Abstract
Objective:
To develop an amino acid prodrug of acetaminophen with comparable therapeutic profile and less hepatotoxicity than acetaminophen.
Materials and Methods:
Acetaminophen prodrug was synthesized by esterification between the carboxyl group of amino acid glycine and hydroxyl group of acetaminophen. Analgesic, antipyretic, ulcer healing, and hepatotoxic activities were performed on Wistar rats in this study.
Results:
Prodrug showed a 44% inhibition in writhings as compared to 53.3% of acetaminophen. Acetaminophen also offered highest antipyretic activity. Prodrug showed gastroprotective and hepatoprotective effects as it reduced the gastric lesions by 32.1% (P < 0.01) and significantly prevented the rise in liver enzymes (serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase and bilirubin). The most notable effect of prodrug was in preventing the depletion of hepatic glutathione (GSH), which is reduced by acetaminophen.
Conclusion:
Prodrug showed hepatoprotective and gastroprotective effects, although the therapeutic efficacy was compromised. Prodrug was successful in preventing a decrease in GSH, thereby exhibiting promising results in the field of prodrug designing to avoid the toxic effects of acetaminophen.
KEY WORDS: Acetaminophen, amino acid, glycine, hepatotoxicity, prodrug
Introduction
Acetaminophen is one of the most widely used drugs in the world and because of this it has also been one of the most widely studied drugs in the field of pharmaceutical sciences, especially in toxicology, pharmacokinetics, and drug metabolism.[1] Acetaminophen, the drug we consider safe, is the leading cause of acute liver failure in the United States, United Kingdom, Australia and New Zealand.[2,3,4] The recommended dose of acetaminophen is believed to be safe, although in few cases liver toxicity in pediatric patients has been reported even after a single acetaminophen dose of 120–150 mg/kg of body weight.[5,6] There were 214 deaths involving overdose of an analgesic agent. In 62 of these cases, acetaminophen was solely responsible agent.[7,8] In another study acetaminophen, otherwise considered to be safe in pregnancy, was linked to infertility in the posterior adult life of the unborn.[9] Oral administration of acetaminophen or in that case most of the phenols have poor bioavailability and hepatic toxicity due to first-pass metabolism in the gastrointestinal tract and liver.[10] The toxic effects of acetaminophen are attributed to the formation of a toxic metabolite N-acetyl-p-benzoquinoneimine, which is detoxified by reaction with glutathione (GSH) leading to depletion of GSH and cell death.[11,12] Using prodrug synthesis as a combat strategy could prove helpful to overcome this barrier. In this context, phenol drugs are attractive targets for prodrug designing because the hydroxyl group is very convenient to attach a wide range of pro-moieties.[13] Majority of the work on phenols for prodrug designing has focused on corresponding ester or ether.[14,15,16] Since amino acids are normal dietary constituents and are nontoxic in moderate doses when compared to other pro-moieties, their incorporation might serve beneficial in overcoming the toxic effects of acetaminophen.
Materials and Methods
Synthesis of Prodrug
Synthesis comprised of a three step process involving amino acid protection, reaction with acetaminophen and the deprotection [Figure 1]. All the chemicals used were purchased from Loba Chemie except for trifluoroacetic acid (TFA) and dichloromethane (DCM), which were purchased from Fisher scientific. Acetaminophen was provided as gift sample by Mankind Pharmaceuticals, Paonta Sahib, Himachal Pradesh.
Figure 1.
