Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease that involves pathological remodeling, vasoconstriction, and thrombosis. Alterations in hemostasis, coagulation, and platelet activation are consistently observed in PAH patients. Microparticles derived from platelets, inflammatory cells, and the endothelium are an increasingly well-recognized signal in a variety of cardiovascular diseases, including PAH. This review will focus on the role of coagulation, thrombosis, platelet activation, and microparticles in the pathology and progression of PAH.
Introduction
Pulmonary arterial hypertension (PAH) is characterized by increased vascular resistance and the progressive loss of the pulmonary circulation [1]. The pathogenesis of PAH involves in situ thrombosis [2], excess vasoconstriction [3], inflammation [4,5], and a loss of the normal balance between apoptosis and proliferation within the intima, media, and adventitia [6]; these pathologic processes combine to narrow the lumen and increase pulmonary vascular resistance, leading to dyspnea and eventual right ventricular heart failure.
Evidence for Thrombosis in PAH
Thrombotic arteriopathy, the significant presence of thrombotic lesions in the pulmonary vasculature, is a common pathological finding in PAH. Thrombosis was first proposed to play a causative role in the mechanism of PAH in 1984 [7]. In this long-term, retrospective, natural history study, they found that 57% of patients who met the criteria for primary pulmonary hypertension exhibited thromboembolic type change upon autopsy. Five years later, an autopsy series from the National Heart, Lung and Blood Institute (NHLBI), Primary Pulmonary Hypertension (PPH) Registry examined the histopathologic features of pulmonary blood vessels from 58 carefully phenotyped patients [8]. Nineteen of these patients had thrombotic lesions (33%), and 9 of the 25 patients with plexiform lesions also had recanalized thrombi. The co-existence of classic plexiform arteriopathy with recanalized thrombi in patients who clearly did not have clinical pulmonary thromboembolic disease cemented a role for thrombosis in the disease pathogenesis. Similarly, three earlier studies also reported high frequencies (20%, 30%, and 56%) of thrombotic lesions in the histopathological classification of hypertensive pulmonary vascular disease [9–11].
Although there is consensus that thrombosis is a common pathological feature of PAH, its role in PAH remains controversial [12]. Some support the view that the activation of coagulation contributes to the pathogenesis of PAH through luminal narrowing (both from the fibrin clot itself and related vascular remodeling, likely driven by proteases, TF, factor Xa, and thrombin). The alternate view is that thrombotic arteriopathy is an epiphenomenon of pulmonary vascular remodeling. Although there is no question that thrombosis is consistently observed in PAH, it is still unclear whether thrombosis contributes to PAH disease progression or evolves as a bystander of the more relevant disease processes.
Evidence for Coagulation in PAH
Tissue factor (TF) is a transmembrane glycoprotein not normally expressed on the endothelium, but abundantly expressed on fibroblasts [13]. TF exposure during injury initiates the coagulation cascade through complex formation with Factor VIIa, which in turn catalyzes the activation of Factor X, leading to generation of thrombin. TF is normally expressed at low levels in the pulmonary vessel wall, and we have shown that it is greatly increased in vascular lesions of PAH patients [14]. Aberrant expression of TF on the luminal surface of arteries would certainly predispose patients to in situ thrombosis, in addition to its role in initiating smooth muscle cell migration and proliferation. We have also proposed a role for TF and downstream thrombin signaling in the development of plexiform lesions by mediating disorganized angiogenesis and endothelial cell migration [15]. Additionally, Bakouboula et al. demonstrated increased TF-expressing endothelial cell microparticles released from the pulmonary circulation of PAH patients, further implicating TF as a key mediator in the vascular injury of PAH [16].
Eisenberg et al. demonstrated evidence for increased thrombin activity (downstream of TF), by observing elevated levels of fibrinopeptide-A (FPA) in PAH patients [17]. FPA is generated when thrombin cleaves fibrinogen to form a fibrin clot and was previously used as a marker of fibrin generation and degradation prior to the availability of the D-dimer assay. Although a separate smaller study did not confirm the FPA data [18], a more recent study using calibrated automated thrombogram demonstrated that PAH patients have increased thrombin generation and exhibit a hypercoagulable state [19]. A limitation of the thrombogram methodology is its failure to account for fibrinolysis, but it is likely that both fibrin generation and fibrinolysis contribute to the hypercoagulable state in PAH. In fact, several studies have shown elevated levels of plasminogen activator inhibitor-1 (PAI), which inhibits the generation of plasmin, thus decreasing fibrin clot degradation [20,21]. Purified fibrinogen from PAH patients has also been shown to be resistant to lysis in vitro compared to patients with chronic thromboembolic pulmonary hypertension (CTEPH) or control subjects who had a prior pulmonary embolism without pulmonary hypertension [22]. This intriguing data suggests that a structural change within the fibrin clot exists, which prevents the interaction with plasmin to facilitate degradation.
