Abstract
Objective:
There is a strong association between depression and anxiety. Duloxetine, an antidepressant agent, is also used in the treatment of anxiety. Hydroxyzine is preferred over benzodiazepines in the treatment of anxiety. Present study was designed to study the impact of a combination of duloxetine with hydroxyzine in treatment of anxiety.
Materials and Methods:
Mice received intraperitoneal injection of normal saline (10 ml/kg), duloxetine alone (10 mg/kg), hydroxyzine alone (10 mg/kg), and hydroxyzine plus duloxetine (5 mg/kg, each).
Results:
The in vivo results (elevated plus maze and light/dark transition tests) showed significant anxiolytic activity with the hydroxyzine treatment than the control group. The brain monoamines were significantly increased in hippocampi, cerebral cortices, and whole brain in drug-treated groups than in the control group. The group receiving the combination showed similar results in the in vivo models and in vitro tests (brain monoamine estimations) than respective monotherapies, with the exception of a greater increase of norepinephrine levels in cerebral cortices in duloxetine-treated group.
Conclusion:
Combination of duloxetine with hydroxyzine is not beneficial in anxiolytic treatment than the respective monotherapies. There is a need to study the pharmacokinetic drug-drug interactions to understand the present study outcomes.
KEY WORDS: Antidepressant drugs, anxiety, duloxetine, hydroxyzine
Introduction
The close association between anxiety and depression is well-established.[1,2] Anxiety disorders are common and have chronic or relapsing course. It has a strong association with personal distress, impaired social and occupational functions, hampered quality-of-life, and overall substantial economic loss.[3] The prevalence of anxiety disorders ranges between 2.4% and 29.8%.[4] Recently, Baldwin et al.[3] reported a substantial unmet public health, clinical, and research needs in the treatment of anxiety disorders. Antidepressants drugs such as duloxetine, a potent reuptake inhibitor of serotonin (5-HT) and norepinephrine (NE), and a weak reuptake inhibitor of dopamine (DA),[5] is approved for the treatment of generalized anxiety disorders.[6] Hydroxyzine is an antagonist of histamine receptors. Double-blind studies showed its efficacy and safety over placebo in the treatment of anxiety. In addition, it can be an effective alternative benzodiazepines.[7] Martin et al.,[8] reported benefits of combining hydroxyzine and 5-HT reuptake inhibitors in learned helpless paradigm representing depression condition. Therefore, the present study aims to assess the anxiolytic effect of a combination of duloxetine and hydroxyzine.
Materials and Methods
Animals
Male Swiss Albino mice (25-30 g) were procured from Bharat Serum Ltd., Thane and housed in Perspex cage. Three mice per cage were housed in a temperature (22-24°C) and humidity (50-60%) controlled central animal house facility under light and dark (12 h: 12 h) illumination cycle. Animals had free access to standard food and water. Experiments were performed between 11.00 h and 14.00 h. Each experimental model had a separate set of animals, randomly distributed into seven groups (n = 6/group). The arena of elevated plus maze (EPM), open field test, and light/dark transition test was wiped with 70% ethyl alcohol solution before placing the animals. Animals were transferred to laboratory 1 h before testing. The present study was performed according to protocols approved by the Institutional Animal Ethics Committee (Approval number: CPCSEA/IAEC/SPTEM-09/2013), Government of India, New Delhi.
Drug Solutions and Treatment
The drugs were administered through intraperitoneal route. Drug solutions were prepared in normal saline (0.9% w/v NaCl). The animals were treated 30 min before each test session. Group I received vehicle treatment (control group) that is, normal saline (10 ml/kg). Group II and III received monotherapies of duloxetine (10 mg/kg; Dr. Reddy's Laboratories Ltd.) and hydroxyzine (10 mg/kg; UCB India Pvt. Ltd.), respectively. Groups IV received combination treatment of duloxetine (5 mg/kg) and hydroxyzine (5 mg/kg).
Anxiety Models
Elevated plus maze test
Two closed and two open arms having dimensions of 30 cm × 5 cm were arranged so that the two closed arms were opposite to each other with an open roof. The height of closed arm walls was 12 cm. Mouse was placed in the center of maze while facing one of the closed arms and the total number of entries and the time spent in the open and enclosed arms were observed for 5 min from a recorded video.[9] The criterion of an entry was the presence of all four paws inside an arm. The parameters such as the frequency of entries in closed (CAE), open arm entries (OAE), total time spent in the closed (CAT), and total time spent in open arms (OAT) were recorded. In addition, the percentage of time spent in the open arms (%OAT= [(open time/300) ×100]) and the percentage of OAE (%OAE= [(open entries/open + closed entries) ×100]) were recorded.[10] These parameters were analyzed using recorded video of each animal by a trained single observer.
