Abstract
Background
Toll-like receptor (TLR)-mediated inflammatory processes are supposed to be involved in pathophysiology of spontaneous abortion and preterm labor. Here, we investigated functional responses of human endometrial stromal cells (ESCs) and whole endometrial cells (WECs) to lipopolysaccharide (LPS) and lipoteichoic acid (LTA).
Methods
Endometrial tissues were obtained from 15 cycling women who underwent laparoscopic tubal ligation. Modulation of TLR2, TLR4 and MyD88 expression and production of pro-inflammatory cytokines by WECs and ESCs in response to LPS and LTA were assessed.
Results
WECs and ESCs expressed significant levels of TLR4 and MyD88 transcripts but, unlike WECs, ESCs failed to express TLR2 gene. Regardless of positive results of Western blotting, ESCs did not express TLR4 at their surface as judged by flow cytometry. Immunofluorescent staining revealed intracellular localization of TLR4 with predominant perinuclear pattern. LPS stimulation marginally increased TLR4 gene expression in both cell types, whereas such treatment significantly upregulated MyD88 gene expression after 8 hr (p < 0.05). At the protein level, however, LPS activation significantly increased TLR4 expression by ESCs (p < 0.05). LTA stimulation of WECs was accompanied with non-significant increase of TLR2 and MyD88 transcripts. LPS and LTA stimulation of WECs caused significant production of IL-6 and IL-8 in a dose-dependent manner (p < 0.05). Similarly, ESCs produced significant amounts of IL-6, IL-8 and also TNF-α in response to LPS activation (p < 0.05).
Conclusion
Our results provided further evidence of initiation of inflammatory processes following endometrial TLR activation by bacterial components which could potentially be harmful to developing fetus.
Keywords: Cytokine, Endometrium, Inflammation, LPS, LTA, Toll-like receptor
Introduction
As a site for implantation of semiallogeneic fetus, endometrium possesses all requirements for controlling devastating immune responses against developing embryo and at the same time hinders outgrowth of ascending pathologic microorganisms by mounting effective innate and adaptive immune responses (1–3). In contrast to the lower parts of female reproductive tract (FRT), endometrium is a microorganism-free environment, a condition which is necessary for safe guarding the embryo (4). Nevertheless, endometrium is potentially susceptible to the ascending expansion of microbes residing in the external genitalia of the pregnant women and such infections could potentially lead to such pregnancy complications as abortion, preterm labor, infertility, and ectopic pregnancy (5–7). Although endometrium is an immunocompetent tissue equipped with different immune cells of adaptive arm of immune system (8–10), such defense mechanisms usually need the rapid action of preset arm of immune system such as Toll-like receptors (TLRs).
TLRs are evolutionarily conserved innate receptors with specificity to microbial determinants and are expressed in different immune and non-immune cells. The main function for TLRs is recognition of pathogen associated molecular patterns (PAMPs) of invading microbes. After ligation, the majority of TLRs induce downstream activation of NFκB and cytokine production in MyD88-dependent pathway (11).
Not only immune cells, but different non-immune cells at the maternal-fetal interface, mainly trophoblasts, express TLRs (12–14). In contrast to placental tissue, data about the expression of TLRs in the human endometrium is limited. Gene expression analysis demonstrated the presence of TLRs in human endometrium (15, 16), a finding which was confirmed by immunohistochemical localization at different regions of human female reproductive tract (16, 17).
Several studies have demonstrated a firm association between pregnancy disorders and intrauterine infections (18, 19). Functional TLR4 has been implicated in preterm labor triggered by administration of heat-killed Escherichia coli to mice (20). There are also some reports on functional consequences of endometrial TLR activation in animal models (21, 22). However, a big gap is present for such data in humans. So, the present study tested the TLR2, TLR4 and MyD88 expression in endometrial stromal cells (ESCs) and whole endometrial cells (WECs) and the inflammatory upshot of TLR2- and TLR4-dependent activation of these cell populations by LTA and LPS, respectively. Furthermore, feedback regulatory mechanism of TLR2, TLR4 and MyD88 expression after ligation with their cognate ligands was scrutinized in primary endometrial cells.
Methods
Study population
Endometrial tissues were obtained from fifteen apparently normal women under 45 years of age referring to a gynecology clinic for hysteroscopic tubal ligation who had no evidence of pathology in their hysteroscopic evaluations. These women had no history of infertility or miscarriage, stillbirth and preeclampsia in their previous pregnancies. Also, participants had no sign of vaginal infection or confounding pathology such as myoma in their endometrium as judged by clinical and ultrasound examinations. All subjects had regular menstrual cycles and had not used hormones for at least 3 months. This study was approved by the institutional review board and ethics committee of Avicenna Research Institute and Tehran University of Medical Sciences and informed consent was taken from all subjects enrolled in the study.
Sample collection and processing
Endometrial biopsies were obtained using endometrial biopsy curette in proliferative phase of menstrual cycle and immediately transferred on ice to the laboratory in tissue culture medium containing antibiotics. Tissue samples were divided into three parts. A small fragment was processed for pathologic examination. The remaining tissue fragment was immersed in RNAlater and frozen at −20°C for RNA extraction. From remaining tissue, single cells were obtained by enzymatic digestion.
