser Tyr292Cys mutant of CorA demonstrating the absence of the higher-mass band of the dimer doublet, an indication that the doublet results from disulfide bond formation at position 292 only or at both position 292 and at position 191. Panel C shows Cu(II)-1,10-phenanthroline-mediated cross-linking at Tyr270Cys and at Ser274Cys in TM2.
In the Tyr292Cys CorA, a doublet was observed in the apparent dimer position at 75 and 82 kDa (Fig. 5A), possibly due to formation of disulfide bonds between two Tyr292Cys residues coupled with formation of a disulfide bond between periplasmic Cys191 residues. Consistent with this hypothesis, involvement of C191 was confirmed by creating a Cys191Ser Tyr292Cys mutant (MM1898) which formed only a single band of 75 kDa (Fig. 5A). Electrophoresis in the presence of 3 mM dithiothreitol diminished the amount of the 75-kDa band but did not eliminate it, similar to results noted above in formaldehyde cross-linking experiments.
DISCUSSION
The CorA Mg2+ transport system is unusual in many respects (10). It lacks significant homology to other known transport systems. Despite forming the primary Mg2+ transport system of a very large number of Bacteria and Archaea, there are no close homologs in eukaryotes, unlike almost every other class of cation transporter. CorA's topology is unusual among transport systems, with only three TM segments, too few to form a Mg2+ channel or pore as a monomeric protein. CorA also features a large periplasmic soluble domain with no apparent leader sequence. Finally, initial cation binding to CorA, presumably via the periplasmic soluble domain, involves coordination with a fully hydrated Mg2+ cation 5 Å in diameter, far larger than any other biologically important cation (6, 11, 12, 19). The results presented in this report indicate that CorA is probably a tetrameric homo-oligomer in which cognate TM domains are relatively close together.
Existence of a CorA tetramer.
The formation of disulfide bonds between residues on two different monomers of CorA provides conclusive evidence that CorA is an oligomer. Cross-linking of full-length CorA in intact cells or membrane fractions with formaldehyde and CS2 indicated the existence of at least a tetramer from both gram-negative and gram-positive species. CorA oligomers were presumably native since identical patterns of cross-linking were seen in intact cells and in membrane preparations. CorA is not an especially abundant protein and hence would not be in a very high concentration in the membrane. Consequently, cross-links between CorA monomers in intact cells and membranes occurred at low relative protein concentrations and therefore would not have been due to adventitious nonspecific protein association.
Formaldehyde cross-linking of the purified CorA-PPD from a gram-negative and an archaeal species also indicated a tetramer. With the purified CorA-PPD, protein concentrations were necessarily much higher, but formaldehyde treatment with varied protein concentrations, multiple time points, and different formaldehyde concentrations always gave tetrameric bands, a strong indication that the cross-links observed were due to normal association of CorA monomers. Although there is only 12% identity in the soluble domain of the Salmonella serovar Typhimurium bacterial and M. jannaschii archaeal CorA molecules, identical cross-linking of the CorA-PPD from both organisms indicates that a similar high order homo-oligomer forms the basic CorA structure of each organism.
In most experiments with the CorA-PPD proteins, the tetrameric band was relatively weak. This might be explained by a weaker association of the PPDs in the absence of the membrane domain. Potential stabilization of the CorA-PPD complex by addition of Mg2+ or the selective CorA inhibitor, Co(III)hexaammine (12), was without effect on oligomeric state. If initial association of the CorA-PPD monomers were relatively loose, formation of initial formaldehyde cross-links would presumably change conformation of the monomers in the resulting cross-linked complex. The strength of association between this cross-linked protein and the remaining monomers would likely be greatly diminished by the cross-linking, stimulating dissociation of the monomers that were not yet cross-linked. If this argument is correct, it indicates that the membrane domain also has an important role in stabilizing the oligomeric structure of the complex.
We were uniformly unsuccessful with any cross-linking reagents tested except formaldehyde and CS2. This is puzzling, especially with cross-linking agents that react with lysine since CorA contains numerous lysine residues. Although agents with different spacing between reactive groups were tested, the simplest explanation for the lack of lysine reactivity is that there are no sufficiently exposed and reactive lysines spaced at appropriate distances for these reagents. Given the very large number of other charged residues, especially aspartate and glutamate, this could suggest that many lysines are not well exposed or not very reactive, their charge possibly being neutralized by interaction with a negatively charged residue.
