Abstract
Etoricoxib is a selective cyclo-oxygenase 2 (COX-2) enzyme inhibitor and is exploited for its analgesic activity in various disease conditions like osteoarthritis, gouty arthritis, acute pain including postoperative dental pain and primary dysmenorrhea, etc. Although highly efficacious in pain management the safety profile of this COX-2 inhibitor is yet to be established in a broader sense. Short-term clinical trials and postmarketing surveillance have shown a very rare incidence of very serious skin reactions like Steven Johnson syndrome or toxic epidermal necrolysis (TEN). In this case report, we summarize regarding a patient who developed TEN after treatment with etoricoxib for osteoarthritis that later resolved in 15 days after withdrawal and symptomatic treatment.
KEY WORDS: Adverse drug reaction, etoricoxib, toxic epidermal necrolysis
Introduction
Toxic epidermal necrolysis (TEN) or Lyell syndrome is a serious cutaneous skin reaction attributed to some drugs. This reaction is characterized by erythema, necrosis and bullous detachment of the epidermis and mucosal membranes. The drugs that could cause the reaction include antibiotics such as sulfonamides, nonsteroidal anti-inflammatory drugs (NSAIDs), allopurinol, antimetabolites like methotrexate, antiretroviral drugs, corticosteroids, chlormezanone, antiepileptics like phenobarbital, phenytoin, carbamazepine, valproic acid etc.[1] Theories proposing the pathogenesis probably attribute it to immunologic reactions, reactive metabolites from certain drugs and genetic aspects.[2]
Etoricoxib is a 30 fold selective cyclo-oxygenase 2 (COX-2) enzyme inhibitor that plays a major role in the inhibition of prostaglandin synthesis especially prostacyclin and hence in pain. This generation of drugs have been developed owing to their enhanced gastrointestinal (GI) tolerability in addition to an effective pain management property both in acute and chronic conditions when compared to traditional NSAIDs.[3] Postmarketing surveillance has reported a few cases of TEN, Steven Johnson syndrome (SJS) and other skin reactions.[4,5,6]
Case Report
A 59-year-old female patient presented with the symptoms of extensive rash all over the body with peeling of skin and fever. Detailed history suggested that the patient consulted a private physician for osteoarthritis and fever a week back for which she has been prescribed etoricoxib 60 mg orally. After taking a single dose of the drug in the evening, she developed maculopapular, erythematous rash with itching. The symptoms turned more severe with the formation of blisters that were later followed by bullous exfoliation of epidermis and necrosis. Concomitant medications include tablet paracetamol 500 mg thrice a day, tablet cefixime 200 mg twice a day, tablet pantaprazole 40 mg one dose a day. The patient presented with above-mentioned condition and was immediately hospitalized in Intensive Care Unit. All the drugs were withdrawn as her condition was serious with hypotension and exfoliation of the skin extending to trunk, face, hands, upper limbs and genital organs [Figure 1]. Nikolsky sign was clearly elicited with a detachment of the epidermis from lower layers when slightly rubbed and extension of existing bullae to the clear skin indicating an active epidermal necrolysis.
Figure 1.
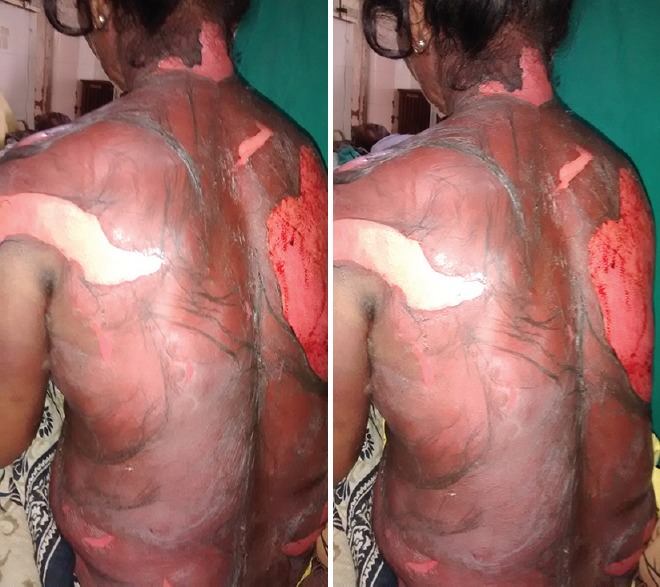
Picture depicting epidermal Loss on shoulders extending to back with etoricoxib
Treatment for the patient was multidirectional involving replacement of fluid loss and also to maintain electrolyte imbalance and antibiotic therapy (injection augmentin 1.2 g intravenously twice a day + injection metronidazole 100 ml thrice a day intravenously). On dermatological consultation parenteral glucocorticoids that is, dexamethasone 8 mg intravenously twice a day were given for 7 days and tablet pentoxifylline 400 mg twice a day were prescribed along with silverex ointment (silver sulfadiazine 1% and chlorhexadine gluconate 0.2%) for topical application. On the 1st day of hospitalization, laboratory results have shown a marginal elevation of serum creatinine levels 1.4 mg% and altered serum electrolytes that is, Na+ 126 mmol/L, K+ 2.9 mmol/L, Cl− 88 mmol/L and blood urea 54 mg/dL. On day 4, the laboratory results were within normal limits with serum creatinine levels 1 mg% and serum electrolytes were in normal range (Na+ 134 mmol/L, K+ 3.6 mmol/L, Cl− 105 mmol/L). Despite fluid replacement, there was continuous hypotension for which dopamine infusion was started at the rate of 5 μg/kg. During the course of treatment, she developed burning sensation in stomach, restlessness and difficulty in micturition. For GI symptoms, she has been symptomatically treated with histamine 2 receptor blockers at 12 hourly. There was rapid improvement in the condition of the patient and hence, on day 12, antibiotic, and glucocorticoid therapy were tapered appropriately [Figure 2]. On day 16, the patient has been discharged with proper instructions regarding a possible relapse.