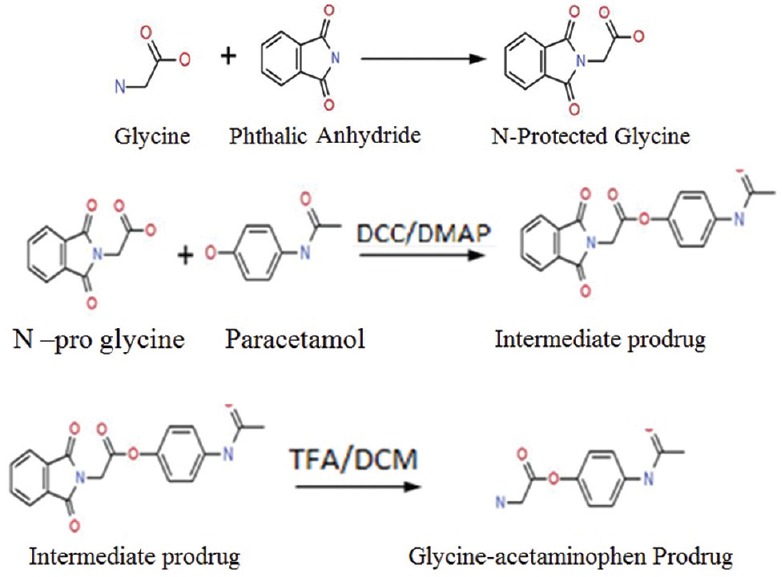
Synthesis of prodrug, a three step process including amino group protection of amino acid using phthaloylation; reaction of acetaminophen with N-protected amino acid and finally the deprotection (DCC=N,N-dicyclohexylcarbodiimide, DMAP=4-dimethylaminopyridine, TFA=Trifluoroacetic acid, DCM=Dichloromethane)
Procedure
N-protected amino acid (1.2 equiv) was added drop wise into the mixture of N, N-dicyclohexylcarbodiimide (1.2 equiv) and 4-dimethylaminopyridine (0.12 equiv) and acetaminophen (1 equiv) in dry dimethylformamide (DMF). The reaction was stirred at room temperature for 24 h. The reaction was monitored by thin layer chromatography (TLC) (hexane/ethyl acetate, 1:1). DMF was removed under high vacuum. The residue was then extracted with ethyl acetate (50 mL) and washed with water (2 mL × 30 mL), saturated NaHCO3(2 mL × 30 mL) and saturated NaCl (1 mL × 30 mL). The organic layer was dried over MgSO4, filtered, and concentrated under vacuum. Crude compounds were separated using column chromatography (hexane/ethyl acetate, 10:1). The fractions were collected and analyzed by TLC for purity. Fractions from each spot were concentrated under vacuum separately. The phthalic group was removed by treating the residues with 5 mL of TFA and 5 mL of DCM for 4 h. After evaporating DCM and most of TFA (95%), cold ether was added to precipitate out the pure compounds. After removing ether, the residues were reconstituted with water and lyophilized.
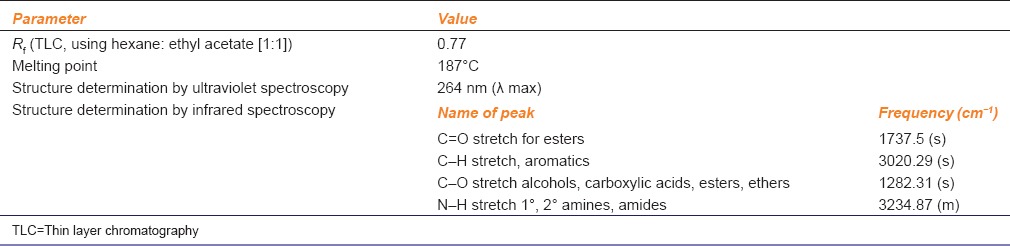
Physiochemical Characterization
Various methods like ultraviolet and infrared spectroscopy were used to characterize and identify the synthesized prodrug [Table 1].
Table 1.
Physiochemical properties of the prodrug
In vivo Analysis
Since the prodrug should not reduce the efficacy of the parent drug, it was important to study the it's pharmacological profile in comparison to acetaminophen.[17] Wistar rats and Balb/C mice of either sex were used in this study. Study consisted of three animal groups (six animals per group) viz: (a) Control group, which received saline (0.9% NaCl) or propylene glycol; (b) standard group, which received acetaminophen and (c) test group which received glycine prodrug in a dose molecularly equivalent to acetaminophen (e.g.,: Ten molecules of prodrug for ten molecules of acetaminophen). All the experiments were conducted in the animal house of VNS Faculty of Pharmacy, approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (Regd no. 778/PO/a/03/CPCSEA).
