Abstract
Background:
We studied the effects of different doses of gold nanoparticles (AuNPs), silver nanoparticles (AgNPs), and zinc oxide nanoparticles (ZnONPs) on oxidative stress markers including glutathione peroxidase (GPX) and catalase (CAT) on male mice.
Materials and Methods:
Male albino mice of Wistar strain (N = 60), weighing 17-32 g, were used for this study. The mice were randomly assigned to three classes such that in each class, there were four groups of which one was control and the other three groups were fed with ZnONPs and AgNPs at 500, 250, and 125 ppm concentration and AuNPs at 100, 50, and 25 ppm concentration for 15 days. The heart blood was taken to measure GPX and CAT enzyme activities at the end of the treatment.
Results:
In male mice treated with AgNPs, the GPX and CAT activities were significantly increased, while significant decreases were seen in the GPX and CAT activities in mice treated with ZnONPs (P < 0.05) and in mice treated with AuNPs (P < 0.05).
Conclusion:
The results of this study showed that AuNPs and ZnONPs caused decreased antioxidant enzyme activities, while nanosilver had the reverse effect and increased the antioxidant enzyme activities and caused decreased stress oxidative.
Keywords: Catalase, glutathione, gold, nanoparticles, silver, zinc oxide
INTRODUCTION
In recent years, nanotechnology has resulted in dramatic changes in different areas such as medicine, cosmetic materials,[1,2] concrete,[3] antibacterial,[4,5] textile, and automotive industries. Potential benefits of nanomaterials in biomedical and industrial applications for human health and environment are now accepted in the literature.[6] In the biological field, research focuses on the effect of size, shape, uptake, and distribution of nanoparticles (NPs).[7] The increased industrial use of NPs can result in frequent exposure through inhalation, ingestion, or dermal contact during manufacture, use, and disposal. Hence, studies are needed to understand the biological effects of exposure to NPs.[8] The term “antioxidant” refers to the chemical material that prevents the use of oxygen. An antioxidant agent acts against the harmful effects of free radicals. Colloidal silver was first used in medicine by Lee in 1889.[9,10] Barath manikanth et al. observed the effective role of gold nanoparticle (AuNP) as an antioxidant agent by inhibiting the formation of reaction oxygen species (ROS) and scavenging the free radicals.[11] Oxidative stress plays a major role in the etiology of several diabetic complications.[12,13,14] This investigation was, therefore, aimed to study the effects of AuNPs, silver nanoparticles (AgNPs), and zinc oxide nanoparticles (ZnONPs) (10 nm) on the oxidative stress markers including glutathione peroxidase (GPX) and catalase (CAT) in the blood cells of mice.
MATERIALS AND METHODS
Male albino mice of Wistar strain (N = 60), weighing 17-32 g, were used for this study. They were supplied by the Medical University of Isfahan and were acclimatized before commencing the experiments at suitable conditions of temperature and light for a period of 2 weeks. The environmental conditions were a temperature of 25-27°C, with a relative humidity of 40-60% and a 12-h light/dark cycle, and the animals had free access to water and food. This study was carried out according to the guidelines approved by the Institutional Animal Ethical Clearance (IAEC) committee. The animals were randomly divided into three classes of which each class consisted of four groups with five animals in each. One group was the control that received 0.3 ml of distilled water and the other three groups were fed with 0.3 ml of ZnONPs and AgNPs at 500, 250, and 125 ppm concentration and AuNPs at 100, 50, and 25 ppm concentration, respectively, for 2 weeks (provided by injection intraperitoneally). The NPs were obtained from the Tehran Notrino Company as a colloidal solution (concentration of 100 ppm) with an average diameter of 10 nm as determined by transmission electron microscopy (TEM). The mice did not show any symptoms of toxicity, such as change in fur color, weight loss, or any other symptoms in terms of morphology and behavior. At the end of the 15-day treatment, all the mice were fasted overnight and were euthanized on the next day to determine the level of toxicity by biochemical analysis. For biochemical analysis, the blood was withdrawn from the hearts of animals to measure the activities of GPX and CAT enzymes at the end of the treatment. The serum was isolated by centrifugation (3000 rpm for 15 min), and the GPX and CAT activities were measured with a spectrophotometer (JENWAY, England). The antioxidant system is comprised of several enzymes such as GPX and CAT, which are responsible for maintaining the balance of oxidative system. Therefore, they prevent an increase in oxidative stress.[15] GPX and CAT activities were assayed according to the method described earlier.[16] The measurements of reduced glutathione and CAT in serum and the mean values of GPX and CAT in mice of treated and control groups were compared.[17] Statistical evaluations were conducted by SPSS version 19.0. Analysis of variance (ANOVA) and Dunnett's test were used to determine the activities of GPX and CAT enzymes, and values of P ≤0.05 relative to control were considered statistically significant.