A second regulatory pathway involves the inhibition of thrombin subsequent to its generation by TF. Thrombomodulin exists in membrane bound and soluble forms and binds thrombin, thus inhibiting its activity. Furthermore, the interaction of thrombomodulin with thrombin activates protein C, which then inhibits further thrombin production (by inactivating factor V). Two studies have demonstrated that PAH patients have lower levels of thrombomodulin compared to controls, consistent with the hypercoagulable state observed in PAH patients [20,23].
The procoagulant phenotype observed in PAH patients suggests a beneficial use of anticoagulation therapy. The American College of Chest Physicians clinical guidelines recommends the use of anticoagulants in PAH patients with a ‘moderate’ degree of certainty [24]. This recommendation is based on limited data from non-randomized trials of warfarin. To date, five observational studies have demonstrated a survival benefit associated with warfarin [7,25–28], while two studies did not observe any significant benefit [29,30]. In light of these studies and expert opinions, the goal of anticoagulation therapy is to reduce the observed hypercoagulable state in PAH patients by using warfarin to maintain an international normalized ratio (INR) of 1.5–2.5 for PAH patients (European authorities generally recommend more intense anticoagulation).
Recently, Delbeck et al. examined the effect of rivaroxaban, an oral direct factor Xa inhibitor, on the prevention of monocrotaline-induced experimental pulmonary hypertension in rats [31]. In this model, rivaroxaban prevented RV dysfunction and hypertrophy without significantly increasing bleeding. In contrast, warfarin and enoxparin dose-dependently increased bleeding in this model. Interestingly, rivaroxaban did not reduce muscularization of the pulmonary arteries. The report is primarily of interest as it underscores the fact that coagulation proteases contribute to the development of pulmonary vascular remodeling in this limited model of human disease.
Evidence for Altered Platelet Activation in PAH
Thrombocytopenia is observed in some patients with PAH, although the etiology is cryptic [32]. It is not clear whether thrombocytopenia originates due to defects in platelet production and/or as a result of peripheral platelet clearance. Herve et al. proposed a mechanism involving pulmonary microangiopathy, whereby platelets shear upon flow through fibrin clots and plexiform lesions [33]. A study of 22 patients with PAH demonstrated that the PAH subjects had significantly higher (p= 0.006) mean platelet volume (MPV) than 25 healthy controls [34]. MPV is a basic measure of platelet production and stimulation, as younger platelets are larger, contain more granule contents and are generally more prothrombotic [35]. Increases in MPV with concomitant decreases in platelet count may indicate platelet consumption in the periphery and a subsequent increase in thombopoiesis. Additionally, a separate study reported higher levels of the thrombopoiesis-stimulating hormone, thrombopoietin, in the pulmonary vasculature [36]. Taken together with controversial data suggesting that thrombopoiesis can occur in the lung from pulmonary megakaryocytes [37], it is possible that PAH patients have both production and destruction of platelets within the lung vasculature.
Hemostasis is a tightly regulated process and an imbalance between stimulatory and inhibitory signals can cause excess or insufficient platelet activation. Patients with PAH are known to have excess stimulatory signals and insufficient inhibitory signals. Thromboxane A2 (TXA2) is a platelet-derived vasoconstrictor and proinflammatory mediator that is elevated in PAH patients [38]. Conversely, decreased endothelial-derived prostacyclin and nitric oxide (NO) in PAH patients can potentiate platelet activation and lower the threshold for activation [39]. There is abundant evidence for a reduction in bioavailable NO within the pulmonary circulation of PAH patients [40,41], and drugs that increase activity in the NO signaling cascade are of benefit to PAH patients [42]. As NO is an important negative regulator of platelet activation, one consequence of decreased NO bioavailability is a likely increase in platelet activation. Similarly, prostacyclin synthase is reduced in the lungs of PAH subjects [43], and prostanoid replacement is a cornerstone of PAH therapy [44]. Prostacyclin was first identified biologically because of its ability to inhibit platelet aggregation [45,46], and experts still consider platelet inhibition to be a part of the mechanism by which prostacyclins favorably influence the pulmonary circulation. Thus, there is ample evidence that the signaling imbalance in the lung (excess thromboxane, insufficient NO and prostacyclin) should have intra-pulmonary platelet activation. We and others postulate that this contributes to the progression of PAH.
