Abstract
Background:
Gold nanoparticles have many industrial applications; moreover, they are photothermic agents for clinical treatment of cancer. This study was provided to investigate the effects associated with different doses of applied gold nanoparticles by injection and contact procedures on the alterations of the serum levels and certain factors in male mice.
Materials and Methods:
72 male mice were randomly assigned into two protocols in terms of touching and injection. The injection protocol was included of five groups: Sham, control, 25, 50, and 100 ppm. They received gold nanoparticles at 25, 50, and 100 ppm concentrations administered in form of 0.3 ml/day for the period of 14 days and that of touching protocol were received 0.2 ml/day gold nanoparticles. Blood sample of which was taken to measure the serum level of creatine kinase phosphate, fasting blood, creatinine, albumin, blood urea nitrogen and eventually, the kidney was dissected for the intent of pathological analysis.
Results:
The serum level of creatine kinase phosphate and fasting blood sugar at middle dose was significantly different (P ≤ 0.05) in touching protocol. In both protocols, the serum level of creatinine in high and medium doses showed a significant difference (P < 0.05) associated with the treated group. In the touching method, in high and medium doses administered to the treated group, the alteration was significant (P ≤ 0.05). In the both protocols, the serum level of albumin in high and medium doses of the treated group showed significant difference (P < 0.05). Thus, the gold nanoparticles could result in undesirable effects upon kidney tissue.
Conclusion:
The result of this study indicated that the administration of gold nanoparticles by touching method was more effective on the serum levels of these factors than that of injection method.
Keywords: CPK, FBS, gold nanoparticle, kidney, kidney factors, male mice
INTRODUCTION
In recent years, nanotechnology has been used in various industries, for example, pharmacology, medicine, and making layer for treatment of different types of cancers.[1,2,3] Nowadays, nanotechnology is involved in different sciences including Physics, Chemistry, and Biology[4] that causes nanoparticles to be cohort or toxic to the environment in different forms and sizes. Gold nanoparticles (AuNPs) have found many applications in recent years.[5] In 2500 B.C, they were used for drug longevity[6] or cancer therapy,[7] and today, AuNPs are used in cosmetics.[8] AuNPs are used as drug carriers because their application is the same as that of liposome in the body.[9] Creatine kinase (CK), formerly known as creatine phosphokinase (CPK), is an intracellular enzyme present in large amounts in skeletal muscles, myocardium, and brain, and in low amounts in other visceral tissues. CK is a dimeric molecule composed of two subunits designated M and B. Combinations of these subunits form the isoenzymes CK-MM, CK-MB, and CK-BB. Trauma, surgery, vascular disease, and metabolic disease of the central nervous system may result in significant elevation of CK. Similarly, several diseases correlate with the muscle (e.g. trauma, metabolic disease such as myxedema, diabetic ketoacidosis, hypothermia, inflammatory disease such as polymyositis, and degenerative disease such as Duchene's muscular dystrophy) may elevate CK levels. Most clinical laboratories use electrophoresis on an arose gel or cellulose acetate combined with band quantification by fluorometric or spectrophotometric techniques.[10,11] Quantification may also be accomplished by elution of the electrophoretic bands. Electrophoretic CK-BB is most mobile, CK-MB is intermediate, and CK-MM is neutral. CK catalyzes the reversible reaction: ADP + creatine phosphate ↔ ATP + creatinine by transferring phosphate groups. A common method of measuring total CK level involves spectrophotometric determination of the foregoing reaction rate. The results vary widely because of differing analytical methodology and also differences due to age, sex, race, and level of physical activity.[12] The kidneys are the organs that serve in several essential regulatory posts in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid-base balance, and regulation of blood pressure (via maintaining salt and water balance). They serve the body as natural filters of the blood, and remove wastes which are diverted to the urinary bladder. In producing urine, the kidneys excrete wastes such as urea and ammonium, furthermore, they are also responsible for the reabsorption of water, glucose, and amino acids. The kidneys also produce hormones including calcitriol, erythropoietin, and the renin enzyme.[13] Normally, fast blood glucose (FBS) levels increase slightly following to the post prandial blood glucose. As a result, your pancreas releases insulin so that your blood glucose level does not exceed from normal. Blood glucose levels that remain high over a long time can impair your eyes, kidneys, nerves, and blood vessels.[14] So, in this study, we try to focus on kidney tissue, FBS and CPK enzyme changes induced by AuNPs. The GNP size and exposure duration affect kidney function of mice. In the present study we aimed to address the particle-size and exposure duration effect on kidney function in an attempt to cover and understand the toxicity of GNPs.