Isolation and culture of WECs and ESCs
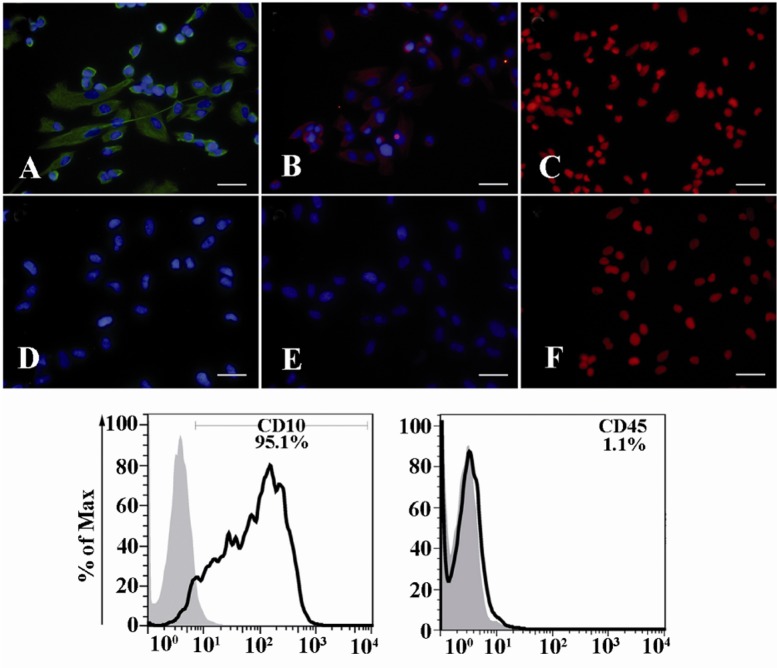
Whole endometrial cells (WECs) and endometrial stromal cells (ESCs) were separated by enzymatic digestion according to the established protocol. In brief, endometrial tissues were minced into small pieces and digested at 37°C for 1.5 hr in collagenase D 0.2% and DNAse 0.03%. Viability of single cell suspension was determined by trypan blue exclusion. Digested single cell suspension was used as WECs. For isolation of ESCs, cells were cultivated in 24-well culture plate at 37°C, 5% CO2 in DMEM-F12 supplemented with 10% fetal calf serum (FCS) and additives. Non-adherent cells were removed by several washes and adherent stromal cells were allowed to propagate. The ESCs were characterized as vimentin+, nestin+, cytokeratin-, CD10+ and CD45- cells (Figure 1) (23).
Figure 1.
Characterization of ESCs. ESCs were purified from endometrial samples and their characteristics were determined as vimentin+; A: Nestin+; b: and cytokeratin-; C: by immunofluorescent staining (upper panel) and CD10+ and CD45- by flow cytometry (lower panel). D-F: are corresponding reagent negative controls for vimentin, Nestin and cytokeratin stainings, respectively. Nuclei were either stained with 7AAD (red) or DAPI (blue). Scale bare: 50 μm
RNA extraction, cDNA synthesis and reverse transcriptase polymerase chain reaction (RT-PCR)
RNA extraction, cDNA synthesis and RT-PCR were performed according to the protocols published elsewhere (24, 25). Oligonucleotide primers used in this study were as follows: β-actin: F;5'-GTG-GGG-CGC-CCC-AGG-CAC-CA-3', R; 3'- CTC- CTT- AAT-GTC-ACG- CAC- GAT-TTC-5', TLR2: F; 5'-GTA-CCT-GTG-GGG-CTC-ATT-GT-3', R; 3'-CTG-CCC-TTG-CAG-ATA-CCA-TT-5', TLR4: F; 5'-GAG-CTT-TAA-TCC-CCT-GAG-GCA-3', R; 3'-GAT-TGG-ATA-AGA-TTG-TGA-GCC-5' and MyD88:F;5'-GAC-CCA-GCA-TTG-AGG-A GG-ATT-G-3', R;3'-GCT-TCT-GAT-GGG-CAC-CTG-GAG-AG-5'. After initial heating at 94°C for 3 min, PCR was performed for 30 (for β-actin) or 35 cycles (for TLR2, TLR4 and MyD 88) with annealing temperatures of 62°C, 54.8°C, 60°C and 60°C for TLR2, TLR4, MyD88 and β-actin, respectively. For all PCR programs, denaturation and extension temperatures of 94°C and 72°C, respectively were applied for 30 s. Final extension for 7 min was performed for all amplifications. PCR systems devoid of template cDNA and no amplification controls (no reverse transcriptase added) were included as negative controls. cDNA prepared from PBMCs served as the positive control.
Flow cytometric analysis of TLR2 and TLR4 expression
For TLR2 staining, 5×105 living cells were incubated with 5 μg/ml of Alexa fluor 488-conjugated anti-TLR2 antibody (BD) diluted in PBS-BSA for 30 min. All incubations were performed on ice. For TLR4 staining, cells were first incubated with biotin-conjugated anti-TLR4 (BD) for 60 min. After three washes, cells were stained with PE-Cy5.5-conjugated streptavidin (Invitrogen, USA) for 30 min. In negative reagent control tubes, isotype matched irrelevant antibodies (BD) were used instead of primary antibodies. Monocyte gate of PBMC served as the positive control. After three washing steps with PBS-BSA, cells were analyzed by Partec flowcytometer (Partec, Germany).
