Abstract
The plant pathogen Agrobacterium tumefaciens forms architecturally complex biofilms on inert surfaces. Adherence of A. tumefaciens C58 was significantly enhanced under phosphate limitation compared to phosphate-replete conditions, despite slower overall growth under low-phosphate conditions. Replacement of Pi with sn-glycerol-3-phosphate and 2-aminoethylphosphonate yielded similar results. The increase in surface interactions under phosphate limitation was observed in both static culture and continuous-culture flow cells. Statistical analysis of confocal micrographs obtained from the flow cell biofilms revealed that phosphate limitation increased both the overall attached biomass and the surface coverage, whereas the maximum thickness of the biofilm was not affected. Functions encoded on the two large plasmids of A. tumefaciens C58, pTiC58 and pAtC58, were not required for the observed phosphate effect. The phosphate concentration at which increased attachment was observed triggered the phosphate limitation response, controlled in many bacteria by the two-component regulatory system PhoR-PhoB. The A. tumefaciens phoB and phoR orthologues could only be disrupted in the presence of plasmid-borne copies of the genes, suggesting that this regulatory system might be essential. Expression of the A. tumefaciens phoB gene from a tightly regulated inducible promoter, however, correlated with the amount of biofilm under both phosphate-limiting and nonlimiting conditions, demonstrating that components of the Pho regulon influence A. tumefaciens surface interactions.
Many microorganisms concentrate at solid-liquid interfaces in the environment, forming adherent multicellular structures generally described as biofilms (see references 15 and 16 for reviews). These adherent cells are often embedded within an extracellular matrix consisting of polysaccharides and other macromolecules. Biofilms can vary considerably in size, depth, and complexity, depending on the bacteria that form them and the prevailing environmental conditions. Bacteria residing within the biofilm are to some extent chemically and physically insulated from environmental stresses such as desiccation, nutrient limitation, and predatory grazing. Sessile bacteria also benefit from stable positioning at the solid-liquid interface, a site of increased chemical and physical activity. Owing to a variety of factors, biofilm populations are also afforded increased tolerance to antimicrobial treatment (56). In aqueous environments biofilms form at solid-liquid boundaries and can be continuously bathed in fluid or experience periodic saturation. In the terrestrial environment, biofilms and smaller multicellular aggregates form within the water film that coats soil particles and can be highly variable in their level of saturation (12). Biofilms are of significant agricultural, industrial, and medical interest because of their ubiquity and recalcitrance. Many host-associated bacteria, including pathogens and symbionts, can form biofilms on host tissues and even within the cells of their host (1).
Agrobacterium tumefaciens, a member of the α-Proteobacteria subdivision, is a plant pathogen that causes crown gall disease on eudicots and is most recognized for its remarkable ability to transfer DNA to plant cell nuclei (for a recent review, see reference 22). As with many other microbial pathogens, attachment to the host tissue is an essential step in the infection process. A. tumefaciens mutants that cannot attach to plants are avirulent, and plant mutants to which A. tumefaciens cannot effectively bind are resistant to infection (17, 65). The mechanism of attachment remains unclear, despite significant study. Several studies suggest that synthesis of cellulose and cyclic β-glucans by the infecting A. tumefaciens is required for stable, productive attachment (17, 41). Likewise a large segment of genes described as the Att cluster was implicated in attachment and virulence (41). However, recent publication of the A. tumefaciens genome sequence revealed that the Att cluster exists on a large plasmid called pAtC58, which is now known to be dispensable for virulence (23, 49, 62). In the terrestrial environment, a large proportion of virulent and avirulent A. tumefaciens strains exists as soil-associated saprophytes, in addition to those bacteria directly associated with plants (7, 46). We postulate that adherent A. tumefaciens populations on inert surfaces and those on plant tissues are both biofilms with a number of properties in common.
In this work we report that limiting inorganic phosphate (Pi) significantly influences the surface interactions of A. tumefaciens. Pi limitation is known to regulate upward of 400 different gene products in Escherichia coli, requiring the PhoR-PhoB two-component system (58, 59). Under Pi-replete conditions, the membrane-associated PhoR sensor kinase is thought to adopt a form with low kinase activity, maintenance of which requires the Pst high-affinity phosphate transport system and PhoU, an additional regulatory protein. Under Pi-limiting conditions, PhoR is somehow released from its repressive form, its autokinase activity is stimulated, and phosphotransfer to the response regulator PhoB ensues (38). Phosphorylated PhoB is active for DNA binding and associates with PHO box DNA sequence elements upstream of Pho regulon genes, thereby regulating transcription of these genes (39). Pho target genes code for a variety of functions beneficial under Pi limitation, including high-affinity phosphate transport, phosphate scavenging, and utilization of alternate phosphorus sources (59).
Phosphorus limitation has been studied considerably less extensively in members of the family Rhizobiaceae than in E. coli. Of those systems in which phosphate transport and the response to phosphate limitation have been examined, Sinorhizobium meliloti is the best understood. There are at least two phosphate transporters in S. meliloti, a high-affinity, low capacity transporter encoded by the phoCDET operon, and a second, low-affinity, high-capacity transporter encoded by the pit gene (2, 4). PhoB is required for transcriptional activation of phoCDET and repression of pit under phosphorus-limiting conditions (3). In contrast to E. coli, there is no evidence of a regulatory function for PhoU in S. meliloti, although the gene is conserved and located directly upstream of phoB. The S. meliloti phoR orthologue is encoded upstream of phoB, although there are no reports on its function (20, 42).
In the present study, we report increased surface-associated A. tumefaciens biomass in Pi-limited medium. The enhanced biofilm formation is mediated through the PhoR-PhoB two-component system and occurs in parallel with other features of the phosphate limitation response. Our findings on phosphate limitation and biofilm formation are consistent with previous studies suggesting that the phosphate limitation response significantly influences plant interactions of A. tumefaciens. These findings also provide an interesting contrast to those reported for the biocontrol agent Pseudomonas aureofaciens, in which biofilm formation is inhibited under phosphate starvation conditions (48).
MATERIALS AND METHODS
Strains, plasmids, reagents, and growth conditions.
All of the strains and plasmids used in this study are described in Table 1. Buffers, antibiotics, and microbiological media were obtained from Fisher Scientific (Pittsburgh, Pa.) and Sigma Chemical Co. (St. Louis, Mo.). DNA manipulations were performed in accordance with standard protocols (53). Plasmids were electroporated into A. tumefaciens by a standard method (44). DNA sequencing was performed with ABI BigDye Terminator version 3.1 on an ABI 3700 sequencer operated by the Indiana Molecular Biology Institute. Oligonucleotides were obtained from Integrated DNA Technologies, Coralville, Iowa (sequences are available upon request). A. tumefaciens derivatives were grown in AT minimal salts medium and 15 mM (NH4)2SO4 with either 0.5% (wt/vol) glucose (ATGN) or 0.5% (wt/vol) mannitol (ATMN) as the carbon source (57). For phosphorus limitation experiments, the phosphate buffer of the AT medium was replaced with 5 mM imidazole buffer, pH 7, and a phosphorus source as specified. AT media with 50 and 500 μM phosphate are abbreviated ATMN-P50 and ATMN-P500, respectively. A crude preparation of the Agrobacterium autoinducer (AAI) N-3-oxooctanoyl-l-homoserine lactone was obtained from an AAI-overproducing A. tumefaciens derivative as described by He et al. (24). Antibiotics were used at the following concentrations (milligrams per liter): for A. tumefaciens, gentamicin, 500; kanamycin (KM), 150; spectinomycin, 50; streptomycin (SM), 2,000; for E. coli, ampicillin, 100; gentamicin, 25; KM, 25; SM, 25.
TABLE 1.