MATERIALS AND METHODS
Synthesis and characterization of gold nanoparticles
AuNps colloid solution of 100 ppm concentration was prepared from Notrino. Co of Tehran. Aucl4 was used as the precursor and subsequently redacted by citrate. Its size and shape were examined by X-ray and transmission electron microscopy (TEM). The range of observed diameter of the synthesized AuNPs was about 10 nm. Next, the required doses were prepared (25 and 50 and 100 ppm). Morphologic studies and examination of the surface of synthesized NPs were performed by the following devices, TU-1901 double-beam UV-visible spectrophotometer, a D/Max-RA X-ray diffractometer using CuKa radiation, and a JEM-200CX TEM. Though the TEM operates on the same basic principles as the light microscope, yet in the former one (i.e. the TEM), rays come from the above to down. This electron microscope consists of a long column on top of which the source of electron rays is mounted. After transmitting through the specimen, electron rays hit a photographic film or screen (built from fluorescent materials) and create an image. Since some rays do not pass through the sample, black spots are left on the image and therefore, the electron microscope images are black-and-white. Slices in a TEM are much thinner than those in a light microscope, and staining techniques are also different.
Animals
A total of 72 male mice of Albino strain, weighting 17-32 g were employed in this study. They were housed individually in stainless steel mesh cages and were acclimatized before the commencing of experiments at suitable conditions of temperature and light for a period of 2 weeks. The environmental conditions were set to the temperature of 25-27°C, relative humidity of 10 ± 60% and a12 h light/dark cycle. They were freely received water and food (Behparvar co, Tehran). This study was carried out according to the guidelines approved by Institutional Animal Ethical Clearance (IAEC).
Optimization of dosage
The animals were randomly divided into nine groups, each consisting of eight mice. Five groups of which were treated by injection method (sham, 25, 50, 100 ppm). The Sham group was fed by normal food and water, and the control group was being injected by 0.3 ml/day distilled water intraperitoneally. The low dose group (25 ppm), middle dose group (50 ppm), and high dose group (100 ppm) all of which were injected by 0.3 ml/day AuNPs intraperitoneally. Four groups were treated by the touching method each was consisted of eight mice (control, 25, 50, 100 ppm). The control group was touched by 0.2 ml/day distilled water, low dose (25 ppm) group, middle dose (50 ppm) group, and high dose (100 ppm) group were touched by 0.2 ml/day AuNPs for 2 weeks. At the end of 18 days of treatment, all the mice were kept starved over night and euthanized on the next day to determine the toxicity through completion of biochemical and histological analyses.
Biochemical analysis
Animals were anaesthetized with Ketamine Chloride that was administered intraperitoneally. 10 ml of their blood was approximately collected by cardiac puncture into Lithium Heparin bottles. Blood samples were centrifuged at 3000xg for 15 minutes for measuring the concentration of alb, BUN, creatinine and FBS and CPK factors were given to auto-analyzer (Hitachi 902) and biochemical kit. Auto-analyzers were mostly used for the routine repetitive medical laboratory analysis. Each of early auto-analyzer instruments tested multiple samples sequentially for individual analysis. Most continuous flow analyzers are sensitive to reactions. The concentration of a colorless solution could not normally be determined by a colorimeter. The addition of a color reagent leads to a color reaction and the absorbance of the colored product can then be measured by a colorimeter.
Histological investigation
At the end of study, the mice were anesthetized with ketamine and the kidneys of the mice were dismembered. They were fixed with a 10% formalin neutral buffer solution and embedded in paraffin. In the next stage, specimens were dehydrated and blocked, then prepared and cut into 5-μm-thick sections. The sections obtained were stained by using Hematoxylin and Eosin and examined under the light microscope. The photomicrographs of which were obtained.
