Abstract
We report a rare case of large cell neuroendocrine carcinoma (LCNEC) of the lung with cancer-associated retinopathy (CAR). To our knowledge, only two cases of LCNEC with CAR have been reported, one in 1995 and another in 2013. CAR, typically associated with small cell lung cancer (SCLC), is one of the paraneoplastic syndromes with deterioration of visual acuity, visual field constriction, and photophobia. CAR is caused by an autoimmune system reaction against the same antigen in the tumor and retinal photoreceptor cells. To diagnose CAR, genetic retinal dystrophies or any other medical causes of retinopathy should be excluded, but there are no standard diagnostic criteria. Anti-retinal antibodies are known to be positive in CAR patients, and anti-recoverin antibodies are thought to be sensitive and specific to CAR. In our case, anti-recoverin antibodies were not detected by serum tests, but CAR could be diagnosed on the basis of ophthalmological findings including clinical symptoms, electroretinographic findings, and visual field tests. CAR with clinical features of rapid visual disorder should be considered in LCNEC patients as well as in SCLC patients.
Key Words: Large cell neuroendocrine carcinoma, Cancer-associated retinopathy, Anti-recoverin antibodies
Introduction
Cancer-associated retinopathy (CAR) is one of the paraneoplastic syndromes caused by the autoimmune reactions against the retinal photoreceptor cells. Sawyer et al. [1] reported the first case of CAR in 1976. The exact incidence of CAR with lung cancer has not been reported. According to some papers, CAR was mainly complicated with small cell lung cancer (SCLC). This is the third case of CAR complicated with large cell neuroendocrine carcinoma (LCNEC).
Case Presentation
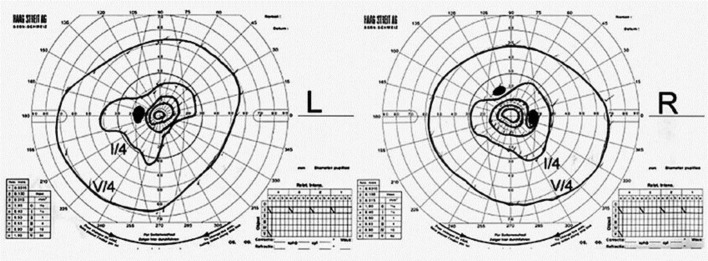
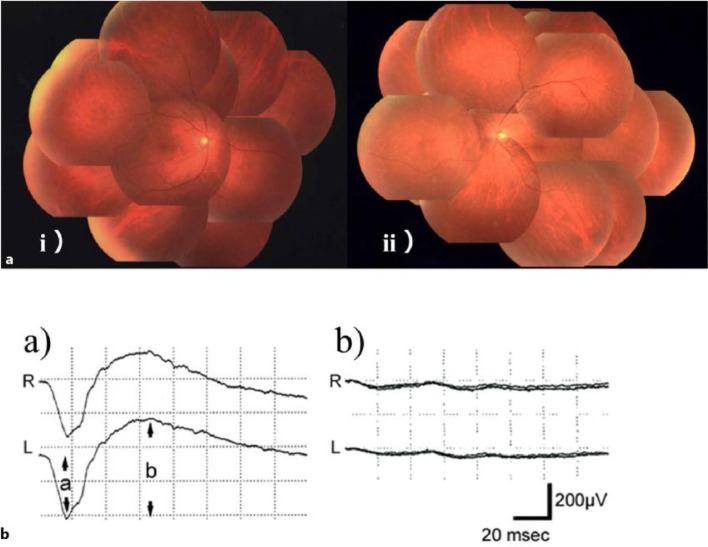
A previously healthy 59-year-old man was referred to our hospital complaining of a rapid visual disorder in the dark, photophobia, and impaired visual field appearing within 1 week in August 2013. He had smoked 20 cigarettes per day for 42 years. His visual field test showed marked constriction of visual field in both eyes (fig. 1). Visual acuities measured in the light were 20/20 in both eyes. On funduscopic examination, no remarkable abnormalities were recognized either in the optic nerves or the macular regions. However, narrowing of the retinal arteries was observed (fig. 2a). Electroretinography (ERG) was performed following the International Society for Clinical Electrophysiology of Vision (ISCEV) standard protocol [2], which demonstrated that photoreceptors, especially rods, were massively damaged. The dark-adapted ERG showed that the amplitudes of a- and b-waves were almost extinguished (fig. 2b). Rapid progression of visual disorder and characteristic ophthalmological findings led us to consider a possibility of CAR.
