Abstract
The cerebral cortex contains dozens of neuronal subtypes grouped in specific layers and areas. Recent studies have revealed how embryonic and induced pluripotent stem cells (PSC) can differentiate into a wide diversity of cortical neurons in vitro, while recapitulating many of the temporal and spatial features that characterize corticogenesis. PSC-derived neurons can integrate into the brain following in vivo transplantation and display patterns of morphology and connectivity specific of cortical neurons. PSC-corticogenesis thus emerges as a robust model that provides new ways to link cortical development, evolution, and disease.
The cerebral cortex is arguably the most complex structure in our brain, and cortical neuron number and diversity are thought to be at the core of its powerful computational capacities. Most (>85%) cortical neurons are excitatory pyramidal neurons, while the remaining 15% are inhibitory interneurons. Pyramidal neurons and interneurons can be further subdivided into many subtypes, characterized by specific patterns of gene expression, morphology and connectivity [1].
Pluripotent stem cells (PSC), whether embryonic (ESC) [2] or induced (iPSC) [3,4], have emerged as a promising tool to model normal brain development and diseases. Here we will review recent data that demonstrate that a substantial fraction of cortical neuron diversity and complexity can be generated in vitro from PSC, while mimicking much of in utero development, revealing that many features of corticogenesis can result from self-organization. We will put special emphasis on studies that used human cells, and the insights that they provide on human brain development, evolution, and disease.
Starting-up: regional patterning and neuronal specification
The cortical primordium emerges in the telencephalon, the anterior-most part of the forebrain. Interestingly, the telencephalic/forebrain identity first develops largely in the absence of any extrinsic morphogenic cues, and is even enhanced through active inhibition of morphogen signals such as Wnts or BMPs [5]. The telencephalon then undergoes patterning along the dorso-ventral axis, leading to the parcellation into several neurogenic niches, including the dorsal telencephalon and the ventrally located ganglionic eminences, which will generate cortical pyramidal neurons and most interneurons, respectively [6–8]. These basic features of corticogenesis are essentially recapitulated during directed differentiation of cortical neurons from PSC. Indeed when PSC are cultured without any added (caudalizing) morphogen or in the presence of selected morphogen inhibitors, most of them differentiate into neural precurors displaying a forebrain/telencephalic identity [9–13] [••14–16]. Moreover, if PSC-forebrain differentiation takes place with little or no SHH signalling, it mostly leads to the generation of dorsal telencephalic progenitors and glutamatergic, cortical pyramidal neurons [9, ••14,15, ••17–20]. In contrast, addition of SHH leads to specification of ventral telencephalic progenitors that will differentiate into both GABAergic and cholinergic neurons [19, • 21–23]. Since the majority of cortical GABAergic interneurons in humans, as in rodents, originate in the subcortical telencephalon [• 24,25], ventralized telencephalic differentiation of human PSC also give rise to cortical interneurons [••26–28].
