Abstract
Background:
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy that results from compression of the median nerve within the carpal tunnel. In most patients, the diagnosis can be proposed based on patient history and clinical symptoms, with physical findings being attributed only in more severely affected patients. The purpose of this study is to introduce a reliable and accurate method for the proper selection of patients with mild carpal tunnel syndrome (CTS), for surgery.
Materials and Methods:
Electerodiagnostic studies are performed before and after placement of the cuff of the sphygmomanometer at the arm (Cuff sign), at a mean arterial pressure, for three minutes. Thirty symptomatic patients with mild findings on electrodiagnostic studies and 49 asymptomatic control hands have been included.
Results:
Fifteen patients reported good pain relief on the first postoperative day (50%), which increased to 21 on the fouteenth postoperative day (70%). The sensory latency changes were significantly higher in the pain relief group, both on the first and fourteenth postoperative days.
Conclusion:
Considering the fact that cooperation of the patients is not necessary and the double effects of direct pressure and ischemia over the proximal parts of the median nerve leads to prolonged latencies, this test is a useful method for decision-making in patients with severe symptoms of CTS, despite the mild electrodiagnostic findings.
Keywords: Carpal tunnel syndrome, median nerve, pain relief scale
INTRODUCTION
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy that results from compression of the median nerve within the carpal tunnel.[1] CTS is a constellation of signs and symptoms presented clinically by a complex of symptoms including pain, numbness, burning, and tingling in the fingers of the affected hand.[2] Neuropathic pain is more prevalent in patients with night pain or a high score on the numerical pain rating scales.[3] It is one of the most common peripheral nerve disorders and continues to account for a majority of reported occupational disorders, with a serious negative influence on the patient's quality of life and ability to work.
The prevalence is estimated and varies from 0.1 to 9.2% in the general population and is gender-based, with a prevalence of 5.8% in women and 0.6% in men.[4,5,6,7,8,9,10,11]
In 1854, Sir James Paget first described the clinical symptoms of CTS in a patient who sustained a fracture of the distal radius. Phalen and colleagues popularized the disorder by calling it to the attention of the medical community with a series of publications that began to appear in 1950.[5] There is still a controversy about the most reliable test that should be performed to diagnose the condition. It is noticeable that early and accurate diagnosis of CTS is essential for effective non-operative management and for valid identification of the surgical candidates.[6]
In most patients, the diagnosis can be proposed based on patient history and clinical symptoms, with the physical findings being attributed only in more severely affected patients.[12,13] Indeed, the diagnosis of CTS has been based both on the clinical findings and the results of electrodiagnostic testing.[5,14,15,16] Proper timing and use of electrodiagnostic studies is very helpful and assists in the appropriate treatment of nerve pathology.[17]
Several clinical tests and provocative maneuvers are used to help in the diagnosis of the CTS. These tests include the Katz hand diagram and provocative tests such as the Phalen's test, the reverse Phalen's test, the Tinel's sign, and the Median nerve compression test. Literature reviews have proposed that the two famous signs (Phalen's test and the Tinel's sign) are of little diagnostic value in the diagnosis of CTS and these tests require subjective reporting by the patient.[18,19,20]
Usually surgery is reserved for moderate/severe cases of CTS; nevertheless, in approximately 20% of the patients with mild CTS, the distress is not relieved with conservative management (methylprednisolone injection).[21] Surgery results in significant improvement of the symptoms with a very low incidence of complications).[22,23]
Identification of the patients’ preferences and matching the identified preferences to the decision-making process result in a better outcome of surgery.[24] The purpose of this study is to introduce a reliable and accurate electrodiagnostic method for the proper selection of patients with mild CTS for surgery, in whom, conservative management has failed.
MATERIALS AND METHODS
Subject selection
This study was performed in the Kashani and Alzahra Hospitals of the Isfahan University of Medical Sciences. Initially, a detailed history, a careful clinical examination, and an extended neurophysiological evaluation were performed, to exclude the presence of other diseases that could be related to CTS. Patients with classic CTS symptoms were included (i.e. subjects who complained of nocturnal or activity-related pain, paresthesia in the hand, and hypoesthesia limited to the distribution of the median nerve). The diagnosis was separately confirmed by two neurologists, who were blinded to each other. Prior to this, at least two injections of methylprednisolone had been performed for all subjects by a physician, but no satisfactory response had been achieved.