Fig. 1.
The Goldman visual field test showed constriction of visual fields in both eyes.
Fig. 2.
Fundus pictures (a) and dark-adapted ERG (b). a Fundus pictures [(i) right eye, (ii) left eye] appear nearly normal with vascular attenuation. b Dark-adapted ERG from a healthy subject (a) and from this case (b). ERG of our patient showed an extinguished pattern.
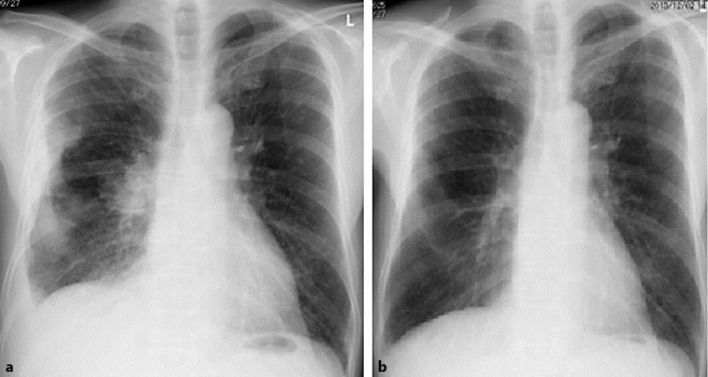
A roentgenogram of the chest and a CT scan showed swelling of the right hilar lymph nodes and pleural thickening in the right middle lobe (fig. 3a). LCNEC was detected in a tissue sample from thickening pleura. From histological and imaging results LCNEC of the lung with CAR was diagnosed. Clinical stage was cT2aN1M1a, stage IV.
Fig. 3.
Roentgenogram of the chest. a Pretreatment and b after 2 cycles of first-line chemotherapy (CDDP and irinotecan). A pretreatment roentgenogram revealed swelling of the right hilum and pleural thickening in the right middle lobe. After chemotherapy, the tumors markedly shrank.
First-line chemotherapy with cisplatin (CDDP) and irinotecan (CPT-11) was performed from September 30, 2013 to March 12, 2014. After 1 cycle of chemotherapy, the patient experienced remarkable tumor shrinkage (fig. 3b). After 2 cycles of chemotherapy, he showed complete response. The visual field test on December 11, 2013, showed improvement of the visual field defect, but photophobia still remained. When regression of the primary lesion was revealed by a follow-up CT scan after 5 cycles of chemotherapy on June 20, 2014, visual disorder had not worsened. Second-line chemotherapy with amrubicin, which is a topoisomerase II inhibitor, was performed from June 25 to January 26, 2015. The best response of amrubicin was stable disease, and the therapy was continued. During this second-line chemotherapy, visual function has been stable.
Discussion
Since the WHO Classification of Tumors 3rd Edition (2004) [3], LCNEC has been added as a subtype of large cell carcinoma. Biological characteristics of LCNEC resemble those of small cell carcinoma [4]. LCNEC produces common antigen to the nervous system and is most likely to cause paraneoplastic neurological disease as well as SCLC.
CAR is one of the paraneoplastic syndromes, which is characterized by the degeneration of retinal photoreceptor cells and caused by an autoimmune reaction against the same antigen in tumor and retinal photoreceptor cells. CAR is typically associated with SCLC rather than non-small SCLC [5]. Only two cases of LCNEC with CAR have been reported so far, one by Stanford et al. in 1995 [6] and another by Isaka et al. in 2013 [7].