Open field test
The open field apparatus consisted of a square white color wooden arena (72 cm × 72 cm × 33 cm). Each animal placed in the center and spent 5 min in arena. The frequency of line crossing, the time spent in, and entries into the central zone of the arena (18 cm × 18 cm) were analyzed using recorded video of each animal by a single trained observer.[11]
Light/dark transition test
The apparatus consisted of a cage having dimensions 21 cm × 42 cm × 25 cm. A partition with door was placed to divide it into two sections of equal size. The first section was white and second was black colored. The light illumination in the first section was kept bright and second was dim. Each mouse was placed separately in the center place of white box while facing door present in the partition. The observation period of each animal was 10 min. Parameters like time spent in light and dark area, percent time spent in light and dark area were analyzed from the recorded video by a trained person.[12]
Estimation of Norepinephrine, Dopamine, and Serotonin by High-performance Liquid Chromatography with Fluorescence Detector Method
Method described by Choudhary et al.[13] and Madepalli et al.[14] was used to estimate NE, DA, and 5-HT levels in the cerebral cortex, hippocampus, and whole brain (whole brain = cerebral cortex + hippocampus + remaining brain tissue). The instruments used in analysis includes high-performance liquid chromatography (Shimadzu, LC-2010C HT, autosampler) with fluorescence detector (RF-20A-prominence, Shimadzu), and a reversed-phase analytical column (KROMASIL 100, C18, 5 μm, 25 mm × 0.46 mm). Euthanasia was performed 1 h after treatment and brain was isolated in ice cold 0.1 M perchloric acid. After recording weight of the brain, cerebral cortex, hippocampus, and remaining brain parts were removed and weighed separately. Samples were homogenized in 2 ml of ice cold 0.1 M perchloric acid and resulting mixture was centrifuged at 20817 ×g (Eppendorf 5810 R, Rotor F-45-30-11) for 30 min (4°C). The supernatant was filtered through 0.45 μm membrane and stored at −80°C until the time of analysis. The chromatographic separation was achieved on reversed-phase analytical column at room temperature, and the acquired data were processed using LC Solution@ software. The mobile phase consists of sodium acetate (0.02 M), ethylenediaminetetraacetic acid (0.2 mM), methanol (16%), di-n-butylamine (0.01%), and heptane sulfonic acid (0.055%). Mobile phase pH was adjusted using phosphoric acid (pH-3.92), filtered through a 0.45-mm membrane (PALL@ Pall corporation, India). Flow rate of mobile phase was kept at 1.3 ml/min. Monoamines were detected at an excitation wavelength of 280 nm and an emission wavelength of 315 nm. Peaks were identified by comparing the retention time of sample and standard. The concentration of each monoamine in the sample was analyzed according their area under curve using their straight line equation. The linearity for NE, DA, and 5-HT was in the range 0.99–0.997 and results were expressed as ng/g of wet weight of tissue.
Statistical Analysis
One-way ANOVA followed by Tukey's honest significant difference post-hoc test was used for the calculation of statistical significance. The GraphpadInStat for 32 bit Windows version 3.06 (GraphPad Software, Inc) was used for statistical assessment. The data were represented as mean ± standard error of mean values (per group n = 6).
Results
Elevated Plus Maze
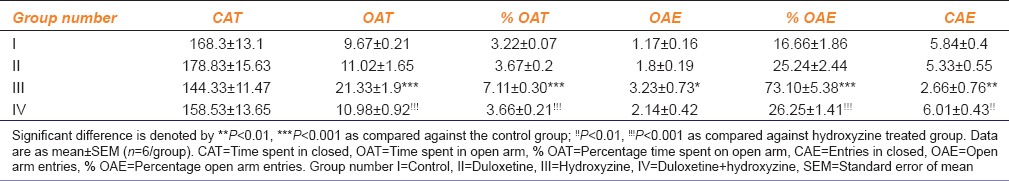
Hydroxyzine treatment showed a significant increase in OAT, %OAT, OAE, %OAE, and decrease in CAE parameters than the control group [Table 1]. Duloxetine and combination treated groups failed to show a significant difference when compared with the control group [Table 1]. Combination treated group showed a significant decrease in OAT, %OAT, %OAE, and increase in CAE parameters when compared with hydroxyzine treated group (P < 0.01) [Table 1].
Table 1.