Western blot analysis of TLR2 and TLR4 expression
Western blotting of TLR2 and TLR4 in ESCs was performed according to the protocol published recently with some modifications (25). Blots were incubated for 2 hr with agitation in goat anti-TLR4 antibody (R&D, USA) at 1 μg/ml or 1:100 dilution of goat anti-TLR2 (Santa Cruz, USA) followed by 45 min incubation in HRP-conjugated rabbit anti-goat Ig (Sinabiotech, Iran). Bands were detected using an ECL Western blotting substrate kit and digital images were obtained with a Gel Logic 2200 imaging system (Kodak, Tokyo, Japan). Membranes were later stripped using Western Re-Probe (Calbiochem, SanDiego, CA, USA), re-incubated for 2 hr with agitation in polyclonal rabbit anti-beta actin antibody (Sigma) as loading control followed by 45 min incubation in HRP-conjugated sheep anti-rabbit Ig (Sinabiotech, Iran) and processed as above. In negative control blots, primary antibodies were substituted by equivalent dilutions of species-matched normal sera. Band densities of PCR and Western blot experiments were quantified as described elsewhere (25).
Immunofluorescent staining
Immunofluorescent staining of TLR2 and TLR4 was performed as described elsewhere (26). Briefly, cells were cyto-spinned and fixed with either ice cold acetone (for TLR2) or natural buffered formalin (for TLR4). Primary antibodies (goat anti-TLR2, 1:100 for overnight or goat anti-TLR4, 10 µg/ml for 3 hr) were added followed by 1:50 dilution of FITC-labeled rabbit anti-goat Ig for 60 min. Cells were then washed and counterstained with 4'-6-diamidino-2-phenylindole (DAPI) for nucleus staining. Fluorescent signal was visualized and photographed by a BX51 Olympus microscope equipped with a DP71 CCD camera.
LPS and LTA treatment of ESCs and WECs
Based on the results of gene and protein expression analysis, WECs were stimulated separately with both LPS and LTA, while ESCs were treated only with LPS. To study the effect of TLR engagement on cytokine production, cells (1×105/well in 96-well plate) were treated with different concentrations of LPS (0.1 ng/ml-10000 ng/ml) or LTA (0.1 ng-1000 ng/ml) for 24 hr and after that cell culture supernatants were collected, centrifuged and stored at −70°C for future analysis. Indeed, to evaluate the effect of LPS and LTA stimulation on TLR4, TLR2 and MyD88 gene expression, WECs were stimulated with LPS (100 ng/ml) or LTA (10000 ng/ml) for 8 hr followed by cell harvesting and gene expression analysis as described above. Control cells received PBS only. ESCs, however, were treated with LPS for extended time periods of 2 hr, 4 hr, 8 hr, 12 hr and 24 hr.
Measurement of cytokines
Concentrations of TNF-α, IL-6 and IL-8 in supernatant of cultured cells were measured using ELISA according to the protocol provided by the manufacturer. The sensitivity of the assays for TNF-α, IL-6 and IL-8 were 7.5, 3.1 and 3.1 pg/ml, respectively.
Statistical analysis
All data are presented as median±range. Pairwise comparisons of gene expression and cytokine production after TLR stimulation was performed using non-parametric Wilcoxon rank test and statistically significant differences were accepted at p < 0.05.
Results
Expression of TLR2, TLR4, and MyD88 in WECs and ESCs
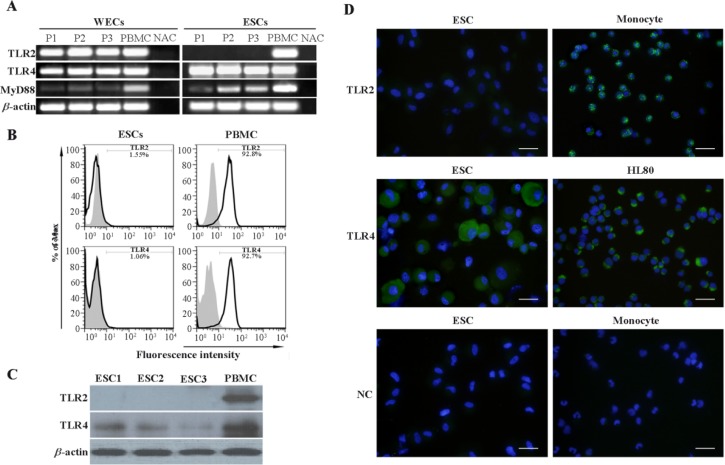
TLR4, TLR2 and MyD88 transcripts were detected in samples derived from human endometrium. Likewise, it was demonstrated that ESCs expressed TLR4 and MyD88 at mRNA level but failed to express TLR2 transcript (Figure 2A). To confirm the results of gene expression analysis of TLR4 and TLR2, three readout systems, flow cytometry, Western blotting and immunofluorescent staining were used. The results of flow cytometric analysis showed that ESCs expressed neither TLR4 nor TLR2 at their surface (Figure 2B). Western blotting clearly demonstrated TLR4, but not TLR2 protein expression by ESCs (Figure 2C). Consistent with flow cytometry results, no surface expression of TLR4 was observed; instead TLR4 was localized in perinuclear compartment. In line with Western blot results, ESCs failed to express TLR2 protein in immunofluorescent staining (Figure 2D).