Patients with evidence of moderate-to-severe changes in the electrodiagnosis and those with weakness and muscle atrophy were excluded. Other exclusion criteria included: The presence of peripheral neuropathy of any origin, other than CTS, median nerve injury, anatomic abnormalities of the wrist or hand, the presence of accompanying cervical radiculopathy, and whether less than two trials of methylprednisolone injections had been performed. Asymptomatic hands of patients and asymptomatic hands of healthy volunteers were entered as controls. After the patients signed an informed consent, a new nerve conduction study (NCS), including the median sensory and motor action potentials and the sural sensory action potential (to rule out polyneuropathy) were performed in the cases and controls.
Nerve conduction tests
The median sensory action potential recording was performed at the base of the middle finger, while median nerve stimulation was applied at a distance of 13 cm from the forearm. Motor action potential latencies were recorded over the thenar area with stimulation at a distance of 8 cm from the forearm. Additionally, we confirmed CTS by comparison studies. On the basis of Bland's classification,[25] we only included patients with very mild CTS (i.e. CTS detected only in two sensitive tests, such as, inching, palm/wrist median/ulnar comparison) and mild CTS (orthodromic sensory conduction velocity from the index finger to the wrist, higher than 40 m/s, with a motor terminal latency, from the wrist to the abductor pollicis brevis, of less than 4.5 ms).
Cuff sign test
The median NCSs were repeated one minute, after applying a blood pressure cuff (BPC) and inflating it to over the diastolic pressure for three minutes. Sensory and motor latencies before and after applying BPC were recorded in the cases and controls. Sensory and motor latencies before and after applying BPC were compared in the symptomatic and control groups, and the sensory and motor latency changes (difference before and after blood pressure cuff application) were compared between two groups.
All the cases underwent open surgical decompression under local anesthesia by a surgeon who was blind to the post cuff electrodiagnostic findings.
After surgery the patients were classified into two groups — ‘satisfied’ and ‘unsatisfied’. Satisfaction was defined by a physician blinded to the surgical results, using a numerical pain rating scale.[26] According to this scale, patients with scores 1 or 2 were regarded as being ‘satisfied’. Satisfaction was sought on the first and fourteenth postoperative days.
Statistical analysis
Sensory and motor latencies before and after applying BPC were compared in the symptomatic and control groups separately using the t-student's test. Sensory and motor latency changes (difference between the pre- and post-blood pressure cuff applications above diastolic pressure) were compared between two groups by the t-student's test as well (the data were normally distributed). Moreover, the sensitivity and specificity of the electrodiagnosis plus cuff maneuver was studied by means of a receiver operating characteristic (ROC) curve. Considering the patients’ satisfaction on the first and fourteenth postoperative days as the reference standard, the area under the ROC curves was calculated for both the output variables, and the points of best performance were recognized as indicators of cutoff values. In all cases, a level of P < 0.05 was considered to be statistically significant.
RESULTS
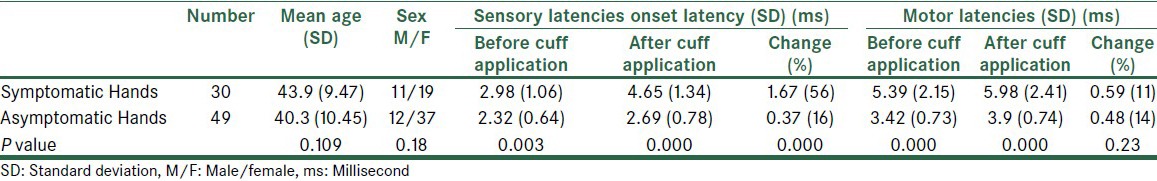
Thirty symptomatic hands with mild electrodiagnostic findings and forty-nine asymptomatic hands (control group) were entered in the study. Characteristic findings and electrodiagnostic findings of both groups are shown in Table 1. The sensory and motor latencies before applying BPC were significantly different between the two groups of symptomatic and asymptomatic hands (P < 0.01); likewise, the latencies were significantly different after BPC application (P < 0.001). Furthermore, the sensory latency change (sensory latency after BPC application minus sensory latency before BPC application) was significantly different between the two groups (P < 0.001); however, this was in contrast to the motor latency change (P > 0.05) [Table 1].
Table 1.