In all experiments, a tetrameric band was readily evident with short times of incubation and at low concentrations of formaldehyde. In cells overexpressing CorA, longer exposure to high concentrations of formaldehyde sometimes gave a higher order band consistent with a pentameric species (Fig. 2). This never occurred with CS2-mediated cross-linking or with formaldehyde cross-linking of the CorA-PPD. Thus, while it is possible that native CorA could be a homopentamer (or higher), the most probable interpretation of our data is that CorA from both Eubacteria and Archaea is a homotetramer.
Membrane domain interactions and structure.
Cross-links between Cys residues in the membrane domain (Fig. 5) further indicate that regions of one monomer are sufficiently close to the same region of another monomer to be able to form a disulfide bond. Unlike substitution at Cys191 or addition of Cys317, however, substitution of an intramembrane residue with a Cys had greater effects on function. When assayed at the wild-type K0.5 for the 63Ni2+ substrate, none of the intramembrane Cys substitution mutants exhibited more than 4% of wild-type activity, although all were expressed in amounts equivalent to wild-type CorA. Regardless, the retention of function indicates that the ability of these residues to cross-link within the membrane is not due to an abnormal protein conformation but to their proximity in a normal, essentially wild-type structure.
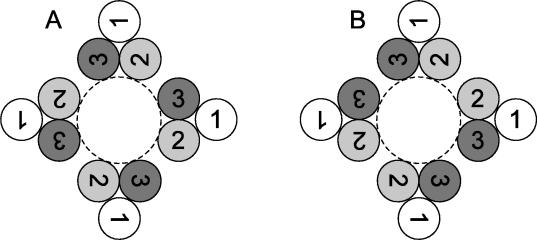
The data indicate that cognate helices of each monomer are sufficiently close to interact. An unambiguous structural interpretation is difficult in the absence of crystallographic information. If the monomers were arranged in a symmetrical pattern (Fig. 6A), cognate residues in the same TM helix of different monomers would not be adjacent to each other, making disulfide bond formation much more difficult and thus rendering a simple symmetrical arrangement unlikely. However, a possible symmetrical arrangement of monomers that would obey constraints imposed by the cross-linking data might be provided by the membrane domain structure of the bacterial mechanosensitive ion channel, MscL (1, 29). The MscL membrane domain consists of five pairs of helices. Each individual helix is tilted both with respect to the other helix of the same monomer and to the pore of the ion channel. Each pair of helices from a given monomer is arranged such that one end of the TM helix lies close to the pore while the other end is tilted away from the pore. The other TM domain of the same monomer is tilted in an opposite manner. In the course of this helix movement as ion flows through the channel, the ends of each helix distant from the pore move in towards the pore while those ends of each helix nearest to the pore move away from it. A recent modeling study (28) of this mechanism of action of MscL is consistent with both structural data (1) and site-directed mutagenesis results (27, 28). A similar arrangement of TM2 and TM3 in a tetrameric CorA is possible with the remaining TM1 segments being peripheral to the pore and would suggest that intramembrane cross-links arise from different conformations of the membrane domain. An alternative arrangement of the membrane helices as a “dimer of dimers” (Fig. 6B) would resolve issues of proximity raised by a strictly symmetrical arrangement. This arrangement is known for several soluble enzymes (8, 32, 34), and studies of the membrane channel EmrE have recently demonstrated such a structure in a membrane ion transporter (14, 33). While the latter EmrE-like model seems more likely based on the multiple intramembrane residues that can form disulfide bonds, MscL-like and EmrE-like structures make distinct predictions about helix and residue proximity that are experimentally testable.
FIG. 6.