Modeling temporal patterns of corticogenesis
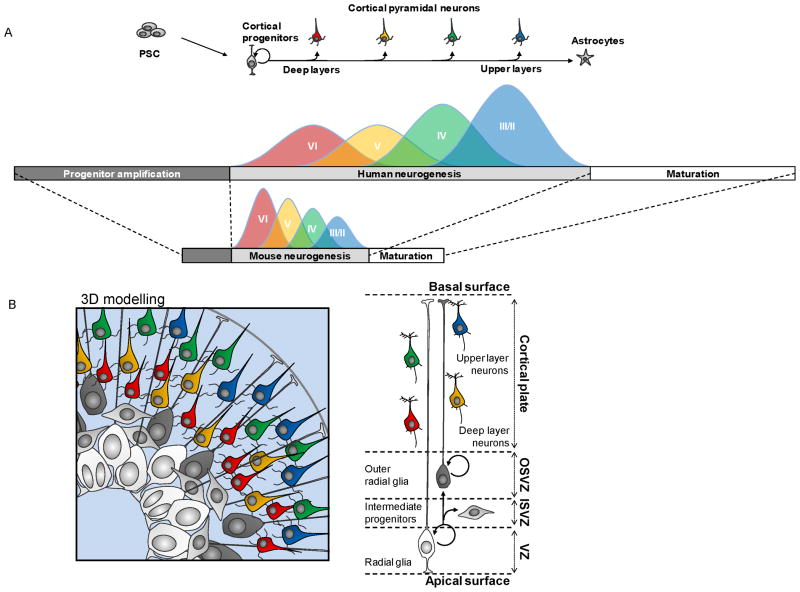
Following early patterning, cortical neurogenesis will start to take place leading to the generation of 6 different neuronal layers, each characterized by specific patterns of gene expression and connectivity [1]. The layer-identity of a cortical neuron is tightly linked to the timing of its generation: this temporal patterning results in the sequential generation of layer-specific types of cortical neurons and is a fundamental process of neuronal diversification [29]. Remarkably, PSC-derived corticogenesis recapitulates this temporal patterning in vitro, leading to the sequential generation of a repertoire of neurons displaying specific molecular markers of all six layers [9,15,17,20,30], similarly to what was previously demonstrated using ex vivo cultures of early cortical progenitors [31]. Intriguingly, the proportion of each layer-specific neuronal subtype varies considerably depending on differentiation conditions. ESC-derived pyramidal neurons obtained in minimal culture conditions (low cell density without any added morphogens) are strongly skewed towards a deep layer identity [9,15], while a higher proportion of upper layer neurons are obtained when PSC are first differentiated at high density [20] or as cell aggregates [17,32], or when the PSC-derived cortical progenitors are transplanted in the mouse brain [15]. These differences should be explored much further, and it may yield new insights on the mechanism that control the timing and rates of production of specific pyramidal neuron subtypes.
While the sequential generation of pyramidal neurons from PSC is a robust feature, observed from ESC and iPSC of mouse and human origin, direct comparison between mouse and human PSC-corticogenesis revealed that it is greatly extended in time with human PSC [15,17,19,20,32], even when using identical culture conditions [15]. Consistent with the protracted period of cortical neurogenesis in humans, human ESC-cortical progenitors start to generate postmitotic neurons after about 4 weeks instead of 6–8 days in the mouse, which is correlated with appearance of radial glia (RG)-like progenitors, the main neurogenic cortical progenitor [33]. Thereafter, mouse ESC-corticogenesis takes 2–3 weeks to be completed, while it takes 10–15 weeks starting from human ESC (Figures 1 and 2) [9]. These temporal specificities are strikingly similar to in vivo corticogenesis [34–38], and they may be directly relevant to the links between development and evolution of the cortex. Indeed, many of the species-specific features of the primate/human cortex, including a larger number and diversity of neurons, are thought to be linked to differences in the mechanisms underlying the generation of cortical neurons [36,38,39]. The mechanisms by which the primate embryonic brain can generate more neurons for prolonged periods might be linked to species-specific properties intrinsic to cortical progenitors, such as differential cell cycle control or tuning of self-renewal vs. terminal differentiation [40]. The emergence of other types of progenitors may also contribute to evolutionary changes in cortical neurogenesis. These progenitors include the recently described “outer” radial glial (oRG) cells [35,37,41–44], which share many features with RG cells, including the potential for self-renewal, but they lack any apical projection. Most strikingly, while human oRG cells can generate neurons directly, their progeny undergoes multiple rounds of divisions before final differentiation, thus providing a mechanism for increased neuronal output and cortical expansion. Importantly, the detection of oRG-like cells was reported following in vitro differentiation from human PSC [••20,45,46] but not from mouse PSC [46], providing further evidence of species-specific features of PSC-corticogenesis directly relevant to evolution.
Figure 1. Modelling temporal and spatial patterning of cortical neuron neurogenesis.