Identification and electrodiagnostic data of asymptomatic and symptomatic carpal tunnel syndrome hands
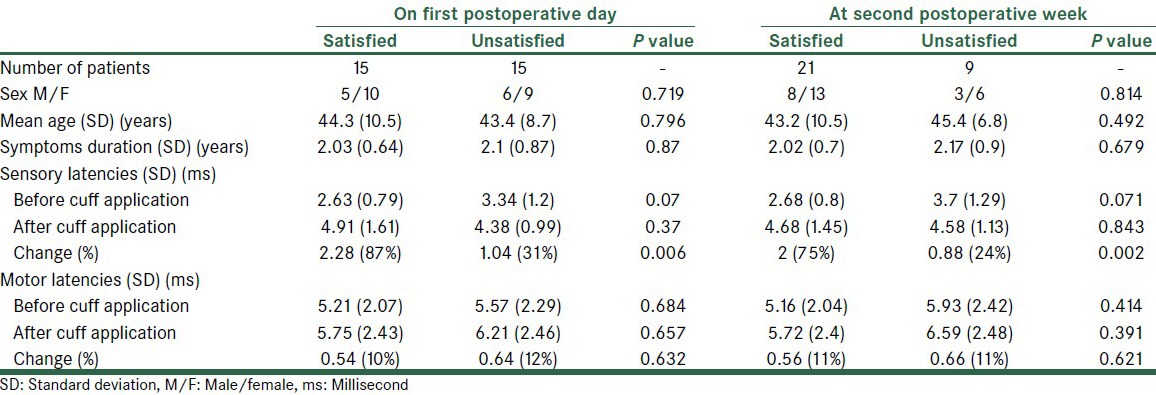
After surgery, satisfactory pain relief was reported by 15 (50%) patients on the first postoperative day, which increased to 21 (70%) on the fourteenth postoperative day. The identification data, duration of symptoms, and electrodiagnostic findings of the both groups (good vs. poor results) are showed in Table 2. Retrospectively, regarding the electrodiagnostic results prior to surgery, we observed that the sensory latency changes had been significantly higher in the satisfied group, both on the first and fourteenth postoperative days (P < 0.01). Conversely, the motor latency difference between the two groups before and after BPC application, as also the motor latency change had not been significantly different (P > 0.05) [Table 2].
Table 2.
Clinical and electrodiagnostic findings in satisfied and unsatisfied patients after carpal tunnel decompression
According to the best performance point on the ROC curves, the cutoff values for sensory latency changes were calculated as 2.05 ms and 1.95 ms for the first postoperative day and fourteenth postoperative day, respectively. In other words, if the sensory latency change after BPC application reached just over 2 ms, it would be a good predictor for benefiting from CTS surgery. The sensitivity and specificity for these points were as follows: 53.3 and 86.7% for a cutoff value on the first postoperative day, and, 73.3 and 93.3% on the fourteenth postoperative day. Figure 1 shows the ROC curve for sensory latency change on the first postoperative day. The area under the ROC curve was 0.796, with a 95% confidence interval of 0.631 to 0.960 (P < 0.05). Figure 2 shows the ROC curve for sensory latency change on the fourteenth postoperative day. The area under the ROC curve was 0.911 with a 95% confidence interval of 0.801 to 0.980 (P < 0.05).
Figure 1.
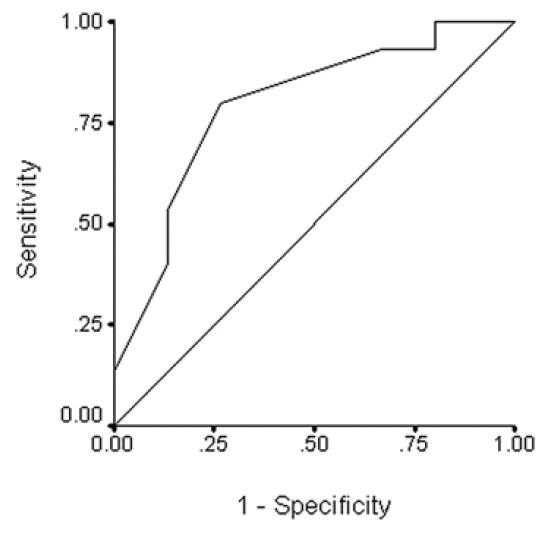
ROC curve on the first postoperative day
Figure 2.