Strains and plasmids used in this study
| Bacterium or plasmid | Relevant feature(s) | Reference(s) |
|---|---|---|
| A. tumefaciens | ||
| C58 | Nopaline-agrocinopine type strain, pTiC58 | 60 |
| NTL4 | Ti plasmidless derivative, NT1 derivative; Tets | 37 |
| MLL2 | ΔexoA C58 derivative | Ramey et al., unpublished |
| TD1 | C58 derivative with pTD102 inserted; Kmr Smr Sucs | This study |
| TD2 | C58 derivative with phoR::Ω-Km; Sms Sucr; only obtained as TD2(pTD103) | This study |
| TD3 | C58 derivative with phoB::pTD104 (phoB+) | This study |
| TD5 | NTL4 derivative; pTi−phoB::pTD105 (phoB); only obtained as TD5(pTD115) | This study |
| E. coli | ||
| S17-1/λpir | λpir; Tra+, cloning host | 29, 54 |
| SY327/λpir | λpir; cloning host | 45 |
| DH5α F′ | Cloning host | 63 |
| TOP10 | Cloning host | Invitrogen |
| Plasmids | ||
| pBluescript II SK(+) | Standard cloning vector; Apr | Stratagene |
| pBBR1MCS-5 | Broad-host-range Plac expression vector; Gmr | 35 |
| pCR2.1-TOPO | TOPO TA Cloning vector; Apr Kmr | Invitrogen |
| pKNG101 | R6K ori; Sucs Smr | 30 |
| pVIK112 | R6K ori; lacZY for transcription fusions; Kmr | 29 |
| pHP45Ω-Km | Ω-Km cassette | 18 |
| pKOK6 | lacZ-Km cassette | 32 |
| pJZ383 | Ptac::gfpmut3; Spr; pVS replicon | J. Zhu; 14 |
| pBER103 | pBluescript II SK(+) carrying PtraI from A. tumefaciens R10 | This study |
| pRHG100 | pBBR1MCS-5 derivative carrying traR from A. tumefaciens R10 | This study |
| pTD102 | pKNG101 carrying phoR::Ω-Km | This study |
| pTD103 | pBBR1MCS-5 derivative carrying phoR | This study |
| pTD104 | pVIK112 carrying 5′-truncated phoB | This study |
| pTD105 | pVIK112 carrying phoB truncated at both ends | This study |
| pTD114 | pBBR1MCS-5 derivative; Plac::traR PtraI | This study |
| pTD115 | pTD114 carrying phoB | This study |
| pTD116 | pTD114 carrying lacZ-Kmr cassette from pKOK6 | This study |
Allelic replacement of phoR.
The phoR gene (University of Washington A. tumefaciens C58 gene no. Atu2735) of A. tumefaciens C58 was PCR amplified from genomic DNA with primers phoR-1 and phoR-2 and cloned into pBluescript II SK(+). A 343-bp NarI fragment within the phoR coding sequence was excised and replaced with the Ω-Km cassette from pHP45Ω-Km (HindIII fragment) (18) after blunting of all DNA ends with the Klenow fragment of DNA polymerase I. This phoR::Ω-Km construct was ligated into R6K suicide plasmid pKNG101 after cleaving with BamHI and XbaI to yield pTD102. Plasmid pTD102 was transformed into E. coli S17-1/λpir and then conjugated into A. tumefaciens C58 as described previously (19). Since pTD102 cannot be replicated in A. tumefaciens, only cells that have the construct recombined into the genome can grow under antibiotic selection. Transconjugants of the resulting strain, TD1 (see Results), were selected on plates with minimal medium containing SM and KM and streak purified, and the insertion of pTD102 into the phoR gene was verified by PCR. Cells were then plated on minimal medium with KM and 5% sucrose in order to select against the sacB gene on the integrated pTD102 plasmid. Sucrose selection should yield cells in which allelic replacement has occurred, leading to loss of the sacB gene along with the functional copy of phoR and the SM resistance gene (see Results). Continued selection for KM will prevent loss of the inserted plasmid by simple reversion of the insertion step and foster the allelic replacement. However, all of the clones isolated by this procedure (>250) were resistant to SM, indicating retention of the plasmid-encoded resistance gene, and those tested by PCR were found to have the normal-length phoR gene, indicating that the sucrose-resistant (Sucr) colonies were the result of mutations in sacB. When the selection was repeated after electroporation of pTD103, a pBBR1MCS-5-derived (35) plasmid containing a functional copy of phoR, SM-sensitive allelic replacement derivatives were readily obtained (see Results) and the interruption of the genomic phoR copy with phoR::Ω-Km was verified by PCR.
Campbell insertion to create a phoB-null mutant.
A central fragment (codons 53 to 183) of the phoB gene (UW C58 gene no. Atu2729) of A. tumefaciens C58 was PCR amplified with primers phoB-2 and phoB-3 from genomic DNA and ligated into pCR2.1-TOPO (Invitrogen, Carlsbad, Calif.). In parallel, a fragment with an intact 3′ end (codons 53 to 228) was amplified with primers phoB-1 and phoB-2 and treated likewise. The phoB fragments were then ligated into suicide vector pVIK112 (29) after restriction digestion with XbaI and XmaI. The resulting constructs, pTD104 (phoB fragment with an intact 3′ end) and pTD105 (internal phoB fragment), which cannot be replicated by A. tumefaciens, were each transformed into E. coli S17-1/λpir and then transferred into A. tumefaciens C58 by conjugation. Transconjugants carrying the plasmid-interrupted genomic copy of phoB were selected on agar plates with minimal medium and KM. The A. tumefaciens C58 derivatives with the pTD104 construct, which recreates a full-length phoB gene upon integration, were readily obtained. Despite repeated attempts, however, no Kmr transconjugants were isolated with an integration of pTD105, which would have resulted in truncation and inactivation of phoB. When the pTD105 construct was conjugated into A. tumefaciens NTL4 carrying plasmid pTD115 carrying a functional phoB copy (see next section), however, the desired phoB mutant was readily obtained (verified by PCR; data not shown).
Construction of an AHL-regulated phoB plasmid.
AHL-responsive controlled-expression vector pTD114 was constructed by using pRHG100, a pBBR1MCS-5 derivative with quorum-sensing transcription factor gene traR from A. tumefaciens R10 expressed from the lac operon promoter (Plac::traR). A short BamHI fragment was deleted from pRHG100 to make the BamHI and SacI sites unique and remove the SmaI/XmaI site. The promoter region and part of the traI gene were PCR amplified from plasmid pBER103 with primers PtraI-5′, containing an engineered SacI site, and PtraI-3′, containing additional NheI, SmaI/XmaI, and XbaI sites. The PCR product was ligated into the SacI and XbaI sites of the pRHG100 derivative mentioned above. The NheI fragment containing part of the traI gene was deleted from the resulting plasmid to yield expression vector pTD114. Plasmid pTD116, which was used to evaluate the functionality of pTD114, was constructed by inserting the BamHI fragment from pKOK6 (32) containing the promoterless lacZ gene and the Kmr marker into the BamHI site in line with PtraI. The phoB gene, including its native Shine-Dalgarno sequence but not its promoter, was amplified from A. tumefaciens C58 genomic DNA with primers phoB-4 and phoB-1 and cloned into the BamHI and XbaI sites of pBluescript II SK(+) by means of restriction sites incorporated into the primer sequences. The integrity of the inserted gene was verified by DNA sequencing. The XmaI/XbaI fragment from this plasmid containing the phoB gene was cloned into the corresponding sites of pTD114 to yield pTD115.
Growth, microscopic observation, and quantitation of coverslip biofilms.