Inflammation and Platelet Activation in PAH
Platelets are the second most numerous cell in the blood and contain many proinflammatory, angiogenic, and prothrombotic mediators, which are released upon activation. Platelets and endothelial cells are the only cells that carry von Willebrand Factor (vWF), a large multimeric glycoprotein involved in facilitating the interaction between the two cell types. Endothelial cells and platelets release vWF upon activation, and circulating vWF levels are known to be increased in PAH patients. Higher levels of vWF independently predicted worse outcome in one large cohort of treatment-naïve patients [47]. In a separate study, higher vWF levels were also associated with decreased survival in a group of PAH patients whose disease was associated with congenital heart defects [48].
CD40L is a proinflammatory mediator contained inside platelets and is expressed on the surface of activated platelets then subsequently cleaved to the soluble form (sCD40L). Approximately 95% of the circulating sCD40L is platelet-derived. Patients with PAH have higher levels of sCD40L and those who do not take warfarin have even higher levels [49]. Additionally, these patients had higher plasma levels of monocyte chemoattractant protein 1 (MCP-1) and interleukin 8 (IL-8). In fact, in vitro treatment of human umbilical vein endothelial cells (HUVECs) with recombinant sCD40L substantially increased MCP-1 and IL-8 levels. These data suggest that sCD40L may interact with its receptor, CD40, on endothelial cells to drive vascular remodeling in PAH, similar to other inflammatory vascular diseases, such as atherosclerosis [50]. Fibroblasts express CD40 and engagement by CD40L can up-regulate cyclooxygenase-2 (COX-2), leading to the production of proinflammatory prostaglandins, cytokines, and connective tissue degrading enzymes [51,52]. This can facilitate immune cell recruitment, inflammation, proliferation, and vascular remodeling.
Serotonin (5-hydroxytryptamine) is synthesized in the central nervous system and is taken up by platelets in the plasma [53]. It can stimulate the second wave of platelet aggregation by activating platelets in combination with ADP after dense granule release. Furthermore, serotonin is a potent vasoconstrictor and over-expression of one of its receptor, 5HT2B, in the pulmonary vasculature in mice and humans has been linked to the development of PAH [54]. Normally, platelets sequester free serotonin, preventing vascular exposure and possibly smooth muscle cell proliferation, vasoconstriction, and thrombosis. There is mounting evidence that serotonin is key to the development of PAH, as increases in plasma serotonin caused by the anorexigen, fenfluramine, markedly increased the chances of developing PAH, and in at least one study, increased serotonin transport was causally linked to smooth muscle cell hypertrophy [55,56]. More recently, a small study demonstrated a negative transpulmonary platelet serotonin gradient in PAH patients, while control subjects exhibited a positive gradient [57]. These data are suggestive that the lungs of PAH patients may take up serotonin from platelets or that altered hemodynamics in the lungs of PAH patients cause degranulation and release of serotonin into the blood. This study did not find any differences between plasma levels of serotonin, while other studies have reported increased plasma concentrations in some cases [58].
It is known that platelets from PAH patients have altered aggregation in vitro, while their in vivo activation status is largely unknown [59]. To address this question, Maeda et al. examined tyrosine phosphorylation of platelets as a marker of in vivo activation [60]. They found a 79% increase in protein-associated tyrosine phosphorylation and a 57% increase in pp125FAK compared to controls. Phosphorylation of pp125FAK is thought to be a late signaling event in platelets, which occurs after αIIbβ3 signaling, and leads to platelet adherence, spreading, and migration [61]. A separate study demonstrated that platelets from PAH patients had diminished aggregation in response to thrombin receptor-activating peptide, TRAP, but not to ADP as compared to controls [59]. This suggests that these platelets have an intrinsic defect in the thrombin-signaling pathway, while the ADP signaling pathway is largely intact. This is particularly interesting in light of the evidence that PAH patients have higher levels of thrombin, which may suggest some level of compensation or “platelet exhaustion” to thrombin stimulation. This is in contrast to the data demonstrating that platelets from PAH patients release more sCD40L in response to TRAP than control platelets[49], further complicating the alterations in platelet signaling in PAH patients.