RESULTS
Microscopic characterization of NPs
The morphology and size of the synthesized NPs were investigated using TEM. The images clearly showed that the average size of the particles was in the order of 10 nm and depicted that they were relatively uniform in diameter and spherical in shape. The assembly was attached with a computer software program to analyze the mean size of the particles in the sample [Figure 1].
Figure 1.
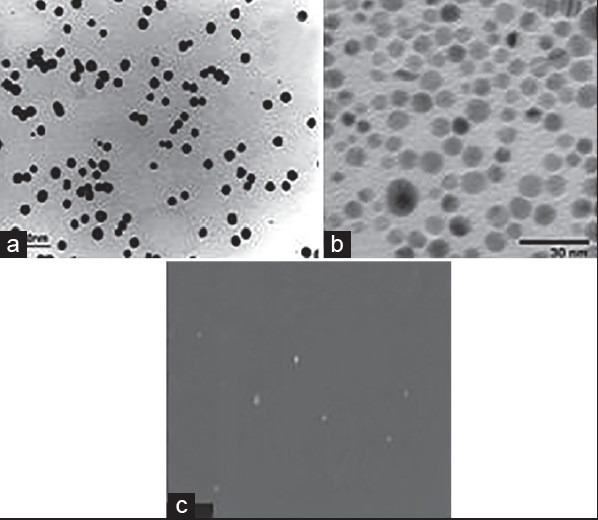
Images of NPs by TEM: (a) AuNPs, (b) AgNPs, (c) ZnONPs
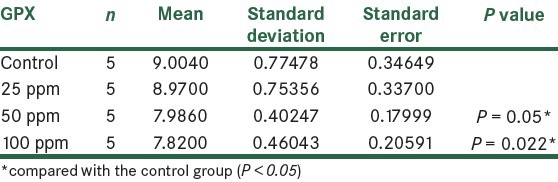
GPX is a tripeptide with a free reductive thiol functional group responsible for the detoxification of peroxides and acts as an important antioxidant in cells. During the detoxification process, GSH (the reduced form) becomes GSSG (oxidized glutathione) which is then recycled to GSH by the enzyme glutathione reductase present in cells. The increase in production of these enzymes results in decrease in destruction of antioxidants such as GSH and CAT.[11] The results showed that the activity of GPX enzyme decreased in all the groups that received AuNPs. In the third and fourth groups that received 50 and 100 ppm NPs, respectively, significant changes were observed statistically (P < 0.05) compared to the control group, as shown in Figure 2 and Table 1.
Figure 2.
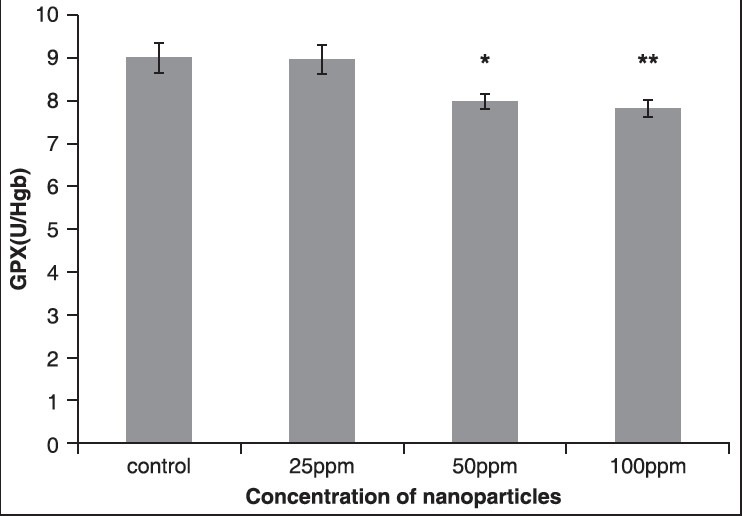
The effect of different concentrations of AuNPs on GPX enzyme
Table 1.