Statistical analysis
Statistical evaluations were conducted by spss19.0. ANOVAs and LSD were performed to investigate the level of alb, BUN, cr, FBS and CPK factors. P values ≤0.05 were considered statistically significant.
RESULTS
The morphology and size of synthesized AuNPs were determined by using transmission electron microscopy (TEM). The images clearly showed that the average size of particles was found to be about 10 nm and this suggested that they are relatively homogeneous in diameter and spherical in shape [Figure 1].
Figure 1.
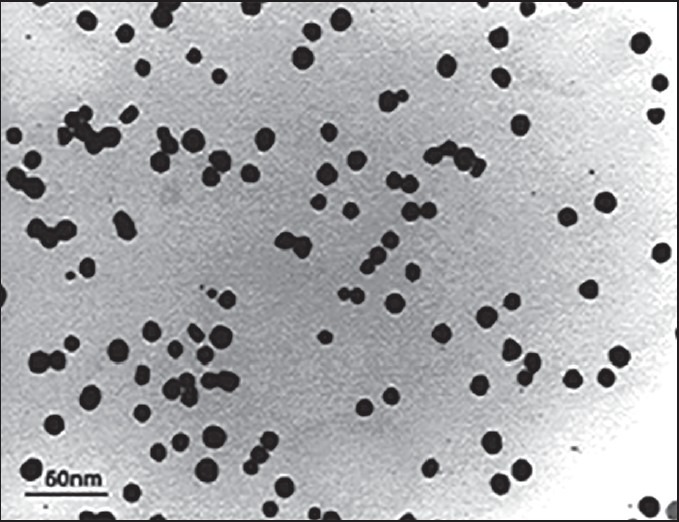
Image of AuNPs by TEM
All the mice survived throughout the experimental period without exhibiting any abnormalities. The mice did not show any symptom of toxicity such as fatigue, loss of appetite, change in fur color, and weight loss. In the injection protocol, the serum level of CPK in experimental groups did not show any significant difference with the sham group but in the touching protocol, in high dose-treated group, the CPK level was significantly different from the control group (P ≤ 0.05). The results also indicated significant differences on FBS levels in nanoparticles-treated groups when compared with the sham group (P ≤ 0.05). However, such observation did not reveal any significant difference in the injection group. In the injection protocol, the serum level of creatinine in high and medium dose-treated groups showed significant difference (P < 0.05) with the sham group and exposing to touching procedure, in high and medium dose-treated groups, the creatinine level was significantly different from the control group (P ≤ 0.05). In the injection protocol, the serum level of BUN in experimental groups did not show significant difference with the sham group but in the touching method, in high and medium dose-treated groups, the BUN level was significantly different from the control group (P ≤ 0.05). In the injection protocol, the serum level of alb in high and medium dose-treated groups showed significant difference (P < 0.05) with the sham group and in the touching method, in high and medium dose-treated groups, the alb level was significantly different from the control group (P ≤ 0.05). As for the achieved results, significant level of the touching protocol is higher than injection protocol, as well as more destructive on factors [Table 1].
Table 1.