(A) PSC cultured under minimal conditions or in the presence of Wnt/Tgfβ/BMP morphogen inhibitors, undergo differentiation towards forebrain/telencephalic identity. In absence or low levels of Shh-signalling, PSC will mostly differentiate into a collection of progenitors of dorsal telencephalon/cortical identity. Subsequent generation of cortical pyramidal neurons follows a temporal patterning, with deep layer neurons being generated earlier than upper layer neurons, eventually followed by a switch to astrocyte production, like in vivo. Human PSC-derived corticogenesis follows a much more protracted time-course than the mouse counterpart, highly reminiscent of the in vivo situation. (B) Schematics of the relationships between the various cellular players of corticogenesis found in vivo. OSVZ/ISVZ, outer/inner subventricular zone. 3D models can recapitulate in a strikingly faithful way the in vivo organization of cortical progenitors and neurons, thereby providing unique tools to study spatial patterning and cytoarchitecture formation.
Figure 2. Comparison of Mouse and Human In Vivo and ESC-based In Vitro PV and SST Interneuron Development.
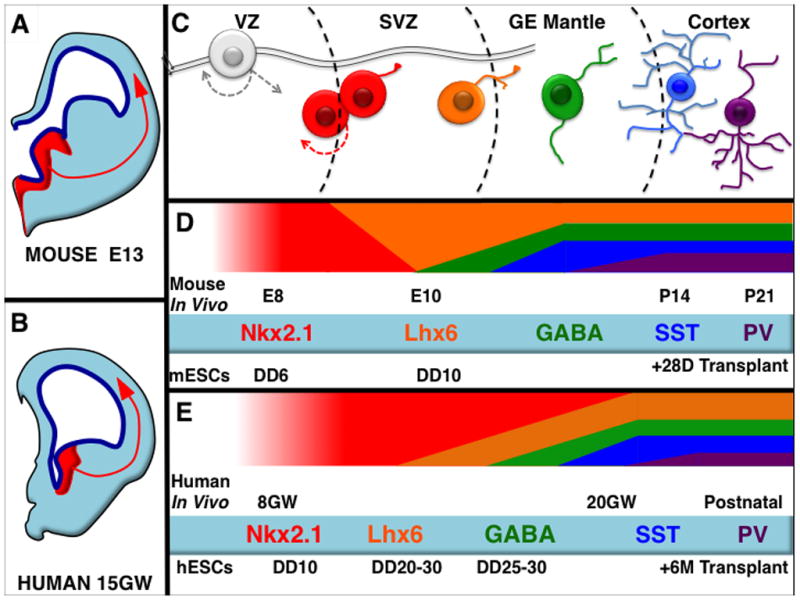
Schematic of a mouse (A) and a human (B) half coronal section at comparable ages during neurodevelopment, embryonic day 13.5 and 15 gestational weeks (not to scale) showing in red the Nkx2.1-expressing medial ganglionic eminence (MGE). The MGE is the progenitor domain for both PV and SST expressing cortical interneurons and is relatively well conserved across species (A, B), as is the progression (C) from Nkx2.1-expressing progenitors, to Lhx6 and then GABA-expressing migratory precursors, then finally to terminally differentiated interneurons. Mouse ESC derived PV and SST expressing cells mature at analogous rates, with both makers detectable by approximately 4 weeks post transplantation into mouse neonatal cortex (D). Conversely human ESC-derived PV and SST expressing cells mature very slowly- also analogously to their in vivo counterparts (E). E=Embryonic Day, DD=Differentiation Day, GW=Gestational Weeks, P=Days After Birth.