Effect of duloxetine and hydroxyzine on the elevated plus maze in mice
Light/Dark Transition Test
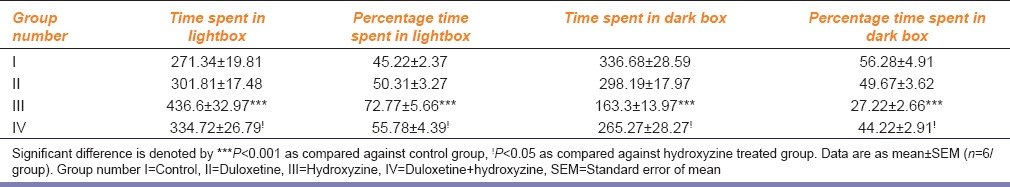
Only hydroxyzine-treated groups showed significant increase in time spent in lightbox, percentage time spent in light box, and decrease in time spent in dark box and percentage time spent in dark box, as compared to control group [Table 2]. Combination treated group showed significant decrease in time spent in lightbox, percentage time spent in light box, and increase in time spent in dark box and percentage time spent in dark box, as compared to hydroxyzine-treated group [Table 2].
Table 2.
Effect of duloxetine and hydroxyzine in light/dark transition test in mice
Brain Monoamine Estimation
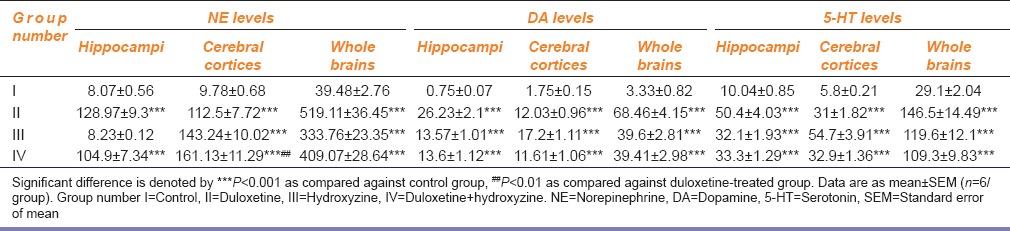
Drug-treated groups showed a significant increase in NE, DA, and 5-HT levels in hippocampi, cerebral cortices, and whole brains, as compared to control group [Table 3]. However, hydroxyzine treatment failed to increase NE levels in hippocampi as compared to control group [Table 3]. With the exception of a significant increase of NE in cerebral cortices, combination treatment failed to produce a significant increase in brain monoamine levels when compared against duloxetine and hydroxyzine treated groups [Table 3].
Table 3.
Effect of duloxetine and hydroxyzine on brain monoamine levels (ng/g) in mice
Discussion
The present study outcomes indicate no benefit of the combination of duloxetine with hydroxyzine over the respective monotherapy. The results of duloxetine monotherapy are inconformity with a previously published report.[13] Unlike the single dose treatment of duloxetine in the present study, Troelsen et al.[13] showed anxiolytic effect of duloxetine with chronic treatment. Results of hydroxyzine monotherapy are also in line with the available reports.[14] Hydroxyzine produces a sedative effect in animals above 15 mg/kg.[15] Therefore, we considered the lower dose of hydroxyzine in the present study. The role of histamine in psychiatric conditions such as anxiety and depression is well-known.[16] Serafim et al.[17] reported an important role of H1 and not H2 receptors in anxiety-like behavior. H1 receptor blockers showed increase in brain levels of noradrenaline and 5-HT and no effect on DA.[18] The results of hydroxyzine treatment induced brain monoamine changes observed in present study [Table 3] are inline with these findings; however, increase in DA levels observed in present study may be due the drug and methodological difference. Similarly, the duloxetine treatment-induced increase in brain monoamine profile [Table 3] is in agreement with previous reports.[19,20] The 5-HT and NE reuptake action of the duloxetine help in treating anxiety associated neurobiological dysfunctions of the serotonergic and noradrenergic system.[6] The duloxetine-induced reduction in 5-HT -transporter density observed with chronic dosing[13] may help in understanding the associated anxiolytic effects. The effect of chronic dosing on anxiety and brain monoamines was not evaluated in this study, which is its limitation. The unavailability of reports of pharmacokinetic interactions between the hydroxyzine and duloxetine limits the understanding of the failure to produce an enhancement of anxiolytic effect by the combination of drugs under study. The absence of an additive effect of hydroxyzine plus duloxetine combination in anxiety needs to be evaluated further. This may help clinicians use this combination rationally and optimally for treatment of anxiety.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Hranov LG. Comorbid anxiety and depression: Illumination of a controversy. Int J Psychiatry Clin Pract. 2007;11:171–89. doi: 10.1080/13651500601127180. [DOI] [PubMed] [Google Scholar]