Biofilms were grown in 12-well polystyrene cell culture dishes (Corning Inc., Corning, N.Y.) containing polyvinyl chloride (PVC) coverslips placed vertically in the wells. Wells were inoculated with a dilute (approximate optical density at 600 nm [OD600] = 0.04) culture in minimal medium and incubated at 28°C for 48 h. Biofilms were visualized macroscopically by crystal violet (CV) staining (50) or microscopically by phase-contrast and epifluorescence microscopy. For each sample, the coverslip was removed from the well and rinsed in a stream of double-distilled H2O to remove planktonic cells. The coverslips were stained by incubation in a 1% (wt/vol) CV solution for at least 10 min and rinsed again with fresh double-distilled H2O to remove excess CV. For microscopy, the coverslips were rinsed and one side was gently scraped clean with a razor blade. The scraped coverslips were placed on a slide and kept hydrated with a drop of AT buffer under a fresh coverslip. Microscopy was carried out with a Nikon E800 with Metamorph software for phase and epifluorescence microscopy. For biofilm quantitation, CV was solubilized from the stained coverslips in 1 ml of dimethyl sulfoxide (DMSO). Both the optical density and the absorbance of the solubilized CV were measured at either 620 or 600 nm in 96-well, flat-bottomed microtiter plates (Thermo Electron Corp. Consumables, Vantaa, Finland) on a Labsystems Multiscan RC microtiter plate reader with appropriate filters.
Flow cell configuration and analyses.
The interaction of A. tumefaciens C58 carrying pJZ383 (Ptac::gfpmut3) for green fluorescent protein expression was examined on a glass slide surface in a once-through flow cell (1 by 4 by 40 mm) configured similarly to that described by Christensen et al. (13). Fresh ATMN-P50 or ATMN-P500 was pumped through the flow cell with a Watson-Marlow low-pulse peristaltic pump. Each chamber of the flow cell was inoculated with 200 μl of a concentrated A. tumefaciens C58 suspension (OD600 = 0.4). The bacteria were incubated within the flow cells in the absence of flow for 30 min, and flow (approximately 3 ml/h) was subsequently initiated. Surface colonization of the glass slide was monitored for several days by confocal scanning laser microscopy (CSLM) with a Zeiss LSM 510 (Carl Zeiss, Jena, Germany). Five stacks of z sections (approximately 1-μm spacing) were taken for each of two flow cell chambers per treatment, and the results shown are the averages of 10 sample image stacks, corresponding to a total surface area of 2.1 · × 105 μm2, sufficient to yield representative quantitative biofilm data (33, 34). Images were acquired with the Zeiss LSM 510 software package and analyzed with the COMSTAT program (25) running in MatLab 6.5. Three-dimensional representations where created with Imaris 3.3 software (Bitplane AG, Zürich, Switzerland).
RESULTS
Phosphorus limitation increases adherence of A. tumefaciens C58 in static culture.
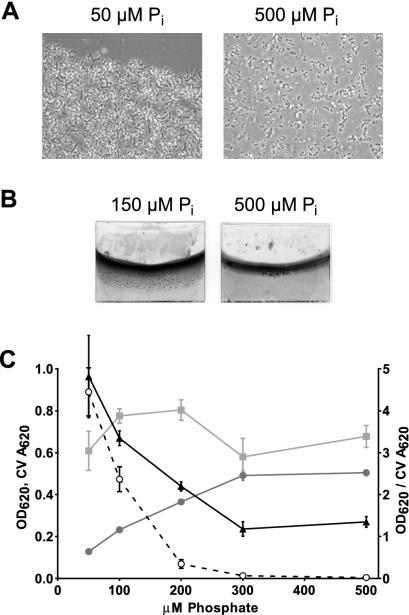
During physiological studies to determine how nutrients influence biofilm formation in A. tumefaciens, we found that surface attachment increased markedly when the phosphate concentration became limiting. A. tumefaciens C58 biofilm cultures were grown on PVC coverslips for 2 days in modified minimal ATMN with phosphate levels ranging from 50 to 500 μM. The OD620 was measured to quantify the cell density in the planktonic phase of the culture. Coverslips were stained with CV, the dye bound to the adherent cell mass on the coverslip was extracted in DMSO, and the A620 of this solution was measured (in later experiments, described below, we used the A600 and OD600 instead). While attachment at 500 μM was comparable to that at higher concentrations (regular AT medium contains 79 mM phosphate buffer), increased adherence was observed at less than 300 μM phosphate both in micrographs (Fig. 1A) and in CV-stained coverslips (Fig. 1B and C). Absolute attached biomass (CV A620) eventually decreased below 150 μM because of the greatly reduced cell density in cultures with lower phosphate concentrations (e.g., by a factor of four between 300 and 50 μM). The increase in CV staining was particularly striking when the absolute amount of biofilm was corrected for planktonic growth by division of the CV A620, reflecting the attached biomass, with the cell density (OD) of the planktonic culture. This ratio (A620/OD620) indicates a continuous increase in relative attached biomass with decreasing phosphate concentration in the medium (Fig. 1C). These observations suggest that Pi limitation leads to increased surface adherence of the cells. Alkaline phosphatase (AP) activity, generally considered a reflection of Pho regulon activation (59), was assayed in cells from the liquid phase of the culture as previously performed with A. tumefaciens by Mantis and Winans (40) and described elsewhere (10). AP activity essentially paralleled the relative biofilm formation by showing an inversely proportional relationship to the phosphate concentration below 300 μM (Fig. 1C). On the basis of these findings, we hypothesized that the phosphate-responsive PhoR-PhoB two-component system, which is known to regulate the response to Pi starvation and induce AP expression in E. coli and other bacteria, acts on one or more target genes that affect biofilm formation in A. tumefaciens.
FIG. 1.
Phosphate concentration influences A. tumefaciens C58 adhesion in static culture. (A) Light micrographs (×100 objective) of A. tumefaciens C58 cells adhering to PVC coverslips and grown for 48 h in 50 or 500 μM phosphate, as indicated. (B) CV staining of adherent A. tumefaciens biofilms on PVC coverslips grown for 48 h in 150 or 500 μM, as indicated. (C) Different cultures of A. tumefaciens C58 grown over a range of phosphate concentrations for 48 h with monitoring of the OD620 of planktonic-phase cultures (filled circles), the AP activity of planktonic cells (open circles), the A620 values of DMSO-solubilized CV from adherent cells (squares), and the CV A620/OD620 ratio (triangles).
The observed enhancement of surface interactions of A. tumefaciens under limiting conditions was independent of the source of phosphorus. The same trends of biofilm formation in response to Pi levels were observed for cultures grown in 2-aminoethylphosphonate and sn-glycerol-3-phosphate as phosphorus sources (data not shown).
Phosphorus limitation in flow cell cultures.
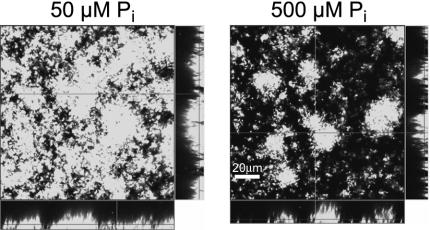
We examined the effect of Pi limitation on adherence to glass in continuous-culture once-through flow cells. Biofilms of A. tumefaciens C58 were grown in flow cells continuously provided with either ATMN-P50 or ATMN-P500. Biofilm formation on the glass coverslip that forms one wall of the flow cells was monitored by CSLM, and image stacks were subjected to statistical evaluation with the COMSTAT program (25) (Table 2). The total biovolume attached to the surface was higher in ATMN-P50 than in ATMN-P500 from the first time point (16 h), but the difference only became significant after about 2 days (P = 2.3 · 10−7, one-tailed heteroscedastic t test). At 62 h postinoculation, the biofilms grown under Pi-limited conditions contained 2.7 times as much biomass as the biofilms grown in ATMN-P500. This effect was almost entirely due to more complete substratum coverage (87% in ATMN-P50 versus 41% in ATMN-P500 at 62 h) because the maximum thickness did not significantly vary between the cultures. The biofilms grown in ATMN-P500 appeared patchy, i.e., consisting of isolated microcolonies, whereas the ATMN-P50 biofilms showed a more complete substratum coverage and therefore appeared much smoother (Fig. 2). This was also reflected in the roughness coefficient, which decreased much more rapidly in the ATMN-P50 cultures. The roughness coefficient at 62 h for ATMN-P50 biofilms was less than half that calculated for ATMN-P500 biofilms, indicating a more homogeneous surface colonization in Pi-limited flow cells. We define the specific properties of these A. tumefaciens surface interactions and the biofilms that result from growth under Pi limitation as the SinPL phenotype (surface interactions under Pi limitation).