A third aspect of corticogenesis that appears to be species-specific is neuronal maturation: once generated human cortical neurons display much prolonged patterns of morphological and functional maturation, such as dendrite patterning and synaptogenesis [47,48]. Similarly, PSC-derived human cortical neurons mature very slowly at the molecular and functional levels [15,20,32]. Even more strikingly, comparison of human vs. mouse PSC-derived cortical neurons transplanted into the mouse neonatal cortex revealed that the human pyramidal neurons follow a species-specific program of delayed neuronal maturation and synaptogenesis [15,49]. For instance, while ESC-derived mouse pyramidal neurons develop full blown and specific axonal and dendritic projections after 4 weeks [9], similarly differentiated and transplanted human neurons take more than 6 months to develop subcortical projections and at least 9 months to develop complex dendrite arborization pattern, dendritic spines, and functional synaptic activity [15]. Similar results are found with GABAergic cortical interneurons derived from human PSCs. While synaptogenesis and reasonably mature-appearing action potentials can be detected within one month of co-culturing with mouse cortical pyramidal neurons [27], transplantation studies into the neocortex of neonatal mice show limited terminal differentiation of these cells even 6 months later [28].
Overall, these data point to cortex-intrinsic mechanisms that control the clock of several key aspects of corticogenesis, for which PSC-based models may provide attractive experimental set-ups to dissect the underlying mechanisms [30], with potentially important relevance to basic mechanisms linking cortical development and evolution.
Importantly, the very slow rate of maturation of cortical cells from human PSC presents a major challenge to the widespread application of this system for the study or treatment of human disease.
Modeling spatial patterns of corticogenesis
The cytoarchitecture of the cortex is crucial to its function, and despite its apparent complexity, key aspects of the patterned, three dimensional (3D) organization of the developing cortex can also be recreated in vitro (Figure 1B). When PSC are cultured as bowls of cells and differentiated into cortical-like progenitors, this leads to robust polarized neurons accumulating at their periphery, following an organization highly reminiscent of a nascent cortical primordium, including a ventricular-like zone and a cortical plate-like mantle region. Using long-term culture systems of 3D differentiation from PSC, the emergence of specific domains of progenitors and even cortical layer-like structures have been reported [45,46]. Most remarkably, using a long-term cortical 3D model, the final position of the neurons within a cortical plate-like structure was found to depend on its neuronal birthdate, where neurons born earlier were found in deeper positions than later ones, thus recapitulating the inside-out pattern characteristic cortical neurogenesis [45]. These data constitute striking demonstration that a cortical-like cytoarchitecture can self-organize in vitro, and it will be fascinating to test how far one can go to model further the complexity of cortical architecture, and perhaps function, in a dish. For example, it may be possible to use focal application of Shh signalling agonists in this system to create an interneuron-generating domain, then use the system to study the migration and integration of cortical interneurons within the developing human cortex.
The patterning of cortical areas is another complex process [6,50] that can be surprisingly modelled using PSC differentiation, combined with intracortical transplantation. Indeed, when mouse or human ESC-derived cortical neurons obtained in minimal culture conditions are grafted in the mouse neonatal cortex, they send most of their axonal projections to targets of the visual and limbic occipital cortex [9,15], with a pattern that is similar to grafted embryonic visual cortical tissue [9,51]. This was observed despite the fact that the cells were transplanted in the frontal cortex, suggesting that the highly selective ‘occipital-like’ pattern of projections was not likely due to respecification of the grafted neurons by the host. In line with this hypothesis, examination of the molecular identity of ESC-derived cortical progenitors and neurons before grafting revealed that most of them expressed markers of the occipital cortex [9]. On the other hand, the areal fate of ESC-derived cortical progenitors can be modified in vitro by the addition of extrinsic cues known to induce frontal cortical fates in vivo [17,45]. Interestingly, the human cortical cells, but not the mouse, tended to lose markers of occipital identity following longer periods post-transplantation, and their axonal projections corresponded to a wider range of areal identities with time [15]. These observations suggest that specific patterns of areal identity may be acquired in vitro, but that following grafting some of the cells can be specified to other areal identities over time, possibly in relation with their relatively early stage of maturation at the time of grafting, and therefore higher susceptibility to extrinsic cues from the host cortex [52]. In any case, these data indicate that areal specification can occur in a specific way from PSC, which has interesting implications for our understanding of arealization mechanisms, and also in the long-run prospect of area-specific cortical repair strategies.