Effects of different doses of AuNPs on blood factors in touching and injection methods
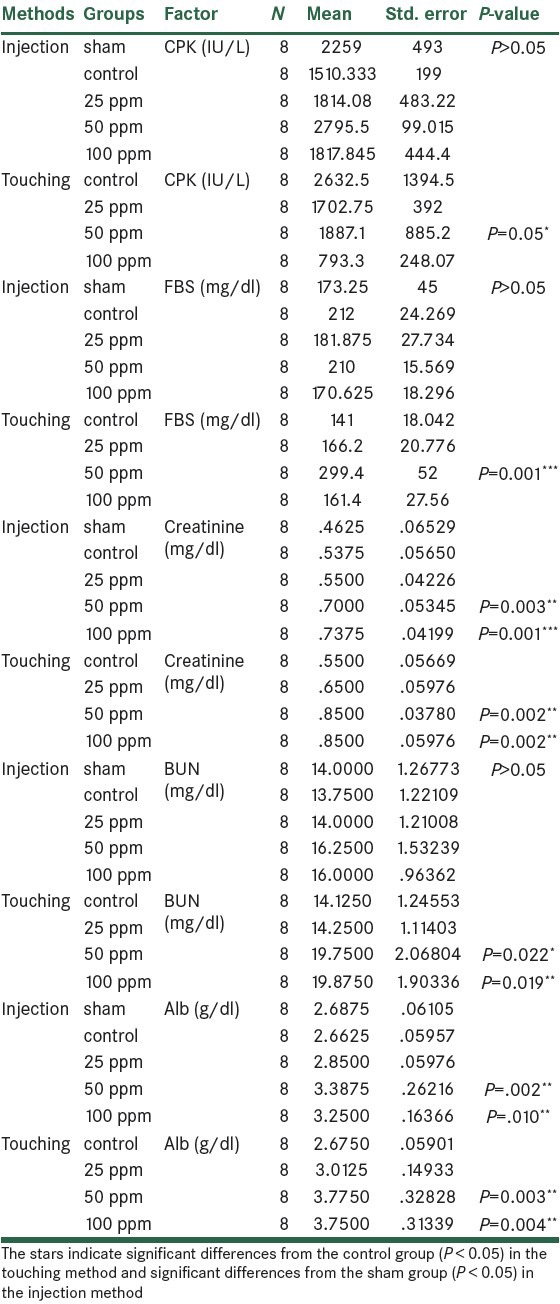
The images of kidney tissue for all groups are demonstrated in Figure 2. In the 25 ppm group, the cortex and medulla sections of kidney showed natural state and glomerular tufts and capsular space in renal corpuscle were distinct. In the 50 ppm group, histopathological alterations in renal cortex were obvious, capsular space in renal corpuscle disappeared and just the glomerular tufts were visible. In the 100 ppm group, the renal cortex and renal corpuscle disappeared and capillary network around the tubules was expanded; so red blood cells were visible inside them [Figure 2].
Figure 2.
Pathological sections after treatment with different doses of AuNPs. The sections were stained with H and E dye
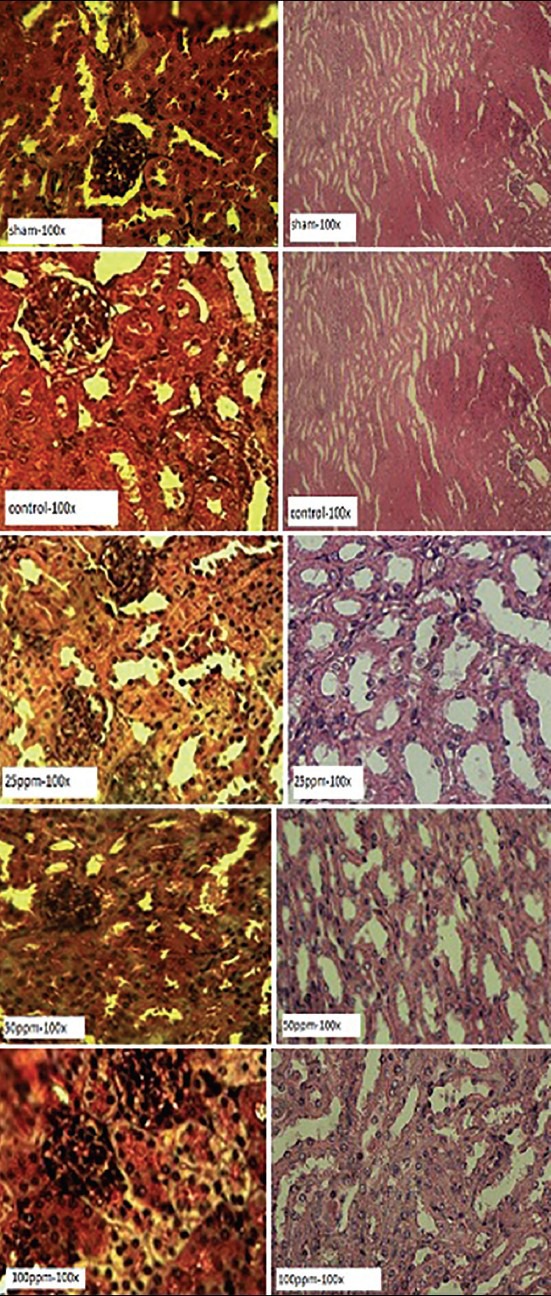
In the touching protocol, histological alterations of the structures belonging to nephron tubules of the 25 ppm group were mostly destroyed, and the capsular space and renal corpuscle showed natural states [Figure 3]. In the 50 ppm group, partly there was demolition of renal cortex such as hypochromia of renal corpuscle and so, glomerular tufts were not distinguished from capsular space. Finally, in the 100 ppm group, histopathological alteration in renal cortex was higher than medulla capsular space and was drained around the glomerulus [Figure 3].