Figure 2.
Assessment of TLR2, TLR4 and MyD88 expression by ESCs and WECs. A: Assessment of TLR2, TLR4 and MyD88 transcripts expression by ESCs and WECs using RT-PCR. B: Flow cytometric analysis of TLR2 and TLR4 expression by ESCs. C: Western blot analysis of TLR2 and TLR4 expression by ESCs. In RT-PCR and Western blot analyses, PBMC was used as positive control. Monocyte gate of PBMC served as positive area in flow cytometry. D: Immunofluorescent staining of TLR2 and TLR4 in ESCs. Monocytes and HL60 cells were used as positive cell controls for TLR2 and TLR4 immunofluorescent stainings, respectively. Reagent negative control (NC) slides received isotype- matched preimmune normal serum. Nuclei were counterstained with DAPI. P1-3: Three representative participants 1-3, ESCs1-3: Endometrial stromal cells from three representative participants, PBMC: Peripheral blood mononuclear cells, NAC: No amplification control
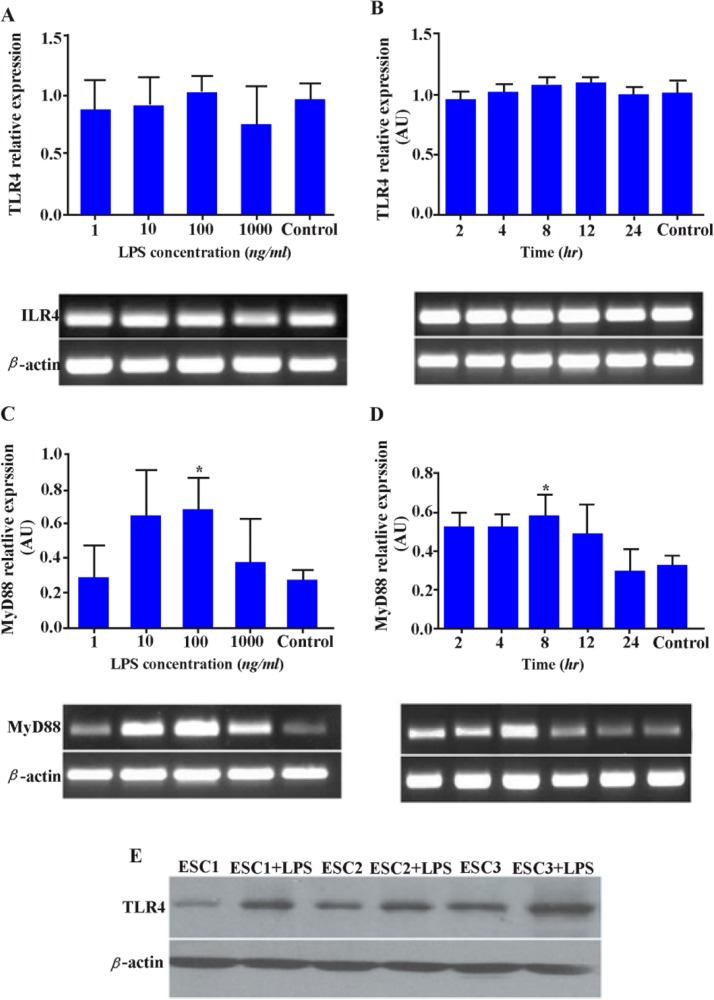
Effect of LPS on TLR4 and MyD88 expression in ESCs
The results showed that different concentrations of LPS at different time intervals had only marginal effect on TLR4 gene up-regulation (Figures 3A, B). At the protein level, however, expression of TLR4 was significantly increased by LPS activation (p < 0.05) (Figure 3E). Treatment of this cell type with 100 ng/ml of LPS significantly increased MyD88 expression up to 8 hr after LPS stimulation (p < 0.05) (Figures 3C, D). More importantly, effect of LPS stimulation on MyD88 expression was gradually subsided thereafter reaching to the control level after 24 hr.
Figure 3.