Modeling pathological corticogenesis
The advent of iPSC technology [4] offers in principle many novel opportunities to model brain diseases, including those that strike the developing cortex [53,54]. So far few studies have relied on iPSC-derived cortical cells to model neurodevelopmental diseases. Among these, one striking example is Timothy syndrome (TS), caused by a mutation in a L-type voltage-gated calcium channel, and leading to developmental delay and autism. Examination of iPSC-derived cortical cells from TS patients revealed several interesting phenotypes. These included, as expected, defects in calcium signaling and neuronal activity, but also more surprising defects such as the generation of specific types of neurons [55]. Together with other studies [56] [57] [46] [58] [59] these findings illustrate that PSC-derived corticogenesis can be used to model some aspects of complex human cortical diseases, particularly in the case of synaptic or electrophysiological abnormalities that are not restricted to a specific neuronal subtype.
Conclusion and perspectives
In sum, recent years have shown tremendous progress in the generation of cortex-like neurons from mouse and human pluripotent cells. Human PSC can generate cortically-patterned tissue both in 2D and 3D cultures, where they replicate key aspects of temporal and spatial patterning. Xenographic transplantation studies with both cortical pyramidal neurons and GABAergic interneurons derived from human PSC corroborate the in vitro studies, suggesting that bona fide cortical neurons are being produced. The protracted differentiation process, and the difficulty in generating and identifying more specific cortical neuronal subtypes, present important technical and conceptual challenges for the use of this technology to study human cortex development and disease. That said, with the generation of transgenic reporter stem cell lines allowing the identification of increasingly specific cell types, and the steady improvement of differentiation protocols, PSC-corticogenesis models may help to transform how we understand and treat diseases of the cerebral cortex.
Highlights.
Pluripotent stem cell differentiation models spatial and temporal patterns of generation of cortical pyramidal and interneurons.
Pluripotent stem cell derived corticogenesis displays species-specific features relevant to brain evolution.
Pluripotent stem cell modelling is a promizing tool to reveal insights on neurodevelopmental diseases.
Acknowledgments
Work from the authors’ lab was was funded by grants from the Belgian FNRS, the Belgian Queen Elizabeth Medical Foundation, the Interuniversity Attraction Poles Program (IUAP), the WELBIO and Programme d’Excellence CIBLES of the Walloon Region, the Fondations Clerdent and de Spoelbergh, and Fondation ULB (to PV). SA was funded by the National Institutes of Mental Health (USA) grants R01 MH066912 and K02 MH070031. We thank members of our labs for critical insights and help for the illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 3.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 7.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 8.Hebert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. Together with [17] this is the first demonstration of efficient generation of cortical neurons in vitro from embryonic stem cells, following a precise temporal pattern similar to in vivo. In addition the authors present evidence that even areal identity of the cortical progenitors can be acquired without influence from the rest of the brain. [DOI] [PubMed] [Google Scholar]
- 10.Bertacchi M, Pandolfini L, Murenu E, Viegi A, Capsoni S, Cellerino A, Messina A, Casarosa S, Cremisi F. The positional identity of mouse ES cell-generated neurons is affected by BMP signaling. Cell Mol Life Sci. 2013;70:1095–1111. doi: 10.1007/s00018-012-1182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juliandi B, Abematsu M, Sanosaka T, Tsujimura K, Smith A, Nakashima K. Induction of superficial cortical layer neurons from mouse embryonic stem cells by valproic acid. Neurosci Res. 2012;72:23–31. doi: 10.1016/j.neures.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nature biotechnology. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 13.Wataya T, Ando S, Muguruma K, Ikeda H, Watanabe K, Eiraku M, Kawada M, Takahashi J, Hashimoto N, Sasai Y. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc Natl Acad Sci U S A. 2008;105:11796–11801. doi: 10.1073/pnas.0803078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. This groundbreaking paper showed that floating embryoid differentiations of mouse embryonic stem cells could be directed to the progenitor states expressing markers consistent with embryonic telencephalon. These could be dorsalized or ventralized, and differentiated into neurons expressing features of cortex or basal ganglia. [DOI] [PubMed] [Google Scholar]