Figure 3.
Pathological sections following to treatment with different doses of AuNPs in touching procedure. The sections were stained with H and E dye
DISCUSSION
The result of the present study confirmed that GNPs cause mild to intensive disorders for the kidney tissue in all of treated groups by two methods consisting of injection and contact, especially in middle and high doses. In a study by Shakibaie et al. in 2012, the toxicity effect of Selenium nanoparticles in mice in doses of 2.5, 5, 10 mg/kg resulted in cues of toxicity including losing the body weight and changes in clinical chemistry parameters. No biochemical changes on CPK enzyme was observed by the administration of 2.5, 5, 10 mg/kg, yet, in 20 mg/kg expose; it resulted in body weight loss of rat.[15] Disclosed by this study, there was no significant difference on CPK enzyme in injection protocol. Research made by Hainfeld et al. in using AuNPs exhibited a non-toxic effect upon the blood chemistry and vital organs.[16] Research made by Sangiliy et al. in using AuNPs in dosage of 2.5 mg/k.b.w per day showed that AuNPs exposure caused an increase in FBS in male mice. This study showed the toxicity of AuNPs in kidney tissue, kidney section showed normal glomerular tubes and renal cortex and gold treatment kidney demonstrated no pathological changes in the animals and any increase in creatinine in their serum[17] and the obtained results are similar to the results gained from touching protocol. Research made by Terentyuk et al. in using AuNPs showed that the kidneys exhibited moderate hyperemia and changes in the glomeruli and in particular, the appearance of single erythrocytes and the proliferation of epithelial cells linked to Bowman's capsule. He used the nanoparticles with 10 nm diameter and found that the cytotoxicity of AuNPs is size dependent[18] that his achieved results are similar to our results. Research made by Junpingchen et al. in using serum oxide nanoparticles study showed that this method results in a decrease in the FBS,[19] but in adverse, our study showed a significant increase in FBS serum. Research made by Yan et al. in using Zinc oxide nanoparticles showed that ZnONPs result in an increase in the levels of glucose after the treatment. These findings showed that ZnONPs may cause impairment in mitochondria and cell membrane of rat kidney, which may contribute to ZnONPs-induced nephrotoxicity;[20] therefore, these results are similar to our results gained from touching protocol. Research made by Kojouri et al. in using selenium nanoparticles showed that the nanoparticles exhibited increases in the level of Cr and BUN after the treatment,[21] and the results we obtained were identical with the results of this study in applying gold nanoparticles. Research made by Giri et al. in using cerium nanoparticles showed that the nanoparticles exhibited increases in level of Cr and BUN after the treatment, however, it led to a decrease in the alb serum level;[22] thus, we obtained similar results in this study in applying gold nanoparticles but in regard with our achieved results, the level of alb serum shows an increase in alb serum level.