Comparison of GPX in the control group and the group treated With AuNPs 14 days post treatment
The results showed that the activity of CAT enzyme decreased in all the groups that received AuNPs. Compared to the control group, in the fourth group that received 100 ppm NPs, respectively, statistically significant changes were seen (P < 0.05), as shown in Figure 3 and Table 2.
Figure 3.
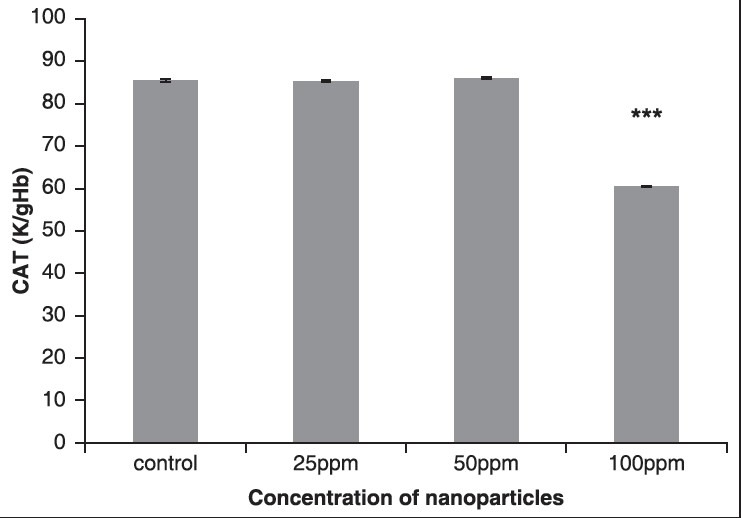
The effect of different concentrations of AuNPs on CAT enzyme
Table 2.
Comparison of CAT in the control group and the group treated with AuNPs 14 days post treatment
The results showed that the activity of GPX enzyme decreased in all the groups that received ZnONPs. Compared to the control group, the fourth group that received 500 ppm ZnONPs exhibited significant change statistically (P < 0.05), as shown in Figure 4 and Table 3.
Figure 4.
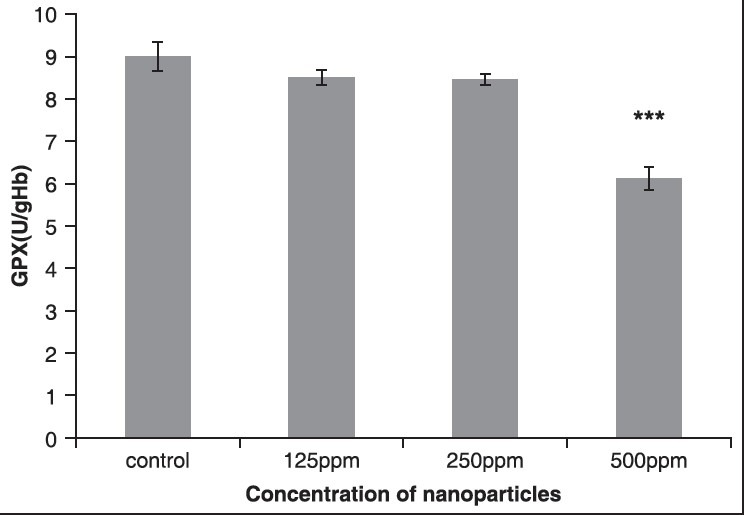
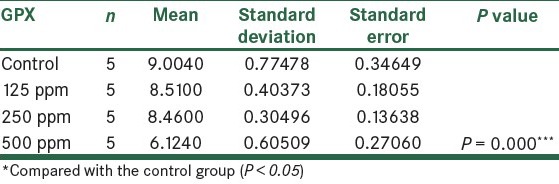
The effect of different concentrations of ZnONPs on GPX enzyme
Table 3.
Comparison of GPX in the control group and the group treated with ZnONPs 14 days post treatment
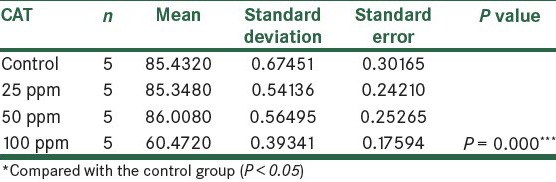
The results showed that the activity of CAT enzyme decreased in all the groups that received ZnONPs. Compared to the control group, in the fourth group that received 500 ppm NPs, respectively, statistically significant changes were observed (P < 0.05), as shown in Figure 5 and Table 4.