There are no standard diagnostic criteria for CAR. To diagnose CAR on physical examination, especially ophthalmological findings are very important, and visual disorder typically occurs before the diagnosis of carcinoma. Progression of visual disturbance (night blindness, photophobia, and constriction of visual field) and deterioration of ERG were identified in this case. The fundus appeared unremarkable with vascular attenuation. Visual disturbance, visual field loss, and attenuated retinal arteriole caliber have been suggested the major triad to consider CAR [8]. Although anti-recoverin antibodies are a useful marker to diagnose CAR [9], a positive rate of anti-recoverin antibodies in the first test is about 60% [10]. In the present case, we could diagnose CAR from characteristic ophthalmologic findings and clinical course, even without the detection of anti-recoverin antibody.
There is no established treatment protocol for CAR. An immunosuppressant, a plasmapheresis, or a combination therapy of these has shown efficacy in some case reports. These reports suggested that the improvement of visual function does not necessarily correlate with the response to chemotherapy [11]. In our case, combination chemotherapy with CDDP and irinotecan as a first-line therapy was performed. As a result, the patient experienced complete response for LCNEC and improvement of visual function, especially of visual field defect.
Conclusion
CAR should be considered in LCNEC patients as well as SCLC patients with clinical features of rapid visual disorder.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Sawyer RA, Selhorst JB, Zimmerman LE, Hoyt WF. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;81:606–613. doi: 10.1016/0002-9394(76)90125-2. [DOI] [PubMed] [Google Scholar]
- 2.Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV Standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC., (eds) Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC Press. 2004 [Google Scholar]
- 4.den Bakker MA, Willemsen S, Grunberg K, Noorduijin LA, van Oosterhout MF, van Suylen RJ, Timens W, Vrugt B, Wiersma-van Tilburg A, Thunnissen FB. Small cell carcinoma of the lung and large cell neuroendocrine carcinoma interobserver variability. Histopathology. 2010;56:356–363. doi: 10.1111/j.1365-2559.2010.03486.x. [DOI] [PubMed] [Google Scholar]
- 5.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 6.Stanford MR, Edelsten CE, Hughes JD, Sanders MD, Brooks CI, Mitchell D, Sheppard MN. Paraneoplastic retinopathy in association with large cell neuroendocrine bronchial carcinoma. Br J Ophthalmol. 1995;79:617–620. doi: 10.1136/bjo.79.6.617-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaka M, Kubota T, Sakai M, Yamane T, Ohnishi H, Yokoyama A. Cancer-associated retinopathy in a patient with large-cell neuroendocrine carcinoma of the lung. Kokyuukigakkaizasshi. 2013;2:39–43. [Google Scholar]
- 8.Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol. 1990;28:162–167. doi: 10.1002/ana.410280208. [DOI] [PubMed] [Google Scholar]
- 9.Thirkill CE, FitzGerald P, Sergott RC, Roth AM, Tyler NK, Keltner JL. Cancer-associated retinopathy (CAR syndrome) with antibodies reacting with retinal, optic-nerve, and cancer cells. N Engl J Med. 1989;321:1589–1594. doi: 10.1056/NEJM198912073212307. [DOI] [PubMed] [Google Scholar]
- 10.Ohguro H, Yokoi Y, Ohguro I, Mamiya K, Ishikawa F, Yamazaki H, Metoki T, Takano Y, Ito T, Nakazawa M. Clinical and immunologic aspects of cancer-associated retinopathy. Am J Ophthalmol. 2004;137:1117–1119. doi: 10.1016/j.ajo.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Kosuke K, Hiroyuki N, Koji K, Hisanaga Y, Gen S, Katsuyuki K, Takeshi M. Cancer-associated retinopathy during treatment for small-cell lung carcinoma. Internal Med. 1999;38:597–601. doi: 10.2169/internalmedicine.38.597. [DOI] [PubMed] [Google Scholar]