Analgesic Activity (Writhing Test)
Pain was induced in mice using 0.1 mL of 0.6% v/v acetic acid intraperitoneally. Control group received 0.9% w/v NaCl. Standard and test groups received their respective drugs orally 10 min prior to acetic acid injection. Standard group received 100 mg/kg of acetaminophen. Test group received 137 mg/kg (molecularly equivalent to 100 mg/kg of acetaminophen) of glycine prodrug. Analgesic activity was calculated by % inhibition of the writhings.
Antipyretic Activity (Brewer's Yeast Induced Hyperpyrexia Method)
Rats were given 10 mg/mL of brewer's yeast suspension to induce pyresis. Body temperature was monitored before injecting brewer's yeast suspension (15% suspension using 0.9% saline) rectally. Post 18 h brewer's yeast injection, the vehicle, standard drug and test drug were administered to different groups. Propylene glycol at dose of 5 mL/kg was administered orally to the control groups of animals and acetaminophen at dose of 150 mg/kg was administered orally to standard group. Test group received 208 mg/kg of glycine prodrug. Rectal temperatures were recorded by clinical thermometer at 0, 1, 2 and 3 h after drug administration and observations were tabulated.
Ulcer Healing Activity (Aspirin Induced Gastric Lesions Method)
Since amino acids have a healing effect on gastric lesions therefore, ulcer healing activity of prodrug was assessed. Each group received 300 mg/kg of aspirin orally to produce gastric lesions. The animals were fasted 24 h before the experiment but had free access to water. The test-substances were administered 30 min before aspirin (300 mg/kg). Acetaminophen was given at its therapeutic dose for fever, that is, 150 mg/kg orally. Test group received 208 mg/kg of glycine prodrug orally.
Hepatotoxicity
Acetaminophen is used as a standard drug to induce hepatotoxicity in experimental animals.[18] Its acute toxicity leads to liver injury or liver transplant due to acute liver failure. After 3 h of fasting, animals were treated with drugs orally. Standard group received acetaminophen at a dose of 2000 mg/kg or 2 g/kg, which is reported to produce hepatic tissue injury. Test group received 2.75 g/kg of glycine prodrug, which is the equivalent dose to that of acetaminophen. Hepatic parameters such as serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), bilirubin and GSH levels were analyzed using biochemical auto analyzer.
Statistical Analysis
Values were represented as mean ± (standard error of mean) SEM. Data were analyzed using one-way analysis of variance and group means were compared by performing Dunnett's multiple comparison test using GraphPad prism v5.03 software (GraphPad Software, Inc). Graphical values are represented as mean ± SEM, *significantly different with P < 0.05, **significantly different with P < 0.01, ***significantly different with P < 0.001, ns: Nonsignificant, a: Significantly different from normal group, b: Significantly different from toxicant group
Results
Spectroscopic tools confirmed the synthesis of prodrug. Ultra-violet spectroscopy presented the compound with a new λ max at 264 nm (graph not shown) while infrared spectroscopic analysis exhibited a C = O stretch for esters at a frequency (cm− 1) 1737.5 (s) the confirming synthesis of a newly synthesized ester prodrug [Table 1]. Prodrug was very competitive matching the therapeutic profile of acetaminophen [Figure 2]. Prodrug reduced the writhing by 44% as compared to 53% by acetaminophen. Acetaminophen hence was a better analgesic than its corresponding prodrug. In terms of antipyretic activity, maximum antipyretic effect for both parent and prodrug was achieved 3 h after treatment. Here also prodrug showed promising results as it was able to reduce the body temperature to 38.3 ± 0.15 (P < 0.01) when compared to a slightly better 37.45 ± 0.1 (P < 0.01) of acetaminophen. The prodrug showed a good ulcer healing activity as it reduced the severity of gastric lesions by 32.1% (P < 0.01). The prodrug protected the rise in hepatic enzymes (SGOT, SGPT, alkaline phosphatase, total bilirubin and direct bilirubin), but most importantly, prevented the depletion of GSH levels (P < 0.01) [Figure 2].
