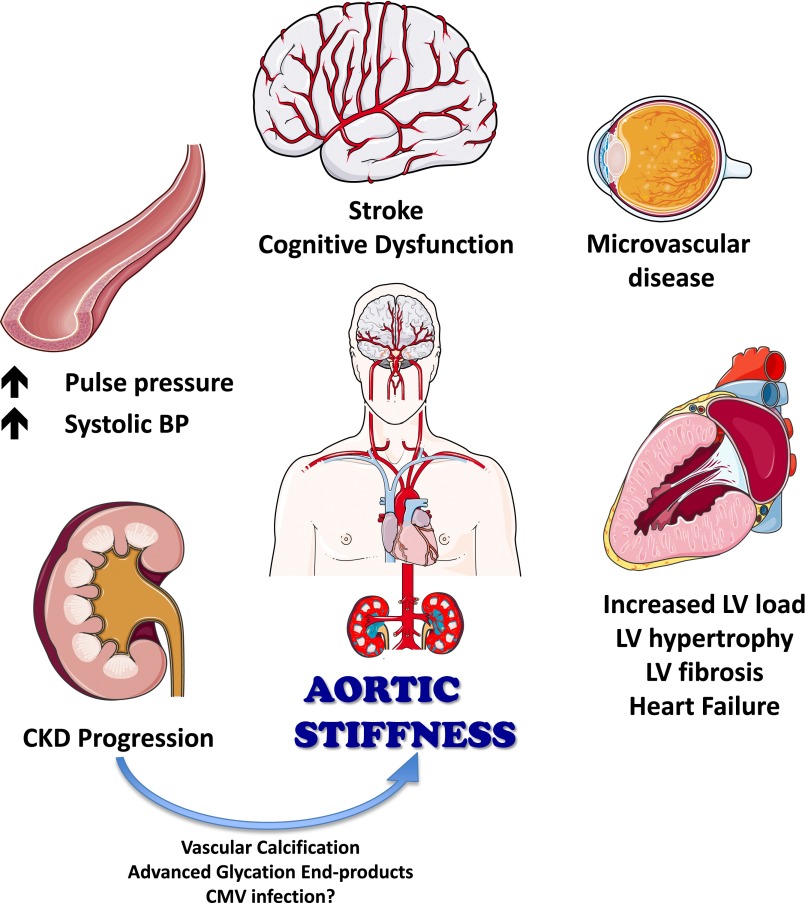
Arterial stiffness is highly relevant to cardiovascular health. Arterial stiffness increases with age and various disease states (1–4), including CKD (5,6). Stiffening of the arterial wall affects the ability of arteries to accommodate the blood ejected by the left ventricle (LV) during each heartbeat. This has important consequences on pressure fluctuations in the arterial tree and on the load opposing LV ejection (LV afterload) (7–9). By virtue of its effect of pressure pulsatility, large artery stiffening promotes excessive penetration of pulsatile pressure into peripheral organs, particularly those in which precapillary resistance is low (e.g., the kidney and the brain) (10,11). The hemodynamic effects of large artery stiffness therefore likely contribute to the development of LV remodeling and failure, the progression of CKD, and microvascular disease in the brain and eye (Figure 1) (5,10–14).
Figure 1.
Consequences of aortic stiffening. CMV, cytomegalovirus; LV, left ventricle.
The Chronic Renal Insufficiency Cohort (CRIC) study demonstrated that, among patients with CKD, carotid-femoral pulse wave velocity (CF-PWV; a measure of large artery stiffness) is much greater (approximately 2 m/s higher) in the presence of diabetes and increases in tandem with the reduction of GFR (with each 10-ml/min per 1.73 m2 decline in GFR being independently associated with an approximately 0.23-m/s increase in CF-PWV) (5). As would be expected from its hemodynamic consequences, CF-PWV was an independent predictor of incident hospitalized heart failure (15) and a faster progression of renal dysfunction (5) in the CRIC cohort. CF-PWV was also an independent correlate of cognitive dysfunction and 24-hour protein excretion (particularly among participants with diabetes).
Although CF-PWV predicts cardiovascular risk in patients without CKD and those with mild-to-moderate CKD (16–18), when patients reach ESRD, large artery stiffness becomes an even stronger risk predictor (16). In ESRD, CF-PWV is a powerful predictor of cardiovascular and all-cause death (19–22); furthermore, longitudinal changes in CF-PWV in response to antihypertensive therapy predict future cardiovascular risk. In a study of 150 hemodialysis patients, aortic pulse wave velocity (PWV) was higher at baseline (11 m/s versus 9.5 m/s) and increased over time despite antihypertensive treatment in those who died during follow-up, compared with a decrease over time (which paralleled the reduction in mean arterial pressure [MAP]) in those who survived (23).
To the degree that large artery stiffness is associated with adverse outcomes and is known to directly lead to hemodynamic perturbations that promote target organ damage (suggesting a cause-effect relation), reducing arterial stiffness is increasingly recognized as an important therapeutic goal. Advanced glycation end-product accumulation, pathways leading to vascular calcification (but not fibroblast growth factor 23), and chronic cytomegalovirus infection have been identified as potential contributors to arterial stiffening in CKD (24). Whereas specific pathways leading to arterial stiffening in ESRD should continue to be investigated (and findings translated into novel therapeutic approaches), a more immediate need exists for a better understanding of the effects of various antihypertensive medications on arterial stiffness in this patient population.
In this issue of CJASN, Georgianos and Agarwal (25) report on the results of a secondary analysis of the Hypertension in Hemodialysis Patients Treated with Atenolol or Lisinopril (HDPAL) study, a randomized, open-label, parallel-arm clinical trial, in which dialysis patients were randomly assigned to receive either lisinopril or atenolol. The investigators performed 44-hour ambulatory BP monitoring (ABPM) and in-office aortic PWV measurements at baseline and after 6 months of randomized therapy. Unexpectedly (and contrary to the original hypothesis), atenolol was superior to lisinopril in reducing aortic PWV. Aortic PWV nonsignificantly increased by +5.5% on lisinopril but significantly decreased by 9.3% on atenolol, with a between-group difference of 14.8% (95% confidence interval, 1.2% to 28.5%). This difference persisted after adjustment for various covariates (including heart rate), but only a statistical trend persisted after adjustment for the change in ambulatory systolic BP. The latter