Effect of LPS on TLR4 and MyD88 expression in ESCs. ESCs were stimulated with different concentrations of LPS for 8 hr or with 100 ng/ml at different time intervals (2-24 hr) and the expression of TLR4; A, B: and MyD88 transcripts; C, D: was then assessed. Control wells received vehicle. Representative TLR4 and MyD88 PCR bands are shown at the bottom of each graph. E: Effect of LPS activation on TLR4 protein expression by ESCs was evaluated by Western blotting. ESCs1-3: Endometrial stromal cells from three representative participants. AU: Arbitrary unit, *: p < 0.05
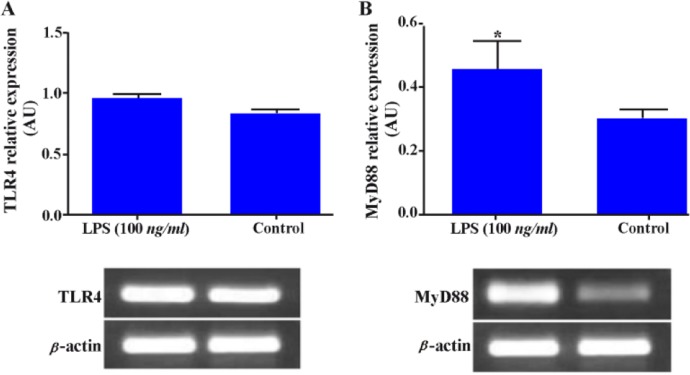
Effect of LPS on TLR4 and MyD88 expression in WECs
Akin to ESCs, expression of TLR4 and MyD88 in WECs was investigated after LPS stimulation. Whole endometrial cells were treated with 100 ng/ml LPS for 8 hr and the expression of TLR4 and MyD88 transcripts were then investigated. In parallel with the results from ESCs, it was demonstrated that LPS treatment marginally increased TLR4 gene expression but significantly intensified the expression of MyD88 transcript (p < 0.05) (Figures 4A, B).
Figure 4.
Effect of LPS on TLR4 and MyD88 gene expression in WECs. WECs were stimulated with LPS (100 ng/ml) for 8 hr and expression of TLR4; A: and MyD88; B: genes was then assayed by RT-PCR. Representative TLR4 and MyD88 PCR bands are shown at the bottom of each graph. AU: Arbitrary unit, *: p < 0.05
Effect of LTA on TLR2 and MyD88 gene expression in WECs
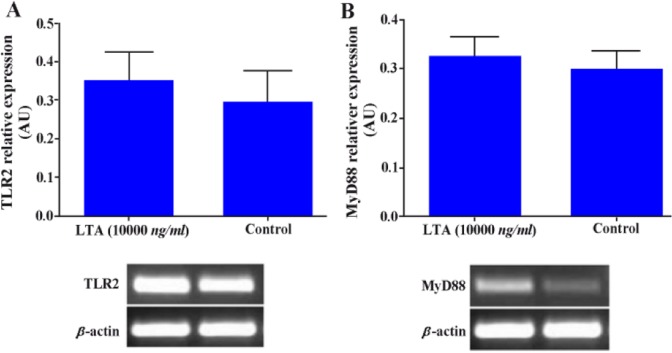
Lower female reproductive tract is more frequently populated with Gram positive bacteria. With this end in view, WECs were also stimulated with LTA to see the effect of such treatment on TLR2 and MyD88 expression. Although such treatment increased the levels of TLR2 and MyD88 gene expression, but this did not reach statistically significant level (Figures 5A, B).
Figure 5.
Effect of LTA on TLR2 and MyD88 gene expression in WECs. WECs were treated with LTA (10000 ng/ml) for 8 hr and expression of TLR2 and MyD88 genes was evaluated by RT-PCR. Representative TLR2 and MyD88 PCR bands are shown at the bottom of each graph. AU: Arbitrary unit
Effect of LPS on cytokine production by ESCs
Endometrial stromal cell cultures were established and analyzed for the potential effect of LPS at different concentrations on pro-inflammatory cytokine production by this cell population. The results showed that ESCs produce significantly higher amounts of IL-6, IL-8 and TNF-α in response to LPS stimulation in a dose-dependent manner (p < 0.05) (Figures 6A–C).
Figure 6.
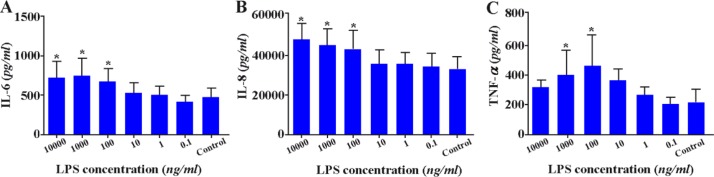
Effect of LPS on cytokine production by ESCs. ESCs were treated with different concentrations of LPS and the levels of IL-6; A:, IL-8; B: and TNF-α; C: were measured by capture ELISA. Control wells received vehicle *: P < 0.05
Effect of LPS and LTA on cytokine production by WECs
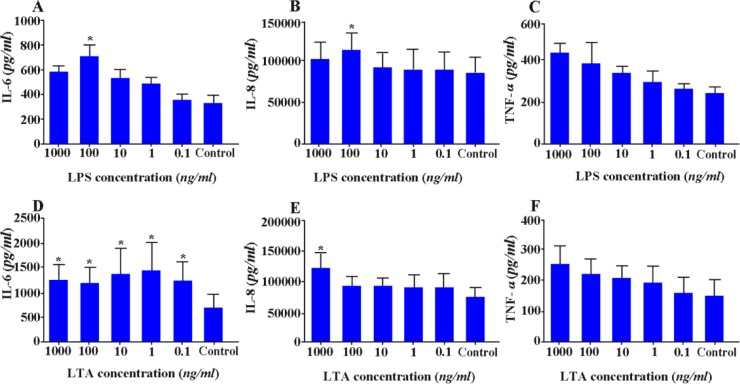
Stimulation with 100 ng/ml LPS and all concentration range of LTA significantly increased production of IL-6 in WECs (p < 0.05) (Figures 7A, D). Similar results for IL-8 production were also achieved when WECs were stimulated with the same concentration of LPS or 1000 ng/ml LTA (p < 0.05) (Figures 7B, E). Although LPS and LTA stimulation increased TNF-α production by WECs, the results were not statistically significant (Figures 7C, F).