Figure 2.
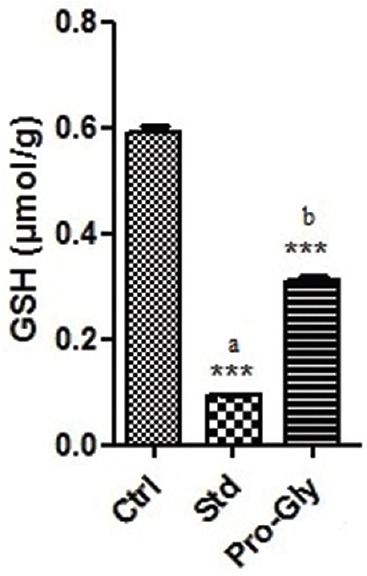
In vivo screening of glycine-acetaminophen prodrug (Pro-gly) showing prevention of glutathione depletion. n = 6 animal per group, graphical values are represents as mean ± standard error of mean, *Significantly different with P < 0.05, **Significantly different with P < 0.01, ***Significantly different with P < 0.001, ns=Nonsignificant, a=Significantly different from normal group, b=Significantly different from toxicant group
Discussion
Acetaminophen toxicity has always been a matter of concern, but till date there has been no effective strategy to overcome this hurdle. Overdosage leads to depletion of GSH levels leading to cell death and ultimately hepatic failure. Prodrug development is a very safe and effective strategy to mask the unwanted properties of a drug. In the case of acetaminophen, many prodrug studies end up analyzing the physiological stability and that too in vitro.[16,19,20] However to date no drug with significant improvement in the therapeutic versus hepatotoxic profile has been obtained. This research is one step forward in the direction of developing a safer analog of acetaminophen. Although acetaminophen exhibited a slightly better therapeutic profile, the prodrug did score in the field of gastroprotective and hepatoprotective actions. Most striking effect was observed with the levels of GSH, as the glycine-acetaminophen prodrug significantly protected the depletion of GSH when compared to its parent drug. To summarize, glycine-acetaminophen prodrug showed remarkable potential in the field of gastric and hepatic complications, but at the cost of therapeutic efficacy. Additional studies using multiple amino acid sequence and peptides as pro-moieties would lead to the emergence of a safer and highly active prodrug of not just acetaminophen but all the drugs associated with high toxicity profile.