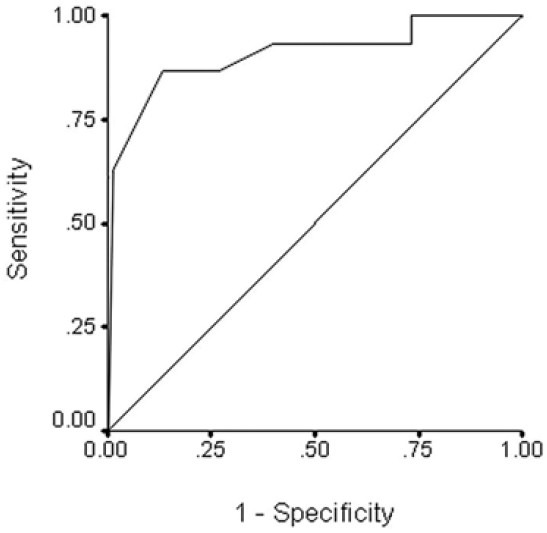
ROC curve on the fourteenth postoperative day
DISCUSSION
The diagnosis and operative decision is still controversial in CTS, especially in mild forms.[27] Operations are ignored in several patients because of mild, equivocal electrodiagnostic findings, instead of an apparent clinical setting because of the uncertainty of prognosis.[28] NCS is an objective diagnostic measurement for CTS. Historically, NCS has been used as the standard test when estimates of sensitivity and specificity for other tests for CTS have been measured. Although electrodiagnostic studies are helpful, many studies[2,29,30,31] have found that NCSs are less than perfect, especially when one decides to estimate the chance of well-being after an operation. Incorrectly assuming NCS as a standard test, results in the errors from the reference test are transferred to the comparison tests.[32,33] The signs and symptoms are not always correlated with electrodiagnostic studies. In a review of CTS patients at the Mayo Clinic, between 1961 and 1980, only 63–80% of the symptomatic patients had abnormal median sensory nerve velocities.[30]
Some new techniques in electrodiagnosis have improved the sensitivity of the diagnosis in the classical and abnormal CTS presentations, although the decision to operate on the mild atypical forms is still difficult.[27,34] Other diagnostic modalities such as MR neurography and nerve sonography are used to resolve the problem with some success, however, they are more sophisticated than the usual electrodiagnostic techniques.[35,36]
Incorrect diagnosis, which is probably common, may lead to the inappropriate use of electrodiagnostic tests, and more importantly, improper treatment, such as surgery. Therefore, an important step in reducing inconsistencies and misdiagnoses would be the development of standardized clinical criteria for CTS that could be applied consistently.[5,19]
As signs and symptoms are more severe during the night mainly due to venous congestion at the carpal tunnel and the ensuing ischemia; therefore, any condition that mimics it, can provoke pain and paresthesia. As a consequence, in this study, we have tried to provoke the pathological process in patients with mild CTS, by applying BPC and producing venous congestion. The results have shown that the sensory and motor latencies, before applying BPC, are significantly different between the two groups of mild CTS and asymptomatic hands. Furthermore, after surgery, in patients with mild CTS, the sensory latency changes between pre- and post-BPC recordings are significantly different between patients with satisfactory and unsatisfactory operative results. This prognostic difference in operative results may be due to the main, primary pathological process of the patients; in other words, the ischemia generated by venous congestion that stems from cuff pressure, increases the sensory latency of the median nerve. Patients with a higher increment in sensory latency after applying BPC have more susceptibility to develop median nerve ischemia in real situations, such as, sleeping and working in positions of wrist flexion, and as a result, gain more advantage of decompression of the carpal tunnel. However, further surveys with a higher sample size, focusing more on NCS variables are required.
The limitations of this study include: Small sample size and a limited follow-up period; however, this study can be a pioneer for conducting new studies.