FIG. 2.
Flow cell biofilms in low and high phosphate concentrations. CSLM images from 62-h A. tumefaciens C58 (pJZ383, Ptac::gfpmut3) biofilms grown on PVC at 50 and 500 μM phosphate. The right side and bottom of each panel are reconstructed vertical cross sections of the biofilm. Images were obtained with a Zeiss LSM 510 microscope and processed with Imaris 3.3 software. See Table 2 for COMSTAT analysis of the biofilms.
Potential essentiality of PhoR-PhoB in A. tumefaciens under phosphate-replete conditions.
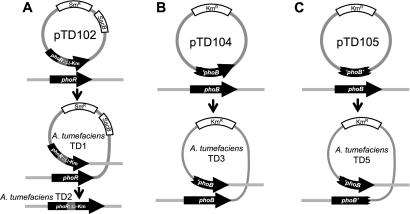
To test the hypothesis of Pho regulon involvement in the SinPL phenotype, a null mutant without a functional PhoR-PhoB system was desired. Allelic replacement of the phoR gene with a phoR::Ω-Km construct was attempted by a two-step process (Fig. 3A). The first step—integration of suicide vector pTD102 into the A. tumefaciens genome by homologous recombination into the phoR gene—readily yielded the desired recombinants. The second step, a subsequent recombination event removing the plasmid backbone carrying the Smr marker—and with it the functional phoR copy—from the genome, was not observed despite counterselection against the sacB sucrose sensitivity marker on the plasmid (a functional sacB gene is lethal in the presence of sucrose [Sucs]). Instead of the desired phoR mutant (Sucr Kmr Sms), only putative sacB mutants (Sucr Kmr Smr) were obtained and confirmed by PCR. The selection was performed on both ATMN and Luria-Bertani plates with the same results. This suggested the possibility that phoR might be essential despite an abundance of phosphate in the medium. The second selection step was therefore repeated after transforming the cells with plasmid pTD103, a plasmid expressing a functional phoR gene from the lac promoter (Plac::phoR). The allelic replacement recombinants were readily obtained in the presence of pTD103. The successful allelic replacement of the chromosomal phoR gene in the presence of the pTD103 plasmid suggests that the failure to obtain such mutants in the absence of the plasmid was not due to a faulty construct and is consistent with the notion that phoR is essential in A. tumefaciens.
FIG. 3.
Mutagenesis constructs for phoR and phoB. (A) Allelic replacement mutagenesis of A. tumefaciens phoR with derivative pTD102 to generate strains TD1 (single recombinant) and TD2 (double recombinant). (B and C) Campbell integration mutagenesis of phoB with pTD104 to retain a functional copy of phoB in TD3 (B) and pTD105 to disrupt phoB in TD5 (C). Single quotes indicate truncation of phoB at either the 5′ end (‘phoB), the 3′ end (phoB'), or both ends (‘phoB').
As an alternative strategy to obtain a mutant deficient in its adaptation to the Pi concentration, we attempted to generate a null mutation of phoB by means of a Campbell insertion (pTD105, Fig. 3C). No Kmr plasmid integrants were obtained. A plasmid that carried a 5′-truncated phoB gene with an intact 3′ end (pTD104), however, integrated readily at phoB. Such recombinants (strain TD3) retain one complete copy of the phoB gene after a single recombination (Fig. 3B). Similarly, pTD105 (internal phoB fragment) could be inserted into the A. tumefaciens NTL4 genome in the presence of a functional phoB copy on expression vector pTD115 (strain TD5, Fig. 3C). These observations are consistent with those made for the phoR gene and suggest that these phosphate-responsive regulators might have essential functions in A. tumefaciens.
Construction and testing of a quorum sensing-controlled expression system.
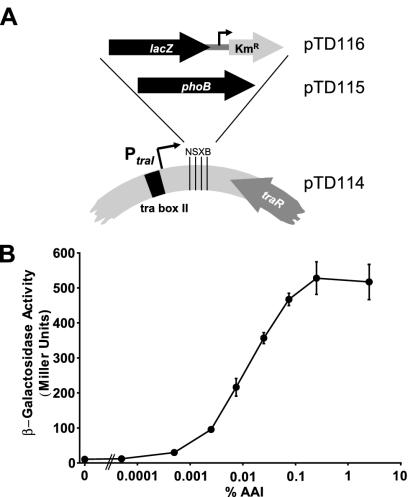
One of the most tightly controlled promoters in A. tumefaciens is that of the traI gene (PtraI) on the virulence plasmid pTi, which encodes the N-3-oxo-octanoyl-l-homoserine lactone synthase (19, 28), the enzyme catalyzing synthesis of the AAI quorum-sensing pheromone. PtraI is controlled in response to AAI levels by the transcriptional activator TraR. We used this activation system to construct expression vector pTD114 (Fig. 4A), which allows AAI-controlled expression of any gene ligated into the cloning site downstream of PtraI. In the absence of a functional traI gene (owing to either a mutation or the absence of pTi), AAI levels depend solely on exogenous addition. The lacZ-Kmr cassette from pKOK6 was cloned into expression vector pTD114 (Fig. 4A) to measure promoter activity in response to AAI concentration. The resulting construct was electroporated into A. tumefaciens NTL4 cells, which lack pTi and therefore have no endogenous quorum-sensing system. Cultures containing a range of AAI concentrations were harvested at mid-exponential phase, and expression of the PtraI::lacZ fusion was assayed by measuring β-galactosidase activity. The response curve (Fig. 4B) shows that PtraI::lacZ expression from the vector is proportional to the log of the AAI concentration over a fixed range, before reaching saturation. From the basal level to maximum induction, a 50-fold increase in expression was achieved.
FIG. 4.
AAI-controlled expression vector system. (A) Schematic representation of pTD115 organization and derivation of pTD115 (PtraI::phoB) and pTD116 (PtraI::lacZ-Km) indicating unique restriction sites (N, NheI; S, SmaI/XmaI; X, XbaI; B, BamHI). (B) β-Galactosidase dose-response curve of A. tumefaciens harboring pTD116 (PtraI::lacZ-Km) as a function of the crude AAI preparation in the medium.
Controlled expression of the phoB regulatory gene modulates adherence.