- 15••.Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J, Bali C, Orduz D, Bilheu A, Herpoel A, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. This paper describes the timely generation of human pyramidal neurons from human pluripotent stem cells, following a time-line strikingly reminiscent of in utero conditions. In addition it shows that human pyramidal neurons transplanted in the mouse display specific patterns of axonal projections and connectivity like bona fide cortical neurons. [DOI] [PubMed] [Google Scholar]
- 16•.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. This paper introduced a more rapid and consistent approach for neural differentiation of human stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. Together with [8] this is the first demonstration of efficient generation of cortical neurons in vitro. In addition the authors present evidence that a complex self-organized cytoarchitecture can emerge in a purely in vitro setting. [DOI] [PubMed] [Google Scholar]
- 18.Danjo T, Eiraku M, Muguruma K, Watanabe K, Kawada M, Yanagawa Y, Rubenstein JL, Sasai Y. Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci. 2011;31:1919–1933. doi: 10.1523/JNEUROSCI.5128-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486. doi: 10.1038/nn.3041. This paper describes the generation of functional pyramidal neurons from human PSC, which involves outer radial glial cell-like progenitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L, Zhou GM, Ayala M, Zhang SC. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat Biotechnol. 2013;31:440–447. doi: 10.1038/nbt.2565. This paper showed that transplants of human stem cell differentiations to Nkx2.1-expressing, medial ganglionic eminence-like progenitors can mature into GABAergic and cholinergic neurons in the hippocampus of adult mice. The transplants also influenced cognition, demonstrating that transplants of human stem cell derived neurons into mammalian cortex can influence complex cortical functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bissonnette CJ, Lyass L, Bhattacharyya BJ, Belmadani A, Miller RJ, Kessler JA. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells. 2011;29:802–811. doi: 10.1002/stem.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Goulburn AL, Alden D, Davis RP, Micallef SJ, Ng ES, Yu QC, Lim SM, Soh CL, Elliott DA, Hatzistavrou T, et al. A targeted NKX2. 1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells. 2011;29:462–473. doi: 10.1002/stem.587. The paper used gene targeting by homologous recombination to insert a GFP reporter into the Nkx2.1 locus of a human embryonic stem cell line. This and subsequent papers using the same line (26,27,28) demonstrate the tremendous utility of using transgenic reporters of fate in the application of pluripotent stem cell technology to study forebrain development. [DOI] [PubMed] [Google Scholar]
- 24•.Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci. 2013 doi: 10.1038/nn.3541. Together with (25), this paper demonstrated that, as in rodents, the majority of cortical interneurons in humans and non-human primates derive from the subcortical telencephalon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, et al. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16:1588–1597. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- 26.Germain ND, Banda EC, Becker S, Naegele JR, Grabel LB. Derivation and Isolation of NKX2. 1-Positive Basal Forebrain Progenitors from Human Embryonic Stem Cells. Stem Cells Dev. 2013;22:1477–1489. doi: 10.1089/scd.2012.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. Together with (28), this paper showed that dual-smad inhibition (16) together with ventralization by Shh signaling can be used to generate putative cortical interneurons. Placed on a culture of mouse cortical pyramidal neurons, these cells differentiate, express interneuron subgroup markers, and undergo functional input and output synaptogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. Like (26) and (27), this paper used the Nkx2.1-GFP hESC line (23) to generate putative cortical interneuron progenitors. Together with (27), these cells were found to undergo extensive non-radial migration after transplantation into rodent neocortex. Remarkably, the authors found that terminal differentiation remained incomplete even 6 months after transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaspard N, Vanderhaeghen P. Laminar fate specification in the cerebral cortex. F1000 Biol Rep. 2011;3:6. doi: 10.3410/B3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiberi L, van den Ameele J, Dimidschstein J, Piccirilli J, Gall D, Herpoel A, Bilheu A, Bonnefont J, Iacovino M, Kyba M, et al. BCL6 controls neurogenesis through Sirt1-dependent epigenetic repression of selective Notch targets. Nat Neurosci. 2012;15:1627–1635. doi: 10.1038/nn.3264. [DOI] [PubMed] [Google Scholar]