CONCLUSION
The results of this study showed that the use of AuNPs in different doses do not show identical results for all states. Results obtained from injection and touching methods were different. The results of this study indicated that the administration of AuNPs by the touching method is more effective on the level of CPK, FBS, alb, BUN, and creatinine in comparison with the injection procedure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kuzma J, Besley JC. Ethics of risk analysis and regulatory review: From bio- to nanotechnology. Nanoethics. 2008;2:149–62. [Google Scholar]
- 2.Yang W, Peters JI, Williams RO., 3rd Inhaled nanoparticles--a current review. Int J Pharm. 2008;356:239–47. doi: 10.1016/j.ijpharm.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Bystrzejewska-Piotrowska G, Golimowski J, Urban PL. Nanoparticles: Their potential toxicity, waste and environmental management. Waste Manag. 2009;29:2587–95. doi: 10.1016/j.wasman.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Babadi VY, Najafi L, Najafi A, Gholami H, Zarji ME, Golzadeh J, et al. Evaluation of iron oxide nanoparticles effects on tissue and enzymes of liver in rats. J Pharm Biomed Sci. 2012;23:1–3. [Google Scholar]
- 5.Higby GJ. Gold in medicine: A review of its use in the West before 1900. GoldBull. 1982;15:130–40. doi: 10.1007/BF03214618. [DOI] [PubMed] [Google Scholar]
- 6.Richards DG, McMillin DL, Mein EA, Nelson CD. Gold and its relationship to neurological/glandular conditions. Int J Neurosci. 2002;112:31–53. doi: 10.1080/00207450212018. [DOI] [PubMed] [Google Scholar]
- 7.Andresen TL, Jensen SS, Kaasgaard T, Jørgensen K. Triggered activation and release of liposomal prodrugs and drugs in cancer tissue by secretory phospholipase A2. Curr Drug Deliv. 2005;2:353–62. doi: 10.2174/156720105774370203. [DOI] [PubMed] [Google Scholar]
- 8.El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239:129–35. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100:13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foreback CC, Chu JW. Creatine kinase isoenzymes: Electrophoretic and quantitative measurements. Crit Rev Clin Lab Sci. 1981;15:187–230. doi: 10.3109/10408368109105871. [DOI] [PubMed] [Google Scholar]
- 11.Grande P, Christiansen C, Pedersen A, Christensen MS. Optimal diagnosis in acute myocardial infarction. A cost-effectiveness study. Circulation. 1980;61:723–8. doi: 10.1161/01.cir.61.4.723. [DOI] [PubMed] [Google Scholar]
- 12.Johnston CC, Bolton EC. Cardiac enzymes. Ann Emerg Med. 1982;11:27–35. doi: 10.1016/s0196-0644(82)80010-3. [DOI] [PubMed] [Google Scholar]
- 13.Desmecht D, Linden A, Amory H, Art T, Lekeux P. Relationship of plasma lactate production to cortisol release following completion of different type of sporting events in horses. Vet Res Commun. 1996;20:371–9. doi: 10.1007/BF00366544. [DOI] [PubMed] [Google Scholar]
- 14.Holemans K, Van Bree R, Verhaeghe J, Meurrens K, Van Assche FA. Maternal semistarvation and streptozotocin-diabetes in rats have different effects on the in vivo glucose uptake by peripheral tissues in their female adult offspring. J Nutr. 1997;127:1371–6. doi: 10.1093/jn/127.7.1371. [DOI] [PubMed] [Google Scholar]
- 15.Shakibaie M, Shahverdi AR, Faramarzi MA, Hassanzadeh GR, Rahimi HR, Sabzevari O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm Biol. 2013;51:58–63. doi: 10.3109/13880209.2012.710241. [DOI] [PubMed] [Google Scholar]
- 16.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: A new X-ray contrast agent. Br J Radiol. 2006;79:248–53. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 17.Barathmanikanth S, Kalishwaralal K, Sriram M, Pandian SR, Youn HS, Eom S, et al. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J Nanobiotechnology. 2010;8:16. doi: 10.1186/1477-3155-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terentyuk G, Akchurin G, Maksimova I, Shantrokha A, Tuchin V, Maslyakova G, et al. Tracking gold nanoparticles in the body. SPIE Newsroom. 2009;10:1–3. [Google Scholar]
- 19.Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1:142–50. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 20.Yan G, Huang Y, Bu Q, Lv L, Deng P, Zhou J, et al. Zinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in rats. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2012;47:577–88. doi: 10.1080/10934529.2012.650576. [DOI] [PubMed] [Google Scholar]
- 21.Kojouri GA, Sharifi S. Preventing effects of nano-selenium particles on serum concentration of blood urea nitrogen, creatinine, and total protein during intense exercise in donkey. J Equine Vet Sci. 2013;33:597–600. [Google Scholar]
- 22.Giri S, Karakoti A, Graham RP, Maguire JL, Reilly CM, Seal S, et al. Nanoceria: A rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PLoS One. 2013;8:e54578. doi: 10.1371/journal.pone.0054578. [DOI] [PMC free article] [PubMed] [Google Scholar]