Genome wide RNA expression profiling in the lungs of PAH patients provides enticing data that platelets may have an altered endogenous activation status [62]. This microarray, confirmed by quantitative PCR, demonstrated elevated levels of the platelet-specific chemokine, platelet factor 4 (PF4), and the surface receptors, purinergic receptor P2Y (P2RY1) and coagulation factor II receptor-like 3 (F2RL3). P2YR1 is expressed on the surface of platelets and functions as a receptor for ADP and ATP while F2RL3 is necessary for platelet activation by thrombin [63,64]. Importantly, PF4 is known to bind to and neutralize heparin-like molecules on endothelial cells, thus promoting coagulation. These data are particularly compelling that platelet activation is mechanistically linked to the development of PAH, as patients with pulmonary hypertension associated with idiopathic pulmonary fibrosis (World Health Organization Group III) did not exhibit increased expression of F2RL3, P2RY1, or PF4.
One randomized, double-blinded, placebo-controlled study evaluated the effectiveness of anti-platelet agents in 19 PAH patients. Patients randomized to receive either aspirin or clopidogrel had significantly diminished platelet aggregation in vitro, suggesting a beneficial anti-platelet effect; aspirin blocked thromboxane production but clopidogrel did not [65]. These patients were not on warfarin, but about half were on intravenous epoprostenol. However, a separate randomized, double-blinded, placebo-controlled study of 92 PAH patients found no beneficial effects of aspirin treatment on a 6-minute walk test at 6 months [66]. Mechanistic studies demonstrated that thromboxane production was suppressed in the aspirin treated group as compared to controls, but platelet aggregation was not inhibited. Although there is considerable evidence to suggest that platelets contribute to the pathophysiology of PAH, the exact mechanisms have yet to be determined, and thus a clear treatment goal is impossible. One possible conclusion from the available data is that thromboxane production is not as important to ongoing disease as platelet aggregation or some other feature of platelet activation.
Role of Microparticles in PAH
Transcellular communication between blood cells, endothelial cells, and smooth muscle cells is almost certainly a vital aspect of pulmonary vascular physiology, and thus by extension, pathophysiology. This can occur through cell-to-cell contact, diffusion of soluble mediators, and by microparticles. Microparticles are small (0.1 to 1 micron) membrane-derived vesicles that are generated from cells through activation or apoptosis [67]. Approximately 80% of blood microparticles are platelet-derived microparticles (PMPs), while the remaining 20% are derived from red blood cells (RMPs), endothelial cells (EMPs), and leukocytes (LMPs). The composition of microparticles is largely determined by the parent cell from which they are generated, as well as the conditions under which they were made (for helpful review, [68]). The number and composition of microparticles play an important role in physiological and pathological processes. Microparticles generally express phosphatidyserine on their surface in addition to various receptors, lipids, and signaling molecules. They can interact with neighboring cells through direct extracellular interactions, and have also been shown to deliver their cytosolic contents to target cells as a means of altering cellular function [69].
Microparticle numbers are known to increase in pathological conditions, such as atherosclerosis [70], type 2 diabetes [71], and cancer [72]. Recently, the number and composition of microparticles have been examined in the context of PAH [73]. Several groups reported increased numbers of circulating PMPs and EMPs in PAH patients compared to controls [74]. Interestingly, one group reported that EMPs and small PMPs (0.3–0.5 microns) were elevated in all PAH patients studied, regardless of the etiology (idiopathic PAH, secondary PAH, or PAH due to BMPR2 mutation), suggesting either a common pathological mechanism or merely a common marker of vascular dysfunction [75].
PMPs are approximately 100 times more prothrombotic than platelets [76]. The presence of phosphatidyserine on their surface acts as a catalytic site for clotting enzyme assembly and thrombin generation. Interestingly, PAH patients have significantly higher levels of procoagulant phosphatidyserine positive microparticles in the pulmonary artery compared to the jugular vein, in addition to CD105+ EMPs [16]. This suggests either increased production of microparticles in the pulmonary arteries or reduced sequestration. In either case, it is reasonable to hypothesize that these procoagulant microparticles exert localized proinflammatory and prothrombotic effects in the lung. Moreover, PAH patients had elevated levels of TF on the surface of microparticles compared to controls in this study, although no differences were observed between the pulmonary arteries and jugular veins. PMPs express high levels on CD40L on their surface, similar to activated platelets. This represents a large pool of circulating CD40L in addition to soluble CD40L, which can act on vascular cells to mediate activation.