Figure 7.
Effect of LPS and LTA on cytokine production by WECs. WECs were treated with different concentrations of LPS; A-C: or LTA; D-F: and the levels of IL-6; A, D: IL-8; B, E: and TNF-α; C, F: were measured by capture ELISA. Control wells received vehicle *: P < 0.05
Discussion
Ascending bacterial infection of the upper FRT is considered to be a major cause of pelvic inflammatory disease (PID) in women (27). Bacterial infection of FRT during pregnancy is also an important cause of abortion and preterm labor (6, 7, 22, 28). Therefore, the present study revealed some new aspects of functional consequences of TLR4 and TLR2 activation by Gram negative and Gram positive bacterial LPS and LTA in human endometrium and endometrial stromal cells. It was demonstrated that ESCs expressed TLR4 and MyD88 transcripts, while WECs also expressed TLR2 mRNA. This finding implies that cells other than ESCs in endometrium express TLR2. Endometrium contains such immune cells as macrophage and dendritic cells (8, 9, 29) that are known to express TLR2 (30, 31).
No specific report was found on TLR2 protein expression by human endometrial stromal cells. Hirata et al. (15) reported TLR2 message expression by ESCs. Fazeli et al. (17) characterized immunolocalization of TLR1-6 in human endometrium by immunohistochemistry. However, they did not specify TLR2 protein expression by this cell type. Interestingly, TLR2 protein was shown not to be expressed by ESCs of dogs at all stages of estrous cycle (32).
In line with our findings, previous studies showed TLR2 and TLR4 genes (3, 16, 33) and proteins (3) expression in primary endometrial tissues. It has been reported that endometrial glands and epithelial cells express TLR4 protein, (17) but the levels of expression were higher in endometrial stromal cells than in endometrial epithelial cells (15), a finding that is in line with what was found in this study.
Positively-identified TLR4 expression in human ESCs by Western blotting was not accompanied by cell surface expression. We found no report on immunolocalization of TLR4 in ESCs. While TLR4 is usually expressed on the cell surface of immune cells, there are a number of reports on intracellular expression of these receptors in various cell types such as intestinal epithelial cells (34). Interestingly, LPS-induced TLR4 signaling in intestinal epithelial cells occurs at the site of the Golgi apparatus and LPS-mediated cellular activation requires ligand internalization that occurs via a lipid raft-dependent formation of clathrin-coated pits and intracellular transport to the Golgi compartment (34). Taken together, these findings on spatial patterns of TLR2 and 4 expressions may imply fine-tuning of innate immunity in the endometrium.
WECs expressed MyD88, a common adaptor protein for TLRs that mediates intracellular signal transduction. Regardless of lacking TLR2 expression, ESCs also exhibited this molecule that might be due to the unique attribute of MyD88 that is not specific to TLR2.
Next, the putative function of bacterial LPS and LTA was tested following engagement with their cognate receptor in WECs and ESCs. The results of the present study showed that LPS increased production of IL-6 and IL-8 in ESCs and WECs and also TNF-α in ESCs. WECs also exhibited increased production of IL-6 and IL-8 in response to LTA.
Endometrium is able to secrete a broad array of cytokines and chemokines including IL-8, MCP-1, macrophage inflammatory protein-1b (MIP-1b), IL-6, TNF-α, granulocyte colony-stimulating factor (G-CSF), granulocyte- macrophage colony-stimulating factor (GM-CSF), and migration inhibitory factor (MIF). These mediators, in turn, recruit and stimulate the immune cells. In response to these cytokine stimulation, immune cells up-regulate the secretion of these and other pro-inflammatory cytokines and induce differentiation of leukocytes to a more functionally active cell, such as phagocytosis for macrophages or antigen presentation for DCs (1). More importantly, such cytokines as IL-6, IL-8 and TNF-α are reported to be constitutively produced and secreted by human endometrial epithelial cells allowing for immediate responsiveness to pathogenic microbes (35). Despite these necessary immunological functions, pro-inflammatory cytokines and chemokines are among the important insults for the developing embryo. High levels of IL-6, IL-8 and MCP-1 in cervicovaginal fluid and amniotic fluid from patients with preterm labor and premature rupture of membranes have been correlated well with amniotic microbial infection (36–38). Increased levels of TNF-α have been associated with pregnancy complications such as menorrhagia, endometriosis, chorioamnionitis, miscarriage, preterm labor, preeclampsia and intrauterine growth retardation (IUGR) (39). Taken together, stimulatory activity of TLR2 and 4 ligands on proinflammatory cytokine production by WECs in general and ESCs in particular may be considered as one mechanistic explanation of bacterial infection-associated pregnancy complications.