Acknowledgment
I am grateful to the VNS Faculty of pharmacy for their invaluable support. I sincerely thank Dr. P. K. Singour (Professor, VNS Faculty of pharmacy) for his valuable guidance in analyzing and interpreting the spectroscopic data.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Chamberlain J. Paracetamol (Acetaminophen). A critical bibliographic review laurie F. Prescott published 1996 taylor and Francis, London x + 708 pages ISBN 0 7438 0136 9 £ 90.00. J Pharm Pharmacol. 1996;48:882. [Google Scholar]
- 2.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 3.Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA. Guidelines for the management of paracetamol poisoning in Australia and New Zealand-explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres. Med J Aust. 2008;188:296–301. doi: 10.5694/j.1326-5377.2008.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins LC, Edwards JN, Dargan PI. Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: A review of the literature. Drug Saf. 2007;30:465–79. doi: 10.2165/00002018-200730060-00002. [DOI] [PubMed] [Google Scholar]
- 5.Henretig FM, Selbst SM, Forrest C, Kearney TK, Orel H, Werner S, Williams TA. Repeated acetaminophen overdosing. Causing hepatotoxicity in children. Clinical reports and literature review. Clin Pediatr (Phila) 1989;28:525–8. doi: 10.1177/000992288902801107. [DOI] [PubMed] [Google Scholar]
- 6.Alander SW, Dowd MD, Bratton SL, Kearns GL. Pediatric acetaminophen overdose: Risk factors associated with hepatocellular injury. Arch Pediatr Adolesc Med. 2000;154:346–50. doi: 10.1001/archpedi.154.4.346. [DOI] [PubMed] [Google Scholar]
- 7.Watson WA, Litovitz TL, Klein-Schwartz W, Rodgers GC, Jr, Youniss J, Reid N, et al. 2003 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2004;22:335–404. doi: 10.1016/j.ajem.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Rowden AK, Norvell J, Eldridge DL, Kirk MA. Updates on acetaminophen toxicity. Med Clin North Am. 2005;89:1145–59. doi: 10.1016/j.mcna.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen DM, Hass U, Lesné L, Lottrup G, Jacobsen PR, Desdoits-Lethimonier C, et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum Reprod. 2011;26:235–44. doi: 10.1093/humrep/deq323. [DOI] [PubMed] [Google Scholar]
- 10.Boyer TD, Rouff SL. Acetaminophen-induced hepatic necrosis and renal failure. JAMA. 1971;218:440–1. [PubMed] [Google Scholar]
- 11.Vermeulen NP, Bessems JG, Van de Straat R. Molecular aspects of paracetamol-induced hepatotoxicity and its mechanism-based prevention. Drug Metab Rev. 1992;24:367–407. doi: 10.3109/03602539208996298. [DOI] [PubMed] [Google Scholar]
- 12.Dahlin DC, Nelson SD. Synthesis, decomposition kinetics, and preliminary toxicological studies of pure N-acetyl-p-benzoquinone imine, a proposed toxic metabolite of acetaminophen. J Med Chem. 1982;25:885–6. doi: 10.1021/jm00350a001. [DOI] [PubMed] [Google Scholar]
- 13.Friis GJ, Bundgaard H, Krogsgaard-Larsen P, Bundgaard H. Amsterdam: Harwood Academic; 1996. A Textbook of Drug Design and Development. [Google Scholar]
- 14.Wasdo SC, Sloan KB. Topical delivery of a model phenolic drug: Alkyloxycarbonyl prodrugs of acetaminophen. Pharm Res. 2004;21:940–6. doi: 10.1023/b:pham.0000029281.12753.25. [DOI] [PubMed] [Google Scholar]
- 15.Majumdar S, Sloan KB. Synthesis, hydrolyses and dermal delivery of N-alkyl-N-alkyloxycarbonylaminomethyl (NANAOCAM) derivatives of phenol, imide and thiol containing drugs. Bioorg Med Chem Lett. 2006;16:3590–4. doi: 10.1016/j.bmcl.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 16.Thomas JD, Sloan KB. In vitro evaluation of alkylcarbonyloxymethyl (ACOM) ethers as novel prodrugs of phenols for topical delivery: ACOM prodrugs of acetaminophen. Int J Pharm. 2008;346:80–8. doi: 10.1016/j.ijpharm.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Vogel HG, Maas J, Gebauer A. New York: Springer; 2011. Drug Discovery and Evaluation: Methods in Clinical Pharmacology. [Google Scholar]
- 18.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 19.Hussain A, Kulkarni P, Perrier D. Prodrug approaches to enhancement of physicochemical properties of drugs IX: Acetaminophen prodrug. J Pharm Sci. 1978;67:545–6. doi: 10.1002/jps.2600670426. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Patel A, Dave R, Yuan X. Development of acetaminophen proline prodrug. Bioorg Med Chem Lett. 2010;20:3851–4. doi: 10.1016/j.bmcl.2010.05.050. [DOI] [PubMed] [Google Scholar]