Patients with PAH also have increased expression and activity of CD39 on the surface of microparticles [77]. CD39 is an extracellular membrane bound ectonucleotidase, which catalyzes the dephosphorylation of ATP to ADP and AMP. ATP has potent vasodilatory effects through its stimulation of NO production. Thus, decreased levels of ATP could increase pulmonary resistance in PAH while increased AMP can contribute to platelet activation. On the other hand, adenosine, which is generated from the dephosphorylation of AMP by CD73, is generally anti-inflammatory, anti-thrombotic, and vasodilatory. Thus, increased CD39 expression on microparticles may contribute to the pathogenesis of PAH at its early stages or may rather represent a compensatory mechanism in a patient with severe disease. The clinical significance of altered microparticle number and composition in PAH is unclear, but the data thus far suggest that this is an important area of research to identify 1) potentially important mechanisms of disease establishment and progression; 2) biomarkers that might show response to therapy; 3) a new target for therapeutic intervention.
Conclusion
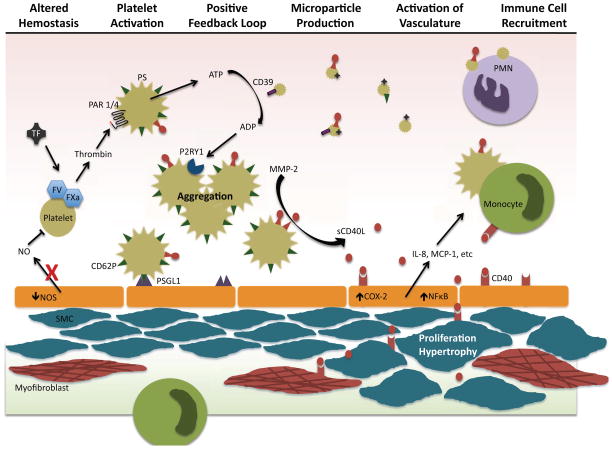
In summary, alterations in hemostasis and coagulation are consistently observed in PAH patients and in preclinical models of PAH. A schematic representation of the potential roles of altered hemostasis, coagulation, and platelet activation in the development and progression of PAH is depicted in Figure 1. Although warfarin is generally recommended for PAH patients, rigorous randomized trials examining its potential benefit are lacking. The recent introduction of novel oral direct factor Xa and thrombin inhibitors offers an attractive strategy for assessing the risks and benefits of anticoagulation therapies in trials with PAH patients. Excess platelet activation has repeatedly been observed, and there are sound theoretical grounds for hypothesizing that excess platelet activation contributes to disease progression. However, further studies are needed to fully understand the role of platelets in the establishment and progression of disease so that we can better design and test a therapeutic approach. Until these studies are completed, we will continue to debate whether platelet activation is primary and contributory or merely an epiphenomenon. Microparticles, platelet, endothelial, and otherwise, are highly pro-thrombotic and often pro-inflammatory. Recent studies suggest that these microscopic structures might be a powerful force in PAH, and several labs are already exploring microparticles for their value as biomarkers or therapeutic targets.
Figure 1. Potential role of platelets in the development and progression of PAH.
Endothelial dysfunction and altered hemostasis leads to an imbalance of inhibitors and activators of platelets. Activated platelets release proinflammatory and prothrombotic mediators and generate highly bioactive platelet microparticles. Activated platelets adhere to endothelial cells via CD62P and its ligand, PSGL1. Large amounts of sCD40L are released from platelets. which induces proinflammatory mediator release, leading to immune cell recruitment. Soluble CD40L further induces smooth muscle cell (SMC) proliferation and hypertrophy. Platelets and platelet microparticles bind to and activate immune cells.
Acknowledgments
This work was supported in part by HL095467, T32A1007285-25 and a grant to the University of Rochester School of Medicine and Dentistry from the Howard Hughes Medical Institute through the Med into Grad Initiative.