CONCLUSION
Sensory latency changes before and after blood pressure cuff application during NCSs have a diagnostic and surgical prognostic significance in patients with mild CTS.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Low HL, Sivasamy VP, Griffiths AP, Redfern RM. Recurrent carpal tunnel syndrome owing to an implantation epidermoid cyst after carpal tunnel decompression: Case report. Neurosurgery. 2007;60:E956. doi: 10.1227/01.NEU.0000255442.67236.0D. [DOI] [PubMed] [Google Scholar]
- 2.MacDermid JC, Wessel J. Clinical diagnosis of carpal tunnel syndrome: A systematic review. J Hand Ther. 2004;17:309–19. doi: 10.1197/j.jht.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Sonohata M, Tsuruta T, Mine H, Asami A, Ishii H, Tsunoda K, et al. Clinical characteristics of neuropathic pain in patients with carpal tunnel syndrome. Hand Surg. 2014;19:43–8. doi: 10.1142/S0218810414500087. [DOI] [PubMed] [Google Scholar]
- 4.de Krom MC, Knipschild PG, Kester AD, Thijs CT, Boekkooi PF, Spaans F. Carpal tunnel syndrome: Prevalence in the general population. J Clin Epidemiol. 1992;45:373–6. doi: 10.1016/0895-4356(92)90038-o. [DOI] [PubMed] [Google Scholar]
- 5.Graham B, Regehr G, Naglie G, Wright JG. Development and validation of diagnostic criteria for carpal tunnel syndrome. J Hand Surg Am. 2006;31:919–24. [PubMed] [Google Scholar]
- 6.Szabo RM, Slater RR, Jr, Farver TB, Stanton DB, Sharman WK. The value of diagnostic testing in carpal tunnel syndrome. J Hand Surg Am. 1999;24:704–14. doi: 10.1053/jhsu.1999.0704. [DOI] [PubMed] [Google Scholar]
- 7.Werner RA, Franzblau A, Gell N, Hartigan AG, Ebersole M, Armstrong TJ. Incidence of carpal tunnel syndrome among automobile assembly workers and assessment of risk factors. J Occup Environ Med. 2005;47:1044–50. doi: 10.1097/01.jom.0000171065.17288.a0. [DOI] [PubMed] [Google Scholar]
- 8.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–8. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 9.Mondelli M, Giannini F, Giacchi M. Carpal tunnel syndrome incidence in a general population. Neurology. 2002;58:289–94. doi: 10.1212/wnl.58.2.289. [DOI] [PubMed] [Google Scholar]
- 10.Padua L, Aprile I, Lo Monaco M, Padua R, Pasqualetti P, Nazzaro M, et al. Italian multicentre study of carpal tunnel syndrome: Clinical-neurophysiological picture and diagnostic pathway in 461 patients and differences between the populations enrolled in the northern, central and southern centres. Italian CTS Study Group. Ital J Neurol Sci. 1999;20:309–13. doi: 10.1007/s100720050046. [DOI] [PubMed] [Google Scholar]
- 11.Faucett J, Blanc PD, Yelin E. The impact of carpal tunnel syndrome on work status: Implications of job characteristics for staying on the job. J Occup Rehabil. 2000;10:55–69. [Google Scholar]
- 12.Hennessey WJ, Falco FJ, Braddom RL, Goldberg G. The influence of age on distal latency comparisons in carpal tunnel syndrome. Muscle Nerve. 1994;17:1215–7. doi: 10.1002/mus.880171014. [DOI] [PubMed] [Google Scholar]
- 13.Nowak M, Noszczyk B. Simple clinical tests in severe carpal tunnel syndrome. Pol Przegl Chir. 2012;84:502–8. doi: 10.2478/v10035-012-0085-1. [DOI] [PubMed] [Google Scholar]
- 14.Katz JN, Larson MG, Fossel AH, Liang MH. Validation of a surveillance case definition of carpal tunnel syndrome. Am J Public Health. 1991;81:189–93. doi: 10.2105/ajph.81.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz JN, Simmons BP. Clinical practice. Carpal tunnel syndrome. N Engl J Med. 2002;346:1807–12. doi: 10.1056/NEJMcp013018. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Watson HK, Carlson L, Lown I, Wollstein R. Use of quantitative abductor pollicis brevis strength testing in patients with carpal tunnel syndrome. Plast Reconstr Surg. 2007;119:1277–83. doi: 10.1097/01.prs.0000254498.49588.2d. [DOI] [PubMed] [Google Scholar]
- 17.Bergquist ER, Hammert WC. Timing and appropriate use of electrodiagnostic studies. Hand Clin. 2013;29:363–70. doi: 10.1016/j.hcl.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Hansen PA, Micklesen P, Robinson LR. Clinical utility of the flick maneuver in diagnosing carpal tunnel syndrome. Am J Phys Med Rehabil. 2004;83:363–7. doi: 10.1097/01.phm.0000124439.14757.99. [DOI] [PubMed] [Google Scholar]