- 2.Starr LR, Hammen C, Connolly NP, Brennan PA. Does relational dysfunction mediate the association between anxiety disorders and later depression? Testing an interpersonal model of comorbidity. Depress Anxiety. 2014;31:77–86. doi: 10.1002/da.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin DS, Pallanti S, Zwanzger P. Developing a European research network to address unmet needs in anxiety disorders. Neurosci Biobehav Rev. 2013;37:2312–7. doi: 10.1016/j.neubiorev.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol Med. 2013;43:897–910. doi: 10.1017/S003329171200147X. [DOI] [PubMed] [Google Scholar]
- 5.Wu WY, Wang G, Ball SG, Desaiah D, Ang QQ. Duloxetine versus placebo in the treatment of patients with generalized anxiety disorder in China. Chin Med J (Engl) 2011;124:3260–8. [PubMed] [Google Scholar]
- 6.Norman TR, Olver JS. Duloxetine in the treatment of generalized anxiety disorder. Neuropsychiatr Dis Treat. 2008;4:1169–80. doi: 10.2147/ndt.s2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llorca PM, Spadone C, Sol O, Danniau A, Bougerol T, Corruble E, et al. Efficacy and safety of hydroxyzine in the treatment of generalized anxiety disorder: A 3-month double-blind study. J Clin Psychiatry. 2002;63:1020–7. doi: 10.4088/jcp.v63n1112. [DOI] [PubMed] [Google Scholar]
- 8.Martin P, Guillou N, Lacroix P, Billardon M. Effects of co-administration of an antidepressant and an anxiolytic drug in the “learned helplessness” paradigm: Importance of hydroxyzine. Encephale. 1996;22:270–9. [PubMed] [Google Scholar]
- 9.Vogel HG, Vogel WH, editors. In: Drug Discovery and Evaluation, Pharmacological Assays. 3rd ed. Heidelberg: Springer-Verlag; 2008. Psychotropic and neurotropic activity; p. 791. [Google Scholar]
- 10.Gianlorenço AC, Canto-de-Souza A, Mattioli R. l-histidine induces state-dependent memory deficit in mice mediated by H (1) receptor. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:91. doi: 10.1016/j.pnpbp.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behav Genet. 1999;26:263–71. [Google Scholar]
- 12.Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 13.Troelsen KB, Nielsen EØ, Mirza NR. Chronic treatment with duloxetine is necessary for an anxiolytic-like response in the mouse zero maze: The role of the serotonin transporter. Psychopharmacology (Berl) 2005;181:741–50. doi: 10.1007/s00213-005-0032-5. [DOI] [PubMed] [Google Scholar]
- 14.Gladney M, Stanley RT, Hendricks SE. Anxiolytic activity of chloral hydrate and hydroxyzine. Pediatr Dent. 1994;16:183–9. [PubMed] [Google Scholar]
- 15.Naghibi B, Rayatnia F. Co-administration of subeffective anxiolytic doses of diazepam and hydroxyzine in elevated zero-maze in mice. Psychiatry Investig. 2011;8:169–73. doi: 10.4306/pi.2011.8.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raber J. Histamine receptors as potential therapeutic targets to treat anxiety and depression. Drug Dev Res. 2005;65:126–32. [Google Scholar]
- 17.Serafim KR, Kishi MS, Cante-de-Souza A, Morrioli R. H1 but not H2 histamine antagonist receptors mediate anxiety-related behaviors and emotional memory deficit in mice subjected to elevated plus-maze testing. Braz J Med Biol Res. 2013;46:440–6. doi: 10.1590/1414-431X20132770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak JZ. Effects of histamine H1- and H2-receptor antagonists on dopamine, noradrenaline and serotonin systems in rat brain. Pol J Pharmacol Pharm. 1980;32:451–61. [PubMed] [Google Scholar]
- 19.Kihara T, Ikeda M. Effects of duloxetine, a new serotonin and norepinephrine uptake inhibitor, on extracellular monoamine levels in rat frontal cortex. J Pharmacol Exp Ther. 1995;272:177–83. [PubMed] [Google Scholar]
- 20.Muneoka K, Shirayama Y, Takigawa M, Shioda S. Brain region-specific effects of short-term treatment with duloxetine, venlafaxine, milnacipran and sertraline on monoamine metabolism in rats. Neurochem Res. 2009;34:542–55. doi: 10.1007/s11064-008-9818-2. [DOI] [PubMed] [Google Scholar]