Footnotes
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Schermuly RT, et al. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8 (8):443–455. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietra GG, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the NHLBI Primary Pulmonary Hypertension Registry. Circulation. 1989;80(5):1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 3.McMurtry IF, et al. Rho kinase-mediated vasoconstriction in pulmonary hypertension. Advances in Experimental Medicine & Biology. 2010;661:299–308. doi: 10.1007/978-1-60761-500-2_19. [DOI] [PubMed] [Google Scholar]
- 4.Price LC, et al. Inflammation in Pulmonary Arterial Hypertension. Chest. 2012;141 (1):210–221. doi: 10.1378/chest.11-0793. [DOI] [PubMed] [Google Scholar]
- 5.Perros F, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185 (3):311–321. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2004;43 (12 Suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Fuster V, et al. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70 (4):580–587. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 8.Pietra GG, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989;80 (5):1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 9.Wagenvoort CAWN. Primary pulmonary hypertension: A pathologic study of the lung vessels in 156 clinically diagnosed cases. Circulation. 1970;42:1163–1184. [Google Scholar]
- 10.Wagenvoort CA. Lung biopsy specimens in the evaluation of pulmonary vascular disease. Chest. 1980;77 (5):614–625. doi: 10.1378/chest.77.5.614. [DOI] [PubMed] [Google Scholar]
- 11.Bjornsson J, Edwards WD. Primary pulmonary hypertension: a histopathologic study of 80 cases. Mayo Clin Proc. 1985;60 (1):16–25. doi: 10.1016/s0025-6196(12)65277-x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SR, et al. Thrombotic arteriopathy and anticoagulation in pulmonary hypertension. Chest. 2006;130 (2):545–552. doi: 10.1378/chest.130.2.545. [DOI] [PubMed] [Google Scholar]
- 13.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arteriosclerosis, Thrombosis & Vascular Biology. 2004;24 (6):1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 14.White RJ, et al. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293 (3):L583–590. doi: 10.1152/ajplung.00321.2006. [DOI] [PubMed] [Google Scholar]
- 15.Meoli DF, White RJ. Thrombin Induces Fibronectin-Specific Migration of Pulmonary Microvascular Endothelial Cells: Requirement of Calcium-Calmodulin Dependent Protein Kinase II. Am J Physiol Lung Cell Mol Physiol. 2009 doi: 10.1152/ajplung.90598.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakouboula B, et al. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177 (5):536–543. doi: 10.1164/rccm.200706-840OC. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg PR, et al. Fibrinopeptide A levels indicative of pulmonary vascular thrombosis in patients with primary pulmonary hypertension. Circulation. 1990;82 (3):841–847. doi: 10.1161/01.cir.82.3.841. [DOI] [PubMed] [Google Scholar]
- 18.Schulman LL, et al. Platelet activation and fibrinopeptide formation in pulmonary hypertension. Chest. 1993;104 (6):1690–1693. doi: 10.1378/chest.104.6.1690. [DOI] [PubMed] [Google Scholar]
- 19.Tournier A, et al. Calibrated automated thrombography demonstrates hypercoagulability in patients with idiopathic pulmonary arterial hypertension. Thromb Res. 2010;126 (6):e418–422. doi: 10.1016/j.thromres.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Welsh CH, et al. Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension. Chest. 1996;110 (3):710–717. doi: 10.1378/chest.110.3.710. [DOI] [PubMed] [Google Scholar]
- 21.Huber K, et al. Fibrinogen, t-PA, and PAI-1 plasma levels in patients with pulmonary hypertension. Am J Respir Crit Care Med. 1994;150 (4):929–933. doi: 10.1164/ajrccm.150.4.7921465. [DOI] [PubMed] [Google Scholar]
- 22.Miniati M, et al. Fibrin resistance to lysis in patients with pulmonary hypertension other than thromboembolic. Am J Respir Crit Care Med. 2010;181 (9):992–996. doi: 10.1164/rccm.200907-1135OC. [DOI] [PubMed] [Google Scholar]
- 23.Sakamaki F, et al. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation. 2000;102 (22):2720–2725. doi: 10.1161/01.cir.102.22.2720. [DOI] [PubMed] [Google Scholar]
- 24.Badesch DB, et al. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131 (6):1917–1928. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 25.Roman A, et al. Clinico-hemodynamic study and treatment of 44 patients with primary pulmonary hypertension. Med Clin (Barc) 2002;118 (20):761–766. doi: 10.1016/s0025-7753(02)72524-4. [DOI] [PubMed] [Google Scholar]
- 26.Kawut SM, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95 (2):199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Ogata M, et al. Effects of a combination therapy of anticoagulant and vasodilator on the long-term prognosis of primary pulmonary hypertension. Jpn Circ J. 1993;57 (1):63–69. doi: 10.1253/jcj.57.63. [DOI] [PubMed] [Google Scholar]