Figure 5.
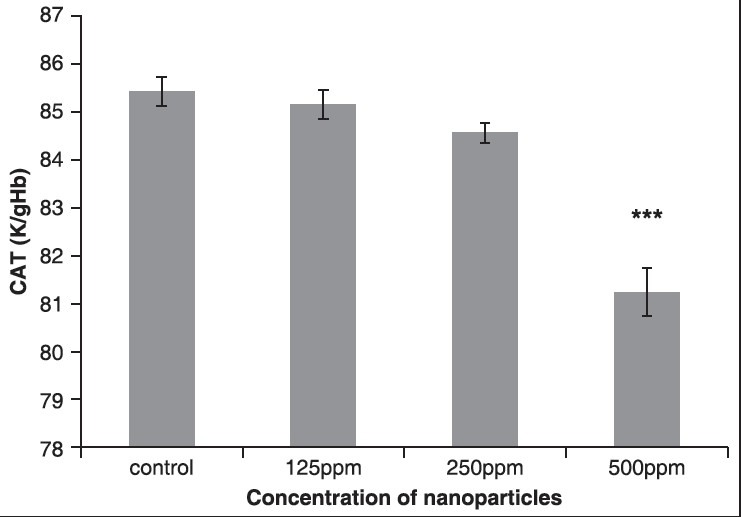
The effect of different concentrations of ZnONPs on CAT enzyme
Table 4.
Comparison of CAT in the control group and the group treated with ZnONPs 14 days post treatment
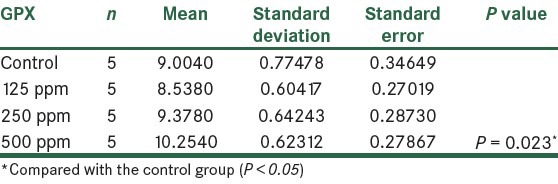
The results showed that the activity of GPX enzyme increased in all the groups that received AgNPs. Compared to the control group, in the fourth group that received 500 ppm NPs, respectively, statistically significant changes were observed (P< 0.05), as shown in Figure 6 and Table 5.
Figure 6.
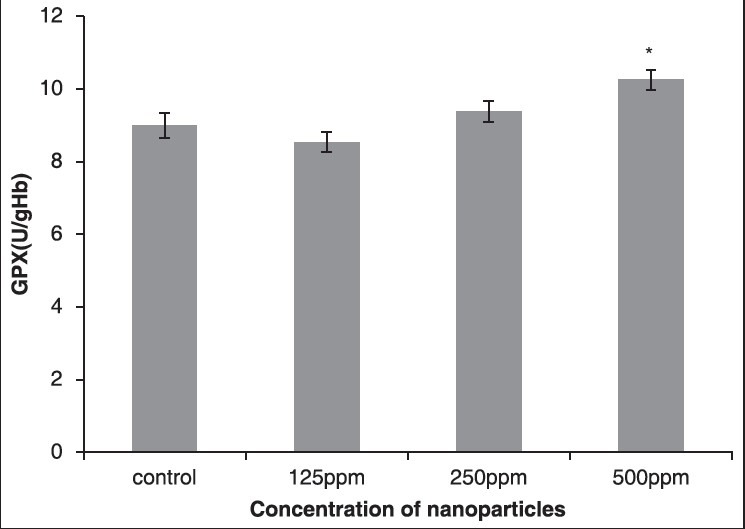
The effect of different concentrations of AgNPs on GPX enzyme
Table 5.
Comparison of GPX in the control group and the group treated with AgNPs 14 days post treatment
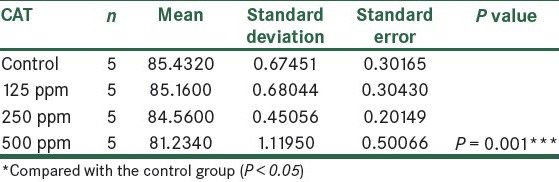
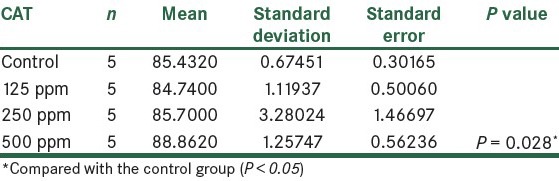
The results showed that the activity of CAT enzyme increased in the third and fourth groups that received AgNPs. Compared to the control group, in the fourth group that received 500 ppm NPs, respectively, statistically significant changes were seen (P < 0.05), as shown in Figure 7 and Table 6.