- 31.Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 32.Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, Horvath TL, Vaccarino FM. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- 35.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010 doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 36.Fish JL, Dehay C, Kennedy H, Huttner WB. Making bigger brains-the evolution of neural-progenitor-cell division. J Cell Sci. 2008;121:2783–2793. doi: 10.1242/jcs.023465. [DOI] [PubMed] [Google Scholar]
- 37.Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010 doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 38.Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 40.Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC, et al. Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus. Cereb Cortex. 2012;22:469–481. doi: 10.1093/cercor/bhr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reillo I, Borrell V. Germinal zones in the developing cerebral cortex of ferret: ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb Cortex. 2012;22:2039–2054. doi: 10.1093/cercor/bhr284. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Moreno F, Vasistha NA, Trevia N, Bourne JA, Molnar Z. Compartmentalization of cerebral cortical germinal zones in a lissencephalic primate and gyrencephalic rodent. Cereb Cortex. 2012;22:482–492. doi: 10.1093/cercor/bhr312. [DOI] [PubMed] [Google Scholar]
- 44.Betizeau M, Cortay V, Patti D, Pfister S, Gautier E, Bellemin-Menard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, et al. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron. 2013;80:442–457. doi: 10.1016/j.neuron.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 45••.Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1315710110. Together with [17] and [46] these studies demonstrate the remarkable degree of self-organization that can be observed from pluripotent stem cells, including most strikingly the generation of spatially organized cortical-plate and germinative zone-like structures, providing unique approaches to model brain development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Defelipe J. The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front Neuroanat. 2011;5:29. doi: 10.3389/fnana.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 50.O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaillard A, Roger M. Early commitment of embryonic neocortical cells to develop area-specific thalamic connections. Cereb Cortex. 2000;10:443–453. doi: 10.1093/cercor/10.5.443. [DOI] [PubMed] [Google Scholar]
- 52.Pinaudeau C, Gaillard A, Roger M. Stage of specification of the spinal cord and tectal projections from cortical grafts. Eur J Neurosci. 2000;12:2486–2496. doi: 10.1046/j.1460-9568.2000.00148.x. [DOI] [PubMed] [Google Scholar]
- 53.Sandoe J, Eggan K. Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nat Neurosci. 2013;16:780–789. doi: 10.1038/nn.3425. [DOI] [PubMed] [Google Scholar]
- 54.Dolmetsch R, Geschwind DH. The Human Brain in a Dish: The Promise of iPSC-Derived Neurons. Cell. 2011;145:831–834. doi: 10.1016/j.cell.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pasca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Pasca AM, Cord B, Palmer TD, Chikahisa S, Nishino S, et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shcheglovitov A, Shcheglovitova O, Yazawa M, Portmann T, Shu R, Sebastiano V, Krawisz A, Froehlich W, Bernstein JA, Hallmayer JF, et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature. 2013 doi: 10.1038/nature12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Wang H, Muffat J, Cheng AW, Orlando DA, Loven J, Kwok SM, Feldman DA, Bateup HS, Gao Q, et al. Global transcriptional and translational repression in human-embryonic-stem-cell-derived rett syndrome neurons. Cell Stem Cell. 2013;13:446–458. doi: 10.1016/j.stem.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y, Kirwan P, Smith J, MacLean G, Orkin SH, Livesey FJ. A human stem cell model of early Alzheimer’s disease pathology in Down syndrome. Sci Transl Med. 2012;4:124ra129. doi: 10.1126/scitranslmed.3003771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krey JF, Pasca SP, Shcheglovitov A, Yazawa M, Schwemberger R, Rasmusson R, Dolmetsch RE. Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat Neurosci. 2013;16:201–209. doi: 10.1038/nn.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]