- 28.Rich S, et al. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327 (2):76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 29.Frank H, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112 (3):714–721. doi: 10.1378/chest.112.3.714. [DOI] [PubMed] [Google Scholar]
- 30.Storstein O, et al. Primary pulmonary hypertension with emphasis on its etiology and treatment. Acta Med Scand. 1966;179 (2):197–212. doi: 10.1111/j.0954-6820.1966.tb05449.x. [DOI] [PubMed] [Google Scholar]
- 31.Delbeck M, et al. A role for coagulation factor Xa in experimental pulmonary arterial hypertension. Cardiovasc Res. 2011;92 (1):159–168. doi: 10.1093/cvr/cvr168. [DOI] [PubMed] [Google Scholar]
- 32.Stuard ID, et al. Microangiopathic hemolytic anemia and thrombocytopenia in primary pulmonary hypertension. N Engl J Med. 1972;287 (17):869–870. doi: 10.1056/NEJM197210262871710. [DOI] [PubMed] [Google Scholar]
- 33.Herve P, et al. Pathobiology of pulmonary hypertension. The role of platelets and thrombosis. Clin Chest Med. 2001;22 (3):451–458. doi: 10.1016/s0272-5231(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 34.Varol E, et al. Platelet indices in patients with pulmonary arterial hypertension. Clin Appl Thromb Hemost. 2011;17 (6):E171–174. doi: 10.1177/1076029610394438. [DOI] [PubMed] [Google Scholar]
- 35.Kamath S, et al. Platelet activation: assessment and quantification. Eur Heart J. 2001;22 (17):1561–1571. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]
- 36.Haznedaroğlu IC, et al. Thrombopoietin inside the pulmonary vessels in patients with and without pulmonary hypertension. Platelets. 2002;13 (7):395–399. doi: 10.1080/0953710021000024000. [DOI] [PubMed] [Google Scholar]
- 37.Weyrich AS, Zimmerman GA. Platelets in lung biology. Annu Rev Physiol. 2013;75:569–591. doi: 10.1146/annurev-physiol-030212-183752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christman BW, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327 (2):70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 39.Ghofrani HA, et al. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5 (8):689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghofrani HA, et al. Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. Journal of the American College of Cardiology. 2004;43(12 Suppl S):68S–72S. doi: 10.1016/j.jacc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Hagan G, Pepke-Zaba J. Pulmonary hypertension, nitric oxide and nitric oxide-releasing compounds. Expert Rev Respir Med. 2011;5 (2):163–171. doi: 10.1586/ers.11.5. [DOI] [PubMed] [Google Scholar]
- 42.Klinger JR, et al. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188 (6):639–646. doi: 10.1164/rccm.201304-0686PP. [DOI] [PubMed] [Google Scholar]
- 43.Tuder RM, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. American Journal of Respiratory & Critical Care Medicine. 1999;159 (6):1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 44.Ferrantino M, White RJ. Inhaled treprostinil sodium for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2011;12 (16):2583–2593. doi: 10.1517/14656566.2011.622269. [DOI] [PubMed] [Google Scholar]
- 45.Moncada S, et al. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263 (5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 46.Whittaker N, et al. The chemical structure of prostaglandin X (prostacyclin) Prostaglandins. 1976;12 (6):915–928. doi: 10.1016/0090-6980(76)90126-x. [DOI] [PubMed] [Google Scholar]
- 47.Kawut SM, et al. von Willebrand factor independently predicts long-term survival in patients with pulmonary arterial hypertension. Chest. 2005;128 (4):2355–2362. doi: 10.1378/chest.128.4.2355. [DOI] [PubMed] [Google Scholar]
- 48.Lopes AA, et al. Plasma von Willebrand factor as a predictor of survival in pulmonary arterial hypertension associated with congenital heart disease. Braz J Med Biol Res. 2011;44 (12):1269–1275. doi: 10.1590/s0100-879x2011007500149. [DOI] [PubMed] [Google Scholar]
- 49.Damås JK, et al. Soluble CD40 ligand in pulmonary arterial hypertension: possible pathogenic role of the interaction between platelets and endothelial cells. Circulation. 2004;110 (8):999–1005. doi: 10.1161/01.CIR.0000139859.68513.FC. [DOI] [PubMed] [Google Scholar]
- 50.Phipps RP. Atherosclerosis: the emerging role of inflammation and the CD40-CD40 ligand system. Proc Natl Acad Sci U S A. 2000;97 (13):6930–6932. doi: 10.1073/pnas.97.13.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fries KM, et al. CD40 expression by human fibroblasts. Clin Immunol Immunopathol. 1995;77 (1):42–51. doi: 10.1016/0090-1229(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, et al. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J Immunol. 1998;160 (3):1053–1057. [PubMed] [Google Scholar]