Figure 7.
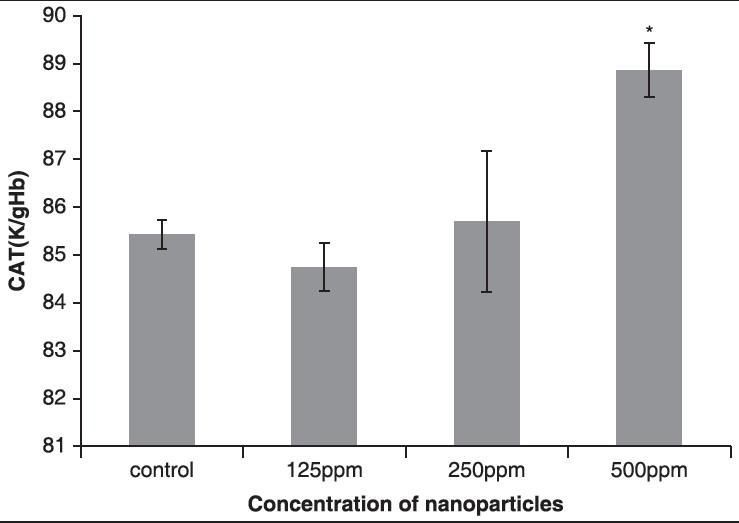
The effect of different concentrations of AgNPs on CAT enzyme
Table 6.
Comparison of CAT in the control group and the groups treated with AgNPs 14 days post treatment
DISCUSSION
The results of this study showed that AgNPs significantly increased the activities of GPX and CAT and the other NPs (ZnONPs and AuNPs) significantly decreased CAT and GPX activities in 2 weeks. The cytotoxicity of AuNPs has been studied in human cells and the results have shown that AuNPs are nontoxic up to 250 mM, while ionic gold exhibits obvious cytotoxicity at 25 mM.[18] Most studies have shown the toxicity of AuNPs of diameter 4-18 nm.[19] Intraperitoneal injection of AuNPs was investigated by Lasagna-Reeves et al. and the result showed a low level of toxicity at the dose range of 320-3200 mg/kg/day.[20] Zhang et al. used AuNPs at the dose of 100 mg/kg which resulted in harm to the tissues and enzymes and they had toxic effect.[21] Hussain et al. observed that AgNPs have toxic effect on the mitochondria of liver and result in the production of ROS and they decrease glutathione in the liver;[22] but in this study, GPX enzyme activity was found to be increased. Hasen and Negley showed that AuNPs result in the production of free radical of peroxidation of unsaturated fatty acid in phospholipid membrane and result in the creation of apoptosis.[23] Halo and Chen reported that the ZnONPs caused significant decrease of GSH and CAT enzymatic activities[24] and a similar result was obtained in this study. The results of the study of Zhaox et al. showed that generation of ROS was significantly increased at a concentration of 50 and 100 mg/L of ZnONPs.[25] At sizes larger than 5 nm, the general assumption is that gold is chemically inert like the bulk; however, reactivity of AuNPs of diameter less than 3 nm is most likely different from that of larger AuNPs.[26] The organ distribution of large AuNPs is size dependent, while small AuNPs of 5-15 nm diameters have wider organ distribution than that of large AuNPs of diameter 50-100 nm.[27]
CONCLUSION
The findings of this preliminary study suggest that AuNPs and ZnONPs of 10 nm diameter significantly decrease CAT and GPX enzyme activities, while AgNPs significantly increase the antioxidant enzyme activities and decrease the level of free radicals.
Footnotes
Source of Support: Expenses of this work were discharged by authors
Conflict of Interest: None declared.