To investigate regulatory effect of ligand stimulation on TLR expression, endometrial cells were treated with LPS and LTA and the expression of TLR4, TLR2 and MyD88 was then quantified. It was demonstrated that LPS treatment significantly increased MyD88 gene expression in WECs and ESCs as early as 8 hr. Impotently, LPS treatment of ESCs significantly increased TLR4 expression at the protein level and not at the gene level. Such discrepancy in the levels of TLR4 gene and protein expression in ESCs following LPS activation could be due to instability of some sorts of mRNA. Importantly, because MyD88 resides in the common signaling pathway of many TLRs, it is conceivable to imagine that increased expression of this adaptor protein following LPS stimulation not only increases the responsiveness of TLR4, but also positively affects the response of other TLRs with the same signaling pathway to their cognate ligands. Fazeli et al. reported that TLR5 stimulation in endometrial cell lines acted to up-regulate TLR5 expression (40). Whether alteration of TLR and MyD88 expression in endometrial cells following activation by TLR ligands is beneficial or harmful for developing embryo is not clear at present and warrants further investigations.
Conclusion
In conclusion, our results provided further evidence of initiation of inflammatory processes following endometrial TLR activation by bacterial components which could potentially be harmful to the developing fetus. TLR-mediated immune responses at the fetomaternal interface may act as a double-edged sword and depending on the condition could result in beneficial or harmful consequences.
Acknowledgement
The present research study is a part of M.Sc. thesis of Mrs. Nesa Rashidi. The authors would like to thank the women participated in this study. This work was supported by grants from Avicenna Research Institute and Tehran University of Medical Sciences.
To cite this article: Rashidi N, Mirahmadian M, Rezania S, Ghasemi J, Kazemnejad S, Mirzadegan E, et al. Lipopolysaccharide- and Lipoteichoic Acid-mediated Pro-inflammatory Cytokine Production and Modulation of TLR2, TLR4 and MyD88 Expression in Human Endometrial Cells. J Reprod Infertil. 2015;16(2):72-81.
Conflict of Interest
The authors of the study have no conflict of interest to report.
References
- 1.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–35. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 2.Johansson M, Lycke NY. Immunology of the human genital tract. Curr Opin Infect Dis. 2003;16(1):43–9. doi: 10.1097/00001432-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Lin Z, Xu J, Jin X, Zhang X, Ge F. Modulation of expression of Toll-like receptors in the human endometrium. Am J Reprod Immunol. 2009;61(5):338–45. doi: 10.1111/j.1600-0897.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- 4.Sheldon IM, Bromfield JJ. Innate immunity in the human endometrium and ovary. Am J Reprod Immunol. 2011;66(Suppl 1):63–71. doi: 10.1111/j.1600-0897.2011.01034.x. [DOI] [PubMed] [Google Scholar]
- 5.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206–15. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 7.McDonald HM, Chambers HM. Intrauterine infection and spontaneous midgestation abortion: is the spectrum of microorganisms similar to that in preterm labor? Infect Dis Obstet Gynecol. 2000;8(5-6):220–7. doi: 10.1155/S1064744900000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Jeddi-Tehrani M. Kinetics of murine decidual dendritic cells. Reproduction. 2007;133(1):275–83. doi: 10.1530/rep.1.01232. [DOI] [PubMed] [Google Scholar]
- 9.Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Jeddi Tehrani M. Analysis of endometrial myeloid and lymphoid dendritic cells during mouse estrous cycle. J Reprod Immunol. 2006;71(1):28–40. doi: 10.1016/j.jri.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Ochiel DO, Fahey JV, Ghosh M, Haddad SN, Wira CR. Innate Immunity in the Female Reproductive Tract: Role of Sex Hormones in Regulating Uterine Epithelial Cell Protection Against Pathogens. Curr Womens Health Rev. 2008;4(2):102–117. doi: 10.2174/157340408784246395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian C, Cao X. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann N Y Acad Sci. 2013;1283:67–74. doi: 10.1111/j.1749-6632.2012.06786.x. [DOI] [PubMed] [Google Scholar]
- 12.Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekstrom ES, et al. Expression and regulation of the pattern recognition receptors Tolllike receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107(1):145–51. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klaffenbach D, Rascher W, Rollinghoff M, Dotsch J, Meissner U, Schnare M. Regulation and signal transduction of toll-like receptors in human chorioncarcinoma cell lines. Am J Reprod Immunol. 2005;53(2):77–84. doi: 10.1111/j.1600-0897.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 14.Mitsunari M, Yoshida S, Shoji T, Tsukihara S, Iwabe T, Harada T, et al. Macrophage-activating lipopeptide-2 induces cyclooxygenase-2 and prostaglandin E(2) via toll-like receptor 2 in human placental trophoblast cells. J Reprod Immunol. 2006;72(1-2):46–59. doi: 10.1016/j.jri.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Hirata T, Osuga Y, Hamasaki K, Hirota Y, Nose E, Morimoto C, et al. Expression of toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. J Reprod Immunol. 2007;74(1-2):53–60. doi: 10.1016/j.jri.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Aflatoonian R, Tuckerman E, Elliott SL, Bruce C, Aflatoonian A, Li TC, et al. Menstrual cycle dependent changes of Toll-like receptors in endometrium. Hum Reprod. 2007;22(2):586–93. doi: 10.1093/humrep/del388. [DOI] [PubMed] [Google Scholar]