- 19.Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol. 1990;17:1495–8. [PubMed] [Google Scholar]
- 20.Phalen GS. The carpal-tunnel syndrome. Seventeen years’ experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Joint Surg Am. 1966;48:211–28. [PubMed] [Google Scholar]
- 21.Dammers JW, Roos Y, Veering MM, Vermeulen M. Injection with methylprednisolone in patients with the carpal tunnel syndrome: A randomised double blind trial testing three different doses. J Neurol. 2006;253:574–7. doi: 10.1007/s00415-005-0062-2. [DOI] [PubMed] [Google Scholar]
- 22.Rahman KU, Rahman S, Khan A, Khan NA, Khan FU, Khan RA, et al. Assessing the complications and effectiveness of open carpal tunnel release in a tertiary care centre in a developing country. Int J Surg Case Rep. 2014;5:209–11. doi: 10.1016/j.ijscr.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chammas M. Carpal tunnel syndrome. Chir Main. 2014;33:75–94. doi: 10.1016/j.main.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Nam KP, Gong HS, Bae KJ, Rhee SH, Lee HJ, Baek GH. The effect of patient involvement in surgical decision making for carpal tunnel release on patient-reported outcome. J Hand Surg Am. 2014;39:493–8. doi: 10.1016/j.jhsa.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Bland JD. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000;23:1280–3. doi: 10.1002/1097-4598(200008)23:8<1280::aid-mus20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 26.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. European Palliative Care Research Collaborative (EPCRC). Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: A systematic literature review. J Pain Symptom Manage. 2011;41:1073–93. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Kilmer DD, Davis BA. Electrodiagnosis in carpal tunnel syndrome. Hand Clin. 2002;18:243–55. doi: 10.1016/s0749-0712(01)00009-9. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira JT. Carpal tunnel syndrome: Controversies regarding clinical and electrodiagnosis and its work-relatedness. Arq Neuropsiquiatr. 2000;58:1142–8. doi: 10.1590/s0004-282x2000000600027. [DOI] [PubMed] [Google Scholar]
- 29.Gerr F, Letz R. The sensitivity and specificity of tests for carpal tunnel syndrome vary with the comparison subjects. J Hand Surg Br. 1998;23:151–5. doi: 10.1016/s0266-7681(98)80163-0. [DOI] [PubMed] [Google Scholar]
- 30.Stevens JC, Sun S, Beard CM, O’Fallon WM, Kurland LT. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38:134–8. doi: 10.1212/wnl.38.1.134. [DOI] [PubMed] [Google Scholar]
- 31.Werner RA, Franzblau A, Albers JW, Buchele H, Armstrong TJ. Use of screening nerve conduction studies for predicting future carpal tunnel syndrome. Occup Environ Med. 1997;54:96–100. doi: 10.1136/oem.54.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buck AA, Gart JJ. Comparison of a screening test and a reference test in epidemiologic studies. I. Indices of agreement and their relation to prevalence. Am J Epidemiol. 1966;83:586–92. doi: 10.1093/oxfordjournals.aje.a120609. [DOI] [PubMed] [Google Scholar]
- 33.Gart JJ, Buck AA. Comparison of a screening test and a reference test in epidemiologic studies. II. A probabilistic model for the comparison of diagnostic tests. Am J Epidemiol. 1966;83:593–602. doi: 10.1093/oxfordjournals.aje.a120610. [DOI] [PubMed] [Google Scholar]
- 34.Padua L, Giannini F, Girlanda P, Insola A, Luchetti R, Lo Monaco M, et al. Usefulness of segmental and comparative tests in the electrodiagnosis of carpal tunnel syndrome: The Italian multicenter study. Italian CTS Study Group. Ital J Neurol Sci. 1999;20:315–20. doi: 10.1007/s100720050047. [DOI] [PubMed] [Google Scholar]
- 35.Beekman R, Visser LH. Sonography in the diagnosis of carpal tunnel syndrome: A critical review of the literature. Muscle Nerve. 2003;27:26–33. doi: 10.1002/mus.10227. [DOI] [PubMed] [Google Scholar]
- 36.Jarvik JG, Yuen E, Kliot M. Diagnosis of carpal tunnel syndrome: Electrodiagnostic and MR imaging evaluation. Neuroimaging Clin N Am. 2004;14:93–102. doi: 10.1016/j.nic.2004.02.002. [DOI] [PubMed] [Google Scholar]