REFERENCES
- 1.Shah MA. Formation of zinc oxide nanoparticles by the reaction of zinc metal with methanol at very low temperature. Afr Phys Rev. 2008;2:106–9. [Google Scholar]
- 2.Ji JH, Bae GN, Yun SH, Jung JH, Noh HS, Kim SS. Evaluation of a silver nanoparticle generator using a small ceramic heater for inactivation of S. epidermidis bioaerosols. Aerosol Sci Technol. 2007;41:786–93. [Google Scholar]
- 3.Dechsakulthorn FB, Hayes A, Bakand S, Joeng L, Winder C. In vitro cytotoxicity assessment of selected nanoparticles using human skin fibroblasts. (Alternatives to Animal Testing and Experimentation. 2006;14:397–400. [Google Scholar]
- 4.Qun Li, Shui-Lin Chen, Wan-Chao Jiang. Durability of nano ZnO antibacterial cotton fabric to sweat. J Appl Polym Sci. 2007;103:412–6. [Google Scholar]
- 5.Saito M. Antibacterial, deodorizing, and UV absorbing materials obtained with zinc oxide (ZnO) coated fabrics. J Ind Text. 1993;23:150–64. [Google Scholar]
- 6.Service RF. American Chemical Society meeting. Nanomaterials show signs of toxicity. Science. 2003;300:243. doi: 10.1126/science.300.5617.243a. [DOI] [PubMed] [Google Scholar]
- 7.Patra HK, Banerjee S, Chaudhuri U, Lahiri P, Dasgupta AK. Cell selective response to gold nanoparticles. Nanomedicine. 2007;3:111–9. doi: 10.1016/j.nano.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian SK, Jittiwat J, Manikandan J, Ong CN, Yu LE, Ong WY. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rat. Biomaterials. 2010;31:2034–42. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 9.Guan ZZ. An experimental study of blood biochemical diagnostic indices for chronic fluorosis. Zhongghua Yua Fang Yi Xue Za Zhi. 1991;25:33–5. [PubMed] [Google Scholar]
- 10.Eisler R. USA: Maryland University; 1996. A review of silver hazards to plants and animals. Proceedings of the 4th international conference transport, fate and effects of silver in the environment; pp. 143–4. [Google Scholar]
- 11.Barathmanikanth S, Kalishwaralal K, Sriram M, Pandian SR, Youn HS, Eom S, et al. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J Nanobiotechnology. 2010;8:16. doi: 10.1186/1477-3155-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–67. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 13.Feldman EL, Stevens MJ, Greene DA. Pathogenesis of diabetic neuropathy. Clin Neurosci. 1997;4:365–70. [PubMed] [Google Scholar]
- 14.Ruggiero D, Lecomte M, Michoud E, Lagarde M, Wiernsperger N. Involvement of cell-cell interactions in the pathogenesis of diabetic retinopathy. Diabetes Metab. 1997;23:30–4. [PubMed] [Google Scholar]
- 15.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 16.Rotruck JT, Pope Al, Ganther HE, Swanson AB. Selenium: Biochemical roles as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 17.Machiedo GW, Powell RJ, Rush BF, Jr, Swislocki NI, Dikdan G. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple system organ failure. Arch Surg. 1989;124:1386–9. doi: 10.1001/archsurg.1989.01410120032007. [DOI] [PubMed] [Google Scholar]
- 18.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–7. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 19.Khan JA, Pillai B, Das TK, Singh Y, Maiti S. Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem. 2007;8:1237–40. doi: 10.1002/cbic.200700165. [DOI] [PubMed] [Google Scholar]
- 20.Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, Olmedo I, Clos A, Sadagopa Ramanujam VM, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393:649–55. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 21.Zhang XD, Wu HY, Wu D, Wang YY, Chang JH, Zhai ZB, et al. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 2010;5:771–81. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen TM, Phillip N. A multifunctional cog in the life and death machine. American Association for the Advancement of Science. 2003;193:31. doi: 10.1126/stke.2003.193.pe31. [DOI] [PubMed] [Google Scholar]
- 23.Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19:975–83. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 24.Hao L, Chen L. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol Environ Saf. 2012;80:103–10. doi: 10.1016/j.ecoenv.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Wang S, Wu Y, You H, Lv L. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquat Toxicol. 2013;136:49–59. doi: 10.1016/j.aquatox.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Tsoli M, Kuhn H, Brandau W, Esche H, Schmid G. Cellular uptake and toxicity of Au55 clusters. Small. 2005;1:841–4. doi: 10.1002/smll.200500104. [DOI] [PubMed] [Google Scholar]
- 27.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–9. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]