Figure 2.
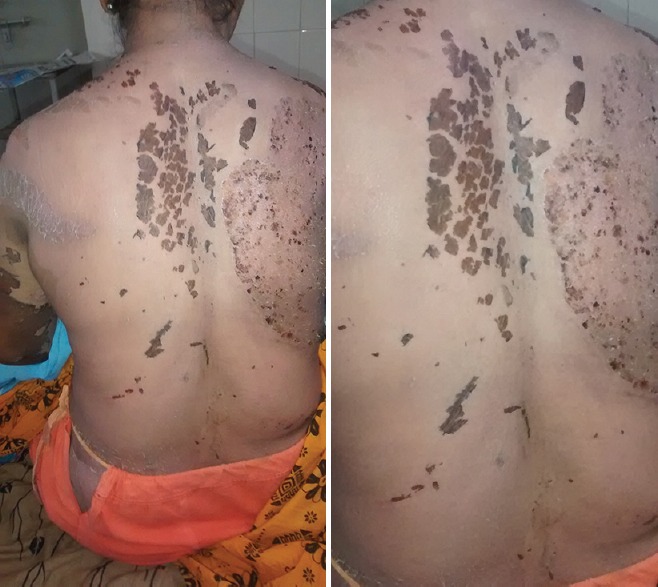
The healed epidermal loss
Discussion
A case report on TEN needs to encompass other related conditions like erythema multiforme and SJS that are also some of the unexpected serious mucocutaneous reactions manifesting due to exposure to certain drugs. All the three reactions differ only in the extent and severity of the skin surface detachment.[2] The incidence of TEN is very less than SJS with female population more vulnerable compared to male.[7] Pathologically, TEN involves necrolysis of keratinocytes at the epidermo-dermal junction associated with lymphocytic infiltrations.[8] The time gap between exposure to drug and exacerbation of the reaction ranges from few hours to a maximum of 22–38 days.[2] In the present case, the patient had developed prodromal phase within an hour evidenced by erythematous rash, fever, arthralgia, myalgia and in 2 days rash turned to flaccid blisters with a serious sloughing of the necrotic skin extending to the extremities, trunk and face with the involvement of genito-urinary areas and there was sparing of oral mucosa, nasal mucosa and conjunctiva.
Even though etoricoxib is highly efficient in the management of pain with many indications, the safety of using this COX-2 inhibitor is still not clear attributing to their suspected unexpected adverse reactions.[4] In some patients who have a history of hypersensitivity, immunologically compromised or genetic susceptibility, the chances of developing a serious mucocutaneous reaction is very high.[9]
In the present case, the causality is “probable” to the drug etoricoxib with a score of seven on Naranjo Causality assessment scale and “probable” on World Health Organization scale to assess causality either. A severity of illness score has been adapted to predict the prognosis of TEN on the day of admission with score of TEN that is, severity-of-illness score for toxic epidermal necrolysis scale which considers seven factors namely age, malignancy, heart rate, body surface area involved on the 1st day, blood urea, serum glucose and serum bicarbonate levels.[10] In this case, the score obtained was three with a predicted mortality of 35.8%. The treatment involving dexamethasone and a secondary choice of pentoxifylline was found to be effective in the management in conditions where there were no other predisposing factors as seen in this case. Even though the chances of developing a serious unexpected reaction with etoricoxib is very rare, it cannot be completely neglected and requires a keen study of the history of the patient before prescribing this drug.
Acknowledgment
Our sincere thanks to Pharmacovigilance Programme of India (PvPI), National Coordinating Centre (NCC), Indian Pharmacopoeia Commission, Ghaziabad, Uttar Pradesh who has extended us every support in bringing this adverse drug reaction projected.
Footnotes
Source of Support: Adverse Drug Reaction Monitoring Center, Kakatiya Medical College, MGM Hospital, Warangal 506007 Telangna, India.
Conflict of Interest: No.
References
- 1.Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128:35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 2.Ellender RP, Peters CW, Albritton HL, Garcia AJ, Kaye AD. Clinical considerations for epidermal necrolysis. Ochsner J. 2014;14:413–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al. VIGOR Study Group. Comparison of upper gastrointestinal toxicity of Rofecoxib and Naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–8. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 4.Kreft B, Wohlrab J, Bramsiepe I, Eismann R, Winkler M, Marsch WC. Etoricoxib-induced toxic epidermal necrolysis: Successful treatment with infliximab. J Dermatol. 2010;37:904–6. doi: 10.1111/j.1346-8138.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- 5.Thirion L, Nikkels AF, Piérard GE. Etoricoxib-induced erythema-multiforme-like eruption. Dermatology. 2008;216:227–8. doi: 10.1159/000112930. [DOI] [PubMed] [Google Scholar]
- 6.Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200–1. doi: 10.2340/00015555-0381. [DOI] [PubMed] [Google Scholar]
- 7.Louden BA, Jorizzo JL. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Callen JP, Jorizzo JL, Bolognia JL, Piette WW, Zone JJ, editors. Dermatological Signs of Internal Disease. 4th ed. Philadelphia, PA: Elsevier; 2009. pp. 63–8. [Google Scholar]
- 8.Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi: 10.1186/1750-1172-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prashant T, Rajnikant P, Arin B, Dheeraj A, Anish C. Toxic epidermal necrolysis: An update. Asian Pac J Trop Dis. 2013;3:85–92. [Google Scholar]
- 10.Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. Scorten: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]