- 53.Beikmann BS, et al. Serotonin uptake is largely mediated by platelets versus lymphocytes in peripheral blood cells. ACS Chem Neurosci. 2013;4 (1):161–170. doi: 10.1021/cn300146w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Launay JM, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8 (10):1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- 55.Voelkel NF. Appetite suppressants and pulmonary hypertension. Thorax. 1997;52(Suppl 3):S63–67. doi: 10.1136/thx.52.2008.s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eddahibi S, et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. Journal of Clinical Investigation. 2001;108(8):1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulrich S, et al. Platelet serotonin content and transpulmonary platelet serotonin gradient in patients with pulmonary hypertension. Respiration. 2011;81 (3):211–216. doi: 10.1159/000314271. [DOI] [PubMed] [Google Scholar]
- 58.Hervé P, et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99 (3):249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- 59.Aytekin M, et al. Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: role of nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2012;302 (6):L512–520. doi: 10.1152/ajplung.00289.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maeda NY, et al. Increased tyrosine phosphorylation of platelet proteins including pp125(FAK) suggests endogenous activation and aggregation in pulmonary hypertension. Clin Appl Thromb Hemost. 2005;11 (4):411–415. doi: 10.1177/107602960501100407. [DOI] [PubMed] [Google Scholar]
- 61.Shattil SJ, et al. Integrin signaling: the platelet paradigm. Blood. 1998;91 (8):2645–2657. [PubMed] [Google Scholar]
- 62.Rajkumar R, et al. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;298 (4):H1235–1248. doi: 10.1152/ajpheart.00254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Léon C, et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J Clin Invest. 1999;104 (12):1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sambrano GR, et al. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413 (6851):74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 65.Robbins IM, et al. A study of aspirin and clopidogrel in idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;27 (3):578–584. doi: 10.1183/09031936.06.00095705. [DOI] [PubMed] [Google Scholar]
- 66.Kawut SM, et al. Randomized clinical trial of aspirin and simvastatin for pulmonary arterial hypertension: ASA-STAT. Circulation. 2011;123 (25):2985–2993. doi: 10.1161/CIRCULATIONAHA.110.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ratajczak J, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20 (9):1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 68.Hargett LA, Bauer NN. On the origin of microparticles: From “platelet dust” to mediators of intercellular communication. Pulm Circ. 2013;3 (2):329–340. doi: 10.4103/2045-8932.114760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahler J, et al. A novel method for overexpression of peroxisome proliferator-activated receptor-γ in megakaryocyte and platelet microparticles achieves transcellular signaling. J Thromb Haemost. 2012;10 (12):2563–2572. doi: 10.1111/jth.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amabile N, et al. Microparticles: key protagonists in cardiovascular disorders. Semin Thromb Hemost. 2010;36 (8):907–916. doi: 10.1055/s-0030-1267044. [DOI] [PubMed] [Google Scholar]
- 71.Koga H, et al. Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur Heart J. 2006;27 (7):817–823. doi: 10.1093/eurheartj/ehi746. [DOI] [PubMed] [Google Scholar]
- 72.Rak J. Microparticles in cancer. Semin Thromb Hemost. 2010;36 (8):888–906. doi: 10.1055/s-0030-1267043. [DOI] [PubMed] [Google Scholar]
- 73.Amabile N, et al. Cellular microparticles in the pathogenesis of pulmonary hypertension. Eur Respir J. 2013;42 (1):272–279. doi: 10.1183/09031936.00087212. [DOI] [PubMed] [Google Scholar]
- 74.Diehl P, et al. Enhanced microparticles in ventricular assist device patients predict platelet, leukocyte and endothelial cell activation. Interact Cardiovasc Thorac Surg. 2010;11 (2):133–137. doi: 10.1510/icvts.2010.232603. [DOI] [PubMed] [Google Scholar]
- 75.Nadaud S, et al. Small platelet microparticle levels are increased in pulmonary arterial hypertension. Eur J Clin Invest. 2013;43 (1):64–71. doi: 10.1111/eci.12018. [DOI] [PubMed] [Google Scholar]
- 76.Sinauridze EI, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97 (3):425–434. [PubMed] [Google Scholar]
- 77.Visovatti SH, et al. Increased CD39 nucleotidase activity on microparticles from patients with idiopathic pulmonary arterial hypertension. PLoS One. 2012;7 (7):e40829. doi: 10.1371/journal.pone.0040829. [DOI] [PMC free article] [PubMed] [Google Scholar]