Arrangements of TM domains of a tetrameric CorA. (A) A tetrameric CorA is symmetrically arranged around a putative Mg2+ pore with TM2 and TM3 forming the pore. TM1 is apparently peripheral to the core (23, 30). This necessarily places the TM2 of one monomer relatively far away from the TM2 of another monomer, likewise for TM3 domains. Cysteine cross-linking data show that TM3 of one monomer can cross-link with TM3 of another monomer, thus implying that they are very close. Similarly, residues in TM2 can cross-link to their cognate residue in TM2 of another monomer. This suggests that such a simple symmetrical arrangement is unlikely (see the text). (B) If TM domains are arranged as a dimer of dimers, a pseudo-twofold axis of symmetry is generated. This arrangement is known for both soluble and membrane proteins. It could satisfy the structural constraints imposed by intramembrane disulfide bond formation.
Acknowledgments
We thank Alan Fields for suggesting the use of sodium tetrathionate, Kathleen Postle for suggesting formaldehyde cross-linking, and Vernon Anderson for assistance with circular dichroism.
This research was supported by National Institutes of Health grant GM39447 to M.E.M. M.A.W. was supported by Cell and Molecular Biology Training Grant GM08056 during part of this work.
REFERENCES
- 1.Chang, G., R. H. Spencer, A. T. Lee, M. T. Barclay, and D. C. Rees. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282:2220-2226. [DOI] [PubMed] [Google Scholar]
- 2.Chou, P. Y., and G. D. Fasman. 1974. Prediction of protein conformation. Biochemistry 13:222-245. [DOI] [PubMed] [Google Scholar]
- 3.Falke, J. J., and D. E. Koshland, Jr. 1987. Global flexibility in a sensory receptor: a site-directed cross-linking approach. Science 237:1596-1600. [DOI] [PubMed] [Google Scholar]
- 4.Garcia, V. E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 5.Garnier, J., J. F. Gibrat, and B. Robson. 1996. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol. 266:540-553. [DOI] [PubMed] [Google Scholar]
- 6.Hmiel, S. P., M. D. Snavely, J. B. Florer, M. E. Maguire, and C. G. Miller. 1989. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J. Bacteriol. 171:4742-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hmiel, S. P., M. D. Snavely, C. G. Miller, and M. E. Maguire. 1986. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 168:1444-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kai, Y., H. Matsumura, and K. Izui. 2003. Phosphoenolpyruvate carboxylase: three-dimensional structure and molecular mechanisms. Arch. Biochem. Biophys. 414:170-179. [DOI] [PubMed] [Google Scholar]
- 9.Kehres, D. G., C. H. Lawyer, and M. E. Maguire. 1998. The CorA magnesium transporter gene family. Microb. Comp. Genomics 43:151-169. [DOI] [PubMed] [Google Scholar]
- 10.Kehres, D. G., and M. E. Maguire. 2002. Structure, properties and regulation of magnesium transport proteins. Biometals 15:261-270. [DOI] [PubMed] [Google Scholar]
- 11.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 12.Kucharski, L. M., W. J. Lubbe, and M. E. Maguire. 2000. Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. J. Biol. Chem. 275:16767-16773. [DOI] [PubMed] [Google Scholar]
- 13.Lynch, B. A., and D. E. Koshland, Jr. 1991. Disulfide cross-linking studies of the transmembrane regions of the aspartate sensory receptor of Escherichia coli. Proc. Natl. Acad. Sci. USA 88:10402-10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma, C., and G. Chang. 2004. Structure of the multidrug resistance efflux transporter EmrE from Escherichia coli. Proc. Natl. Acad. Sci. USA 101:2852-2857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Milligan, D. L., and D. E. Koshland, Jr. 1988. Site-directed cross-linking: establishing the dimeric structure of the aspartate receptor of bacterial chemotaxis. J. Biol. Chem. 263:6268-6275. [PubMed] [Google Scholar]
- 16.Prossnitz, E., K. Nikaido, S. J. Ulbrich, and G. Ferro-Luzzi Ames. 1988. Formaldehyde and photoactivatable cross-linking of the periplasmic binding protein to a membrane component of the histidine transport system of Salmonella typhimurium. J. Biol. Chem. 263:17917-17920. [PubMed] [Google Scholar]
- 17.Schmid, F. X. 1990. Spectral methods of characterizing protein conformation and conformation changes, p. 251-285. In T. E. Creighton (ed.), Protein structure: a practical approach. IRL Press, New York, N.Y.