- 17.Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20(5):1372–8. doi: 10.1093/humrep/deh775. [DOI] [PubMed] [Google Scholar]
- 18.Aldo PB, Krikun G, Visintin I, Lockwood C, Romero R, Mor G. A novel three-dimensional in vitro system to study trophoblast-endothelium cell interactions. Am J Reprod Immunol. 2007;58(2):98–110. doi: 10.1111/j.1600-0897.2007.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arechavaleta-Velasco F, Gomez L, Ma Y, Zhao J, McGrath CM, Sammel MD, et al. Adverse reproductive outcomes in urban women with adenoassociated virus-2 infections in early pregnancy. Hum Reprod. 2008;23(1):29–36. doi: 10.1093/humrep/dem360. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69(6):1957–63. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- 21.Herath S, Fischer DP, Werling D, Williams EJ, Lilly ST, Dobson H, et al. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology. 2006;147(1):562–70. doi: 10.1210/en.2005-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheldon IM, Roberts MH. Toll-like receptor 4 mediates the response of epithelial and stromal cells to lipopolysaccharide in the endometrium. PLoS One. 2010;5(9):e12906. doi: 10.1371/journal.pone.0012906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavakoli M, Jeddi-Tehrani M, Salek-Moghaddam A, Rajaei S, Mohammadzadeh A, Sheikhhasani S, et al. Effects of 1,25(OH)2 vitamin D3 on cytokine production by endometrial cells of women with recurrent spontaneous abortion. Fertil Steril. 2011;96(3):751–7. doi: 10.1016/j.fertnstert.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 24.Zarnani AH, Shahbazi M, Salek-Moghaddam A, Zareie M, Tavakoli M, Ghasemi J, et al. Vitamin D3 receptor is expressed in the endometrium of cycling mice throughout the estrous cycle. Fertil Steril. 2010;93(8):2738–43. doi: 10.1016/j.fertnstert.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 25.Shahbazi M, Jeddi-Tehrani M, Zareie M, Salek-Moghaddam A, Akhondi MM, Bahmanpoor M, et al. Expression profiling of vitamin D receptor in placenta, decidua and ovary of pregnant mice. Placenta. 2011;32(9):657–64. doi: 10.1016/j.placenta.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Delbandi AA, Mahmoudi M, Shervin A, Akbari E, Jeddi-Tehrani M, Sankian M, et al. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertil Steril. 2013;100(3):761–9. doi: 10.1016/j.fertnstert.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 27.Ross JD. An update on pelvic inflammatory disease. Sex Transm Infect. 2002;78(1):18–9. doi: 10.1136/sti.78.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamicpituitary- adrenal axis. Am J Obstet Gynecol. 2000;182(6):1404–13. doi: 10.1067/mob.2000.106180. [DOI] [PubMed] [Google Scholar]
- 29.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 30.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kabelitz D, Wesch D, Oberg HH. Regulation of regulatory T cells: role of dendritic cells and tolllike receptors. Crit Rev Immunol. 2006;26(4):291–306. doi: 10.1615/critrevimmunol.v26.i4.10. [DOI] [PubMed] [Google Scholar]
- 32.Chotimanukul S, Sirivaidyapong S. The localization of Toll-like receptor 2 (TLR2) in the endometrium and the cervix of dogs at different stages of the oestrous cycle and with pyometra. Reprod Domest Anim. 2012;47(Suppl 6):351–5. doi: 10.1111/rda.12104. [DOI] [PubMed] [Google Scholar]
- 33.Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72(10):5799–806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198(8):1225–35. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20(6):1439–46. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsson B, Holst RM, Wennerholm UB, Andersson B, Lilja H, Hagberg H. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol. 2003;189(4):1161–7. doi: 10.1067/s0002-9378(03)00594-5. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda Y, Kouno S, Nakano H. Effects of antibiotic treatment on the concentrations of interleukin- 6 and interleukin-8 in cervicovaginal fluid. Fetal Diagn Ther. 2002;17(4):228–32. doi: 10.1159/000059374. [DOI] [PubMed] [Google Scholar]
- 38.Goepfert AR, Goldenberg RL, Andrews WW, Hauth JC, Mercer B, Iams J, et al. The Preterm Prediction Study: association between cervical interleukin 6 concentration and spontaneous preterm birth. Am J Obstet Gynecol. 2001;184(3):483–8. doi: 10.1067/mob.2001.109653. [DOI] [PubMed] [Google Scholar]
- 39.Haider S, Knofler M. Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta. 2009;30(2):111–23. doi: 10.1016/j.placenta.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aboussahoud W, Aflatoonian R, Bruce C, Elliott S, Ward J, Newton S, et al. Expression and function of Toll-like receptors in human endometrial epithelial cell lines. J Reprod Immunol. 2010;84(1):41–51. doi: 10.1016/j.jri.2009.09.008. [DOI] [PubMed] [Google Scholar]