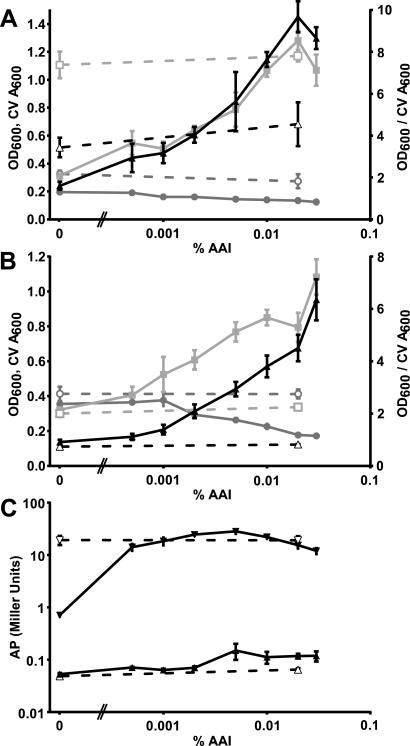
Plasmid pTD115 was constructed by placing a functional phoB gene under control of the traI promoter in pTD114 (Fig. 4A). PVC coverslip cultures with ATMN-P50 and ATMN-P500 were inoculated with A. tumefaciens TD5 (NTL4 phoB::Kmr) carrying plasmid pTD115 (Plac::traR PtraI::phoB). Each assay was performed in triplicate, and biofilm formation under various concentrations of a crude AAI preparation was assessed by CV staining and measuring CV A600 values, again in parallel with the OD600 values of the planktonic phase. AP activity was also determined from the planktonic phase of the culture. No effect of AAI was observed in the absence of the pTD115 plasmid (dashed lines, Fig. 5A, B, and C). Under phosphate-limiting conditions (50 μM Pi), growth dropped by more than 30% but CV A600 values increased fourfold, from no induction to optimal induction (0.02% [vol/vol] AAI, Fig. 5A). Relative to the cell density of the planktonic culture, the increase was sixfold. Strong phoB induction beyond the optimum of 0.02% (vol/vol) AAI led to a decrease in both growth and adhered biomass (Fig. 5A). The AP activity increased with increasing phoB induction until reaching a maximum at 0.005% (vol/vol) AAI, well before the maximal biofilm formation (Fig. 5C). Under phosphate-replete conditions (500 μM Pi), the cell density dropped by more than 50% between no induction (0% AAI) and the maximum induction level tested (0.03% [vol/vol] AAI). CV A600 values, on the other hand, increased more than threefold, whereas biofilm relative to cell density increased sevenfold (Fig. 5B). In contrast to the Pi-limited cultures, at high AAI levels (>0.02%) there was no decrease in biofilm formation. AP activity was lower by a factor of about 190, and without AAI induction, the values were close to the limit of detection. Although induction appears clearly less pronounced under these circumstances, the general trend is similar to that observed under phosphate limitation (Fig. 5C). It should be noted that under Pi-replete conditions a much smaller percentage of the PhoB protein gets activated by phosphorylation than under Pi starvation, which explains the differences between Fig. 5A and B. Despite the lack of the external stimulus (Pi starvation), overexpression of PhoB led to induction of both biofilm formation and AP activity at 500 μM, as it did at 50 μM.
FIG. 5.
Controlled expression of phoB influences biofilm formation at high and low phosphate concentrations. Dose-response curves of A. tumefaciens TD5 (phoB::pTD105) harboring pTD115 (PtraI::phoB) over a range of AAI concentrations after 48 h of growth at 50 (A) or 500 (B) μM phosphate are shown. In panels A and B, the OD600 of planktonic cells (filled circles), the A600 values of DMSO-solubilized CV from adherent cells (filled squares), and the CV A600/OD600 ratio (filled triangles) are shown. Dashed lines with open symbols correspond to filled symbols but are for A. tumefaciens NTL4 (wild-type phoB) without pTD115. (C) AP activities in response to AAI dose at 50 (inverted triangles) and 500 (upright triangles) μM phosphate in either A. tumefaciens TD5(pTD115) (filled symbols) or A. tumefaciens NTL4 without pTD115 (open symbols).
The SinPL phenotype is independent of plasmids pTiC58 and pAtC58.
The A. tumefaciens C58 genome contains two large curable plasmids, tumor-inducing plasmid pTiC58 and pAtC58, a 0.54-Mb plasmid that is dispensable for virulence. To determine whether functions encoded on either of these two plasmids are required for the SinPL phenotype, plasmidless strain UIA5, which is isogenic to C58 but cured of pTiC58 and pAtC58, was tested for biofilm formation in ATMN-P50 and ATMN-P500. Although the absolute amount of biofilm formation differed between UIA5 and C58, they demonstrated similar responses to Pi limitation (Table 3), leading to the conclusion that neither plasmid pTiC58 nor pAtC58 (a collective 0.76 Mb of the 5.67-Mb A. tumefaciens C58 genome) is required for the phosphorus limitation effect on biofilm formation.
TABLE 3.
SinPL effect of plasmidless strain UIA5
| Strain | [Pi] (μM) | CV A600 | OD600 | A600/OD600 |
|---|---|---|---|---|
| C58a | 50 | 1.23 (0.02)b | 0.10 (<0.01) | 12.2 (0.2) |
| 500 | 0.97 (0.07) | 0.39 (0.01) | 2.5 (0.1) | |
| UIA5 | 50 | 1.10 (0.03) | 0.24 (0.01) | 4.6 (0.2) |
| 500 | 0.80 (0.07) | 0.38 (0.01) | 2.1 (0.2) |
Wild type.
Standard errors of the means are in parentheses.
DISCUSSION
In this study we have found that A. tumefaciens C58 forms more robust biofilms under Pi limitation than under Pi-saturating conditions, with greater adherent biomass, average thickness, and surface coverage. The more uniform distribution of biomass over the surface under Pi limitation is reflected in a decreased roughness coefficient (Table 2). We abbreviate the overall surface interaction phenotype exhibiting these features under Pi limitation as SinPL. Comparisons of early-stage adherent populations in flow cells (16 h postinoculation) suggest an early trend in which the Pi-limited cultures already have adopted the SinPL phenotype, although the biovolume and average thickness are not significantly different from those of the Pi-replete biofilms. At later stages the SinPL phenotype is much more pronounced and significantly different for all parameters except maximum thickness. This stimulatory effect of Pi limitation is independent of the source of phosphorus in the culture (Pi, 2-aminoethylphosphonate, or sn-glycerol-3-phosphate). The SinPL phenotype is clearly observed for biofilms grown in static culture on PVC surfaces and in continuous-culture format on the borosilicate glass slides of flow cells. Induction of the SinPL phenotype parallels the general Pi limitation response, as indicated by induction of AP activity, suggesting that the functions mediating this response may be a component of the Pho regulon.
TABLE 2.
COMSTAT analysis of A. tumefaciensa flow cell biofilms
| Time (h) | [Pi] (μM) | Biomassb | Biofilm thickness (μm)
|
Substratum coverage (%) | Roughness coefficientc | |
|---|---|---|---|---|---|---|
| Avg | Max | |||||
| 16 | 50 | 0.51 (0.08) | 0.41 (0.07) | 12.26 (0.91) | 14.7 (1.8) | 1.73 (0.04) |
| 500 | 0.37 (0.05) | 0.32 (0.04) | 14.42 (1.13) | 6.7 (0.5) | 1.85 (0.01) | |
| 38 | 50 | 1.93 (0.33) | 1.70 (0.31) | 18.92 (1.24) | 37.4 (4.5) | 1.26 (0.08) |
| 500 | 1.38 (0.14) | 1.23 (0.15) | 19.16 (0.83) | 24.9 (1.2) | 1.50 (0.04) | |
| 62 | 50 | 6.98 (0.43) | 6.40 (0.42) | 22.37 (2.13) | 86.7 (1.7) | 0.53 (0.02) |
| 500 | 2.55 (0.16) | 2.31 (0.15) | 20.43 (1.59) | 41.3 (2.3) | 1.23 (0.04) | |
A. tumefaciens C58 harboring pJZ383 (Ptac::gfpmut3). Values are averages of 10 fields of view from two separate flow cells. Standard errors of the means are in parentheses.
Cubic micrometer of fluorescent material per square micrometer of surface.
Dimensionless coefficient that reflects biofilm heterogeneity as variability in biofilm thickness (25).
The simple genetic experiment of disrupting the key regulators of the Pho regulon, phoR and phoB, in the A. tumefaciens C58 genome was unexpectedly complicated. Our findings suggest that the phoR-phoB two-component regulatory system is essential in A. tumefaciens, even under phosphate-replete conditions. We developed a novel controlled-expression system based on the A. tumefaciens TraR quorum-sensing regulator that allowed us to tightly regulate phoB expression and which may be of utility in studying other processes in bacteria that do not produce endogenous AHLs. Experiments in which expression of phoB was under the control of TraR in a genetic background carrying a phoB-null mutation clearly implicate the PhoR-PhoB two-component system in development of the SinPL phenotype. Elevated expression of phoB increases the adherent biomass as measured by DMSO-solubilized CV-stained biofilms under both high- and low-phosphate conditions, and this increase is coincident with that of AP. These findings are consistent with a model in which the Pi limitation response, as transduced via PhoB, is directly responsible for the transition to the SinPL phenotype.
Influence of phosphate limitation on biofilm formation.
In the plant biocontrol agent P. aureofaciens, phosphate limitation is reported to diminish surface interactions and biofilm development (48). In addition, mutations that lead to constitutive induction of the Pi limitation response through PhoR-PhoB (pstA- and pstC-null mutants) prevent biofilm formation in vitro and reduce the antifungal properties of P. aureofaciens. Conversely, phoB and phoR mutants form apparently normal biofilms on PVC, even under Pi-limiting conditions. The present report of enhanced biofilm formation by Pi-limited A. tumefaciens or strains that artificially induce the Pho regulon stands in contrast to the findings from P. aureofaciens. This contrast may reflect the differential host interactions of the two genera—commensal biocontrol agent and invasive pathogen, respectively. It should also be noted that two other strains tested in the P. aureofaciens study, P. aeruginosa and Serratia entomophila, did not show differential biofilm formation in Pi-limiting cultures (48).
Another aspect of phosphate metabolism, polyphosphate synthesis via polyphosphate kinase, is reported to be essential for biofilm formation and virulence in P. aeruginosa (51). The biofilm requirement was traced to an inhibitory effect on both the Las and Rhl quorum-sensing systems. A. tumefaciens has a very well-studied AHL quorum-sensing system, based on the AHL-responsive TraR transcriptional regulator (19, 28, 64). All recognized components of the TraR quorum-sensing system are encoded on the Ti plasmid. We find that both A. tumefaciens NTL4, a derivative cured of the Ti plasmid, and A. tumefaciens UIA5, cured of the Ti plasmid and the 0.543-Mb pAtC58 plasmid (formerly known as the cryptic plasmid), demonstrate the SinPL phenotype under Pi limitation. Likewise, A. tumefaciens C58 derivatives that constitutively express TraR target genes are not notably different from wild-type C58 for biofilm formation (T. Danhorn and C. Fuqua, unpublished data). A direct requirement of the A. tumefaciens ppk gene for the SinPL phenotype through quorum sensing is therefore unlikely, although the fluctuations of internal phosphate pools in such a mutant might affect A. tumefaciens biofilm formation.
PhoR and PhoB in A. tumefaciens.
PhoR and PhoB have not been studied in A. tumefaciens and have not been identified in random genetic screens for regulatory mutants (perhaps because of the essentiality we report here). The region encoding PhoR and PhoB in A. tumefaciens C58 is highly conserved with the PhoR-PhoB region in S. meliloti, and therefore similarities between the PhoR-PhoB systems in these closely related bacteria seem likely. In light of this facile similarity, our inability to obtain null mutants in the A. tumefaciens phoR and phoB genes, except in the presence of plasmid-borne copies of these genes, is surprising. These findings suggest that PhoR and PhoB may be essential in A. tumefaciens under the conditions applied in this study (minimal and complex media) despite ample phosphorus. In contrast, phoB-null mutants of S. meliloti are fully viable and are competent for root nodulation (3). In the present study we circumvented this problem by expressing phoB under the tight regulation of TraR. Surprisingly, the TD5 phoB-null mutant that carries our PtraI::phoB traR plasmid (pTD115) is not suppressed for growth, even when no AHL inducer is provided. The normal growth of this derivative suggests that although PhoB, and by analogy PhoR, may have essential functions in A. tumefaciens, basal expression from PtraI on the pTD114 derivative is sufficient to fully satisfy this requirement. Plasmid pTD114 was originally derived from pBBR1MCS-5, a broad-host-range plasmid that maintains a high copy number in A. tumefaciens (Danhorn and Fuqua, unpublished, and reference 35). We are currently constructing a strain with an integrated single copy of our quorum sensing-controlled phoB expression system to further explore the effect of copy number. A. tumefaciens would be the first bacterium for which the PhoR-PhoB two-component system is required under phosphate-replete conditions (B. L. Wanner, personal communication). The mechanistic basis of this apparent essentiality is not yet clear but presumably would be due to one or more regulatory targets of PhoR and PhoB that are required for growth in standard laboratory culture.
Influence of Pi limitation on bacterial cell surface properties.
Several cell surface properties of A. tumefaciens and other members of the family Rhizobiaceae are influenced by phosphate. Exopolysaccharide production by S. meliloti is regulated through the PhoB response regulator, which activates production of galactoglucan (EPS II) under phosphate limitation by direct activation of the exp genes (43, 52). Conversely, succinoglycan (SCG; also referred to as EPS I in S. meliloti) is preferentially synthesized at high levels of phosphate. A. tumefaciens C58 is not known to synthesize galactoglucan and does not encode exp orthologues but does synthesize succinoglycan via the exo-exs gene products (11). Phosphorus levels could plausibly regulate SCG synthesis in A. tumefaciens, but preliminary experiments with an exoA-null mutant unable to synthesize SCG suggest that this exopolysaccharide is not required for biofilm formation and therefore cannot be responsible for the SinPL phenotype we observe (B. Ramey et al., unpublished data).
Cyclic β-1,2-glucans are important periplasmically localized cell surface polysaccharides produced by members of the family Rhizobiaceae. In A. tumefaciens, the cyclic β-1,2-glucans are known to be required for plant attachment and are thought to have a role in osmoregulation (17). Under phosphorus-replete conditions, the cyclic β-1,2-glucans are a mixture of anionic derivatives with sn-1-phosphoglycerol substituents and neutrally charged derivatives (8). Under phosphorus limitation, the composition switches to predominantly neutral derivatives. Synthesis and export of cyclic β-1,2-glucans to the periplasm are directed by the chvAB genes in A. tumefaciens (9). In addition to deficiencies in plant attachment and virulence, a chvB-null mutant was recently reported to be unable to associate with and transform human cells, while wild-type A. tumefaciens was able to do so, albeit inefficiently (36). Furthermore, individual chvA- and chvB-null mutants do not develop into biofilms on inert surfaces (Danhorn and Fuqua, unpublished). Taking these observations into consideration, the SinPL phenotype we observe may be linked to programmed modification of cyclic β-1,2-glucans during Pi limitation.
In several bacterial species Pi limitation stimulates the synthesis of phosphate-free membrane lipids such as sulfolipids, ornithine-containing lipids, and diacylglyceryl-N,N,N-trimethylhomoserine (DGTS), presumably to conserve Pi during new membrane synthesis (5, 47). Phospholipids comprise 95% of the extractable membrane lipids in S. meliloti grown in Pi-replete medium with no detectable DGTS (21). In contrast, membranes of S. meliloti grown in 20 μM phosphate are greater than 50% DGTS and phospholipids are reduced to 31% of the total lipid content. This dramatic incorporation of DGTS requires phoB, and membranes of S. meliloti phoB mutants contain no detectable DGTS. Incorporation of positively charged DGTS lipid instead of phospholipids, which carry negatively charged phosphate groups, will influence the net charge of the cell envelope and could influence cell surface interactions. In the related photosynthetic bacterium Rhodobacter sphaeroides, DGTS head group synthesis is mediated by enzymes encoded within the btaAB operon. These enzymes are conserved among diverse α-Proteobacteria, including S. meliloti and A. tumefaciens (31). Inspection of the A. tumefaciens C58 sequence upstream of the operon encoding its BtaA (Atu2119) and BtaB (Atu2120) orthologues reveals a strong consensus PHO box, a putative binding site for PhoB. This arrangement is suggestive of a similar Pi-dependent response on DGTS synthesis in A. tumefaciens C58.
Changes in cell envelope composition in response to phosphorus starvation are not limited to gram-negative bacteria. Several Bacillus species replace the phosphate-containing teichoic acids of their cell walls with teichuronic acid. In Bacillus subtilis, this reaction is controlled by the Pho regulon (55).
Phosphorus in the terrestrial environment.
Phosphorus is a major limiting nutrient in the bulk soil environment, generally present at bioavailable levels that average 1 μM and range from 0.1 to 10 μM (26). We observed a significant increase in the adherent growth of A. tumefaciens in culture medium with Pi concentrations at or below 50 μM, relative to that at the higher Pi concentrations typically used in standard laboratory medium (e.g., 79 mM in AT minimal medium; reference 57). It therefore seems likely that the more avid biofilm formation (SinPL) we observe in limiting phosphorus would be operational for A. tumefaciens residing saprophytically in most soils. Plants sequester 10 to 20 mM Pi in their root tissues (6), but a zone of depleted Pi, generated through the active Pi uptake mechanisms of the plant, extends 0.2 to 1 mm from the plant-soil interface, creating a decreasing gradient toward the plant surface (27). Relatively low Pi availability is inherent to the plant surface, while high Pi concentrations are available within the tissue. Pi limitation has also been demonstrated to potentiate activation of A. tumefaciens virulence via elevated expression of the regulatory gene virG through a PHO box (61). Our findings on the SinPL phenotype add to the list of features of A. tumefaciens that are activated under limiting Pi and have the potential to promote the disease process in plants. The SinPL phenotype may be beneficial during adaptation of colonizing bacteria to the low Pi concentration at the plant surface and also facilitate adherence to soil constituents in the terrestrial environment.
Acknowledgments
We acknowledge Yves Brun and Ellen Quardokus for helpful discussions regarding phosphate-responsive regulation and Bronwyn Ramey for assistance with flow cells and COMSTAT analysis, as well as critical reading of the manuscript. Thanks also to other members of the Fuqua laboratory for their input.
T.D. was supported by a 1-year Indiana University Floyd Microbiology Fellowship. M.G. received grants from the Danish Technical Research Council and the Villum Kann-Rasmussen Foundation. M.R.P. receives research funding through the National Science Foundation (MCB 0133-833). This project was supported by a grant from the U.S. Department of Agriculture to C.F. (CRI 2002-35319-12636).
REFERENCES
- 1.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 2.Bardin, S., S. Dan, M. Osteras, and T. M. Finan. 1996. A phosphate transport system is required for symbiotic nitrogen fixation by Rhizobium meliloti. J. Bacteriol. 178:4540-4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardin, S. D., and T. M. Finan. 1998. Regulation of phosphate assimilation in Rhizobium (Sinorhizobium) meliloti. Genetics 148:1689-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardin, S. D., R. T. Voegele, and T. M. Finan. 1998. Phosphate assimilation in Rhizobium (Sinorhizobium) meliloti: identification of a pit-like gene. J. Bacteriol. 180:4219-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benning, C., Z.-H. Huang, and D. A. Gage. 1995. Accumulation of a novel glycolipid and a betaine lipid in the cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 317:103-111. [DOI] [PubMed] [Google Scholar]
- 6.Bieleski, R. L. 1973. Phosphate pools, phosphate transport, and phosphate availability. Annu. Rev. Plant. Physiol. 24:225-252. [Google Scholar]
- 7.Bouzar, H., and L. W. Moore. 1987. Isolation of different Agrobacterium biovars from a natural oak savanna and tallgrass prairie. Appl. Environ. Microbiol. 53:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breedveld, M. W., A. J. Benesi, M. L. Marco, and K. J. Miller. 1995. Effect of phosphate limitation on synthesis of periplasmic cyclic β-(1,2)-glucans. Appl. Environ. Microbiol. 61:1045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breedveld, M. W., and K. J. Miller. 1994. Cyclic β-glucans of members of the family Rhizobiaceae. Microbiol. Rev. 58:145-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkman, E., and J. Beckwith. 1975. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and φ80 transducing phages. J. Mol. Biol. 96:307-316. [DOI] [PubMed] [Google Scholar]
- 11.Cangelosi, G. A., L. Hung, V. Puvanesarajah, G. Stacey, D. A. Ozga, J. A. Leigh, and E. W. Nester. 1987. Common loci for Agrobacterium tumefaciens and Rhizobium meliloti exopolysaccharide synthesis and their roles in plant interactions. J. Bacteriol. 169:2086-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, W. S., and L. J. Halverson. 2003. Reduced water availability influences the dynamics, development, and ultrastructural properties of Pseudomonas putida biofilms. J. Bacteriol. 185:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen, B. B., C. Sternberg, J. B. Andersen, R. J. J. Palmer, A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. [DOI] [PubMed] [Google Scholar]
- 14.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 15.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas, C. J., W. Halperin, and E. W. Nester. 1982. Agrobacterium tumefaciens mutants affected for attachment to plant cells. J. Bacteriol. 152:1265-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fellay, R., J. Frey, and H. Kirsch. 1984. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 19.Fuqua, W. C., and S. C. Winans. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176:2796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galibert, F., T. M. Finan, S. R. Long, A. Pühler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 21.Geiger, O., V. Rohrs, B. Weissenmayer, T. M. Finan, and J. E. Thomas-Oates. 1999. The regulator gene phoB mediates phosphate stress-controlled synthesis of the membrane lipid diacylglyceryl-N,N,N-trimethylhomoserine in Rhizobium (Sinorhizobium) meliloti. Mol. Microbiol. 32:63-73. [DOI] [PubMed] [Google Scholar]
- 22.Gelvin, S. B. 2003. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 67:16-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodner, B., G. Hinkle, S. Gattung, N. Miller, M. Blanchard, B. Qurollo, B. S. Goldman, Y. Cao, M. Askenazi, C. Halling, L. Mullin, K. Houmiel, J. Gordon, M. Vaudin, O. Iartchouk, A. Epp, F. Liu, C. Wollam, M. Allinger, D. Doughty, C. Scott, C. Lappas, B. Markelz, C. Flanagan, C. Crowell, J. Gurson, C. Lomo, C. Sear, G. Strub, C. Cielo, and S. Slater. 2001. Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294:2323-2328. [DOI] [PubMed] [Google Scholar]
- 24.He, X., W. Chang, D. L. Pierce, L. O. Seib, J. Wagner, and C. Fuqua. 2003. Quorum sensing in Rhizobium sp. strain NGR234 regulates conjugal transfer (tra) gene expression and influences growth rate. J. Bacteriol. 185:809-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heydorn, A., A. Toftgaard Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. Kjaer Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 26.Hinsinger, P. 2001. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes. Plant Soil. 237:173-195. [Google Scholar]
- 27.Holford, I. C. R. 1997. Soil phosphorus: its measurement and its uptake by plants. Aust. J. Soil Res. 35:227-239. [Google Scholar]
- 28.Hwang, I., P.-L. Li, L. Zhang, K. R. Piper, D. M. Cook, M. E. Tate, and S. K. Farrand. 1994. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc. Natl. Acad. Sci. USA 91:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalogeraki, V. S., and S. C. Winans. 1997. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188:69-75. [DOI] [PubMed] [Google Scholar]
- 30.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 31.Klug, R. M., and C. Benning. 2001. Two enzymes of diacylglyceryl-O-4′-(N,N,N,-trimethyl)-homoserine biosynthesis are encoded by btaA and btaB in the purple bacterium Rhodobacter sphaeroides. Proc. Natl. Acad. Sci. USA 98:5910-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kokotek, W., and W. Lotz. 1989. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene 84:467-471. [DOI] [PubMed] [Google Scholar]
- 33.Korber, D. R., J. R. Lawrence, M. J. Hendry, and D. E. Caldwell. 1993. Analysis of spatial variability within Mot+ and Mot− Pseudomonas fluorescens biofilms using representative elements. Biofouling 7:339-358. [Google Scholar]
- 34.Korber, D. R., J. R. Lawrence, M. J. Hendry, and D. E. Caldwell. 1992. Programs for determining statistically representative areas of microbial biofilms. Binary 4:204-210. [Google Scholar]
- 35.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. I. Roop, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 36.Kunik, T., T. Tzfira, Y. Kapulnik, Y. Gafni, C. Dingwall, and V. Citovsky. 2001. Genetic transformation of HeLa cells by Agrobacterium. Proc. Natl. Acad. Sci. USA 98:1871-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo, Z.-Q., T. E. Clemente, and S. K. Farrand. 2001. Construction of a derivative of Agrobacterium tumefaciens C58 that does not mutate to tetracycline resistance. Mol. Plant-Microbe Interact. 14:98-103. [DOI] [PubMed] [Google Scholar]
- 38.Makino, K., H. Shinagawa, M. Amemura, T. Kawamoto, M. Yamada, and A. Nakata. 1989. Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J. Mol. Biol. 210:551-559. [DOI] [PubMed] [Google Scholar]
- 39.Makino, K., H. Shinagawa, M. Amemura, S. Kimura, A. Nakata, and A. Ishihama. 1988. Regulation of the phosphate regulon of Escherichia coli: activation of pstS transcription by PhoB protein in vitro. J. Mol. Biol. 203:85-95. [DOI] [PubMed] [Google Scholar]
- 40.Mantis, N. J., and S. C. Winans. 1993. The chromosomal response regulatory gene chvI of Agrobacterium tumefaciens complements an Escherichia coli phoB mutation and is required for virulence. J. Bacteriol. 175:6626-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthysse, A. G. 1987. Characterization of nonattaching mutants of Agrobacterium tumefaciens. J. Bacteriol. 169:313-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDermott, T. R. 2000. Phosphorus assimilation and regulation in the rhizobia, p. 529-548. In E. W. Triplett (ed.), Prokaryotic nitrogen fixation: a model system for the analysis of a biological process. Horizon Scientific Press, Norfolk, England.
- 43.Mendrygal, K. E., and J. E. Gonzalez. 2000. Environmental regulation of exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 182:599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mersereau, M., G. J. Pazour, and A. Das. 1990. Efficient transformation of Agrobacterium tumefaciens by electroporation. Gene 90:149-151. [DOI] [PubMed] [Google Scholar]
- 45.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mills, A. L., and D. K. Powelson. 1996. Bacterial interactions with surfaces in soils, p. 25-57. In M. Fletcher (ed.), Bacterial adhesion: molecular and ecological diversity. Wiley-Liss, New York, N.Y.
- 47.Minnikin, D. E., H. Abdolrahimzadeh, and J. Baddiley. 1974. Replacement of acidic phospholipids by acidic glycolipids in Pseudomonas diminuta. Nature 249:268-269. [DOI] [PubMed] [Google Scholar]
- 48.Monds, R. D., M. W. Silby, and H. K. Mahanty. 2001. Expression of the Pho regulon negatively regulates biofilm formation by Pseudomonas aureofaciens PA147-2. Mol. Microbiol. 42:415-426. [DOI] [PubMed] [Google Scholar]
- 49.Nair, G. R., Z. Liu, and A. N. Binns. 2003. Reexamining the role of the accessory plasmid pAtC58 in the virulence of Agrobacterium tumefaciens strain C58. Plant Physiol. 133:989-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 51.Rashid, M. H., K. Rumbaugh, L. Passador, D. G. Davies, A. N. Hamood, B. H. Iglewski, and A. Kornberg. 2000. Polyphosphate kinase is essential for biofilm development, quorum sensing, and virulence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:9636-9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rüberg, S., A. Pühler, and A. Becker. 1999. Biosynthesis of the exopolysaccharide galactoglucan in Sinorhizobium meliloti is subject to a complex control by the phosphate-dependent regulator PhoB and the proteins ExpG and MucR. Microbiology 145:603-611. [DOI] [PubMed] [Google Scholar]
- 53.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 54.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 55.Soldo, B., V. Lazarevic, M. Pagni, and D. Karamata. 1999. Teichuronic acid operon of Bacillus subtilis 168. Mol. Microbiol. 31:795-805. [DOI] [PubMed] [Google Scholar]
- 56.Stewart, P. S. 2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292:107-113. [DOI] [PubMed] [Google Scholar]
- 57.Tempé, J., A. Petit, M. Holsters, M. Van Montagu, and J. Schell. 1977. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: possible relation to transformation in crown gall. Proc. Natl. Acad. Sci. USA 74:2848-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Bogelen, R. A., E. R. Olson, B. L. Wanner, and F. C. Neidhardt. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J. Bacteriol. 178:4344-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wanner, B. L. 1996. P assimilation and control of the Pho regulon, p. 1357-1381. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. 2. ASM Press, Washington, D.C.
- 60.Watson, B., T. C. Currier, M. P. Gordon, M. D. Chilton, and E. W. Nester. 1975. Plasmid required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 123:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winans, S. C. 1990. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J. Bacteriol. 172:2433-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wood, D. W., J. C. Setulab, R. Kaul, D. E. Monks, J. P. Kitajima, V. K. Okura, Y. Zhou, L. Chen, G. E. Wood, N. F. J. Almeida, L. Woo, Y. Chen, I. T. Paulsen, J. A. Eisen, P. D. Karp, D. S. Dovee, P. Chapman, J. Clendenning, G. Deatherage, W. Gillet, C. Grant, T. Kutyavin, R. Levy, M.-J. Li., E. McClellund, A. Palmieri, C. Raymond, G. Rouse, C. Saenphimmachak, Z. Wu, P. Romero, D. Gordon, S. Zhang, H. Yoo, Y. Tao, P. Biddle, M. Jung, W. Krespan, M. Perry, B. Gordon-Kamm, L. Liao, S. Kim, C. Hendrick, Z.-Y. Zhao, M. Dolan, F. Chumley, S. V. Tingey, J.-F. Tomb, M. Gordon, M. V. Olson, and E. W. Nester. 2001. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294:2317-2323. [DOI] [PubMed] [Google Scholar]
- 63.Woodcock, D. M., P. J. Crowther, J. Doherty, S. Jefferson, E. DeCruz, M. Noyer-Weidner, S. S. Smith, M. Z. Michael, and M. W. Graham. 1989. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 17:3469-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang, L., P. J. Murphy, A. Kerr, and M. E. Tate. 1993. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature 362:446-448. [DOI] [PubMed] [Google Scholar]
- 65.Zhu, Y., J. Nam, J. M. Humara, K. S. Mysore, L. Y. Lee, H. Cao, L. Valentine, J. Li, A. D. Kaiser, A. L. Kopecky, H. H. Hwang, S. Bhattacharjee, P. K. Rao, T. Tzfira, J. Rajagopal, H. Yi, Veena, B. S. Yadav, Y. M. Crane, K. Lin, Y. Larcher, M. J. Gelvin, M. Knue, C. Ramos, X. Zhao, S. J. Davis, S. I. Kim, C. T. Ranjith-Kumar, Y. J. Choi, V. K. Hallan, S. Chattopadhyay, X. Sui, A. Ziemienowicz, A. G. Matthysse, V. Citovsky, B. Hohn, and S. B. Gelvin. 2003. Identification of Arabidopsis rat mutants. Plant Physiol. 132:494-505. [DOI] [PMC free article] [PubMed] [Google Scholar]