- 18.Skare, J. T., B. M. Ahmer, C. L. Seachord, R. P. Darveau, and K. Postle. 1993. Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J. Biol. Chem. 268:16302-16308. [PubMed] [Google Scholar]
- 19.Smith, R. L., J. L. Banks, M. D. Snavely, and M. E. Maguire. 1993. Sequence and topology of the CorA magnesium transport systems of Salmonella typhimurium and Escherichia coli. Identification of a new class of transport protein. J. Biol. Chem. 268:14071-14080. [PubMed] [Google Scholar]
- 20.Smith, R. L., E. Gottlieb, L. M. Kucharski, and M. E. Maguire. 1998. Functional similarity between archaeal and bacterial CorA magnesium transporters. J. Bacteriol. 180:2788-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, R. L., and M. E. Maguire. 1995. Distribution of the CorA Mg2+ transport system in gram-negative bacteria. J. Bacteriol. 177:1638-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, R. L., and M. E. Maguire. 1998. Microbial magnesium transport: unusual transporters searching for identity. Mol. Microbiol. 28:217-226. [DOI] [PubMed] [Google Scholar]
- 23.Smith, R. L., M. A. Szegedy, C. Walker, R. M. Wiet, A. Redpath, M. L. Kaczmarek, L. M. Kucharski, and M. E. Maguire. 1998. The CorA magnesium transport protein of Salmonella typhimurium: mutagenesis of conserved residues in the third transmembrane segment identifies part of a Mg2+ pore. J. Biol. Chem. 273:28663-28669. [DOI] [PubMed] [Google Scholar]
- 24.Snavely, M. D., J. B. Florer, C. G. Miller, and M. E. Maguire. 1989. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J. Bacteriol. 171:4761-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snavely, M. D., S. A. Gravina, T. T. Cheung, C. G. Miller, and M. E. Maguire. 1991. Magnesium transport in Salmonella typhimurium: regulation of mgtA and mgtB expression. J. Biol. Chem. 266:824-829. [PubMed] [Google Scholar]
- 26.Soncini, F. C., V. E. Garcia, F. Solomon, and E. A. Groisman. 1996. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J. Bacteriol. 178:5092-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukharev, S., M. Betanzos, C. S. Chiang, and H. R. Guy. 2001. The gating mechanism of the large mechanosensitive channel MscL. Nature 409:720-724. [DOI] [PubMed] [Google Scholar]
- 28.Sukharev, S., S. R. Durell, and H. R. Guy. 2001. Structural models of the MscL gating mechanism. Biophys. J. 81:917-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sukharev, S. I., P. Blount, B. Martinac, F. R. Blattner, and C. Kung. 1994. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 368:265-268. [DOI] [PubMed] [Google Scholar]
- 30.Szegedy, M. A., and M. E. Maguire. 1999. The CorA Mg2+ transport protein of Salmonella typhimurium. Mutagenesis of conserved residues in the second membrane domain. J. Biol. Chem. 274:36973-36979. [DOI] [PubMed] [Google Scholar]
- 31.Tao, T., P. F. Grulich, L. M. Kucharski, R. L. Smith, and M. E. Maguire. 1998. Magnesium transport in Salmonella typhimurium: biphasic time and magnesium dependence of the transcription of the mgtA and mgtCB loci. Microbiology 144:655-664. [DOI] [PubMed] [Google Scholar]
- 32.Thoden, J. B., Z. Zhuang, D. Dunaway-Mariano, and H. M. Holden. 2003. The structure of 4-hydroxybenzoyl-CoA thioesterase from Arthrobacter sp. strain SU. J. Biol. Chem. 278:43709-43716. [DOI] [PubMed] [Google Scholar]
- 33.Ubarretxena-Belandia, I., J. M. Baldwin, S. Schuldiner, and C. G. Tate. 2003. Three-dimensional structure of the bacterial multidrug transporter EmrE shows it is an asymmetric homodimer. EMBO J. 22:6175-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, R. G., C. E. Andersson, T. Skarina, E. Evdokimova, A. M. Edwards, A. Joachimiak, A. Savchenko, and S. L. Mowbray. 2003. The 2.2 Å resolution structure of RpiB/AlsB from Escherichia coli illustrates a new approach to the ribose-5-phosphate isomerase reaction. J. Mol. Biol. 332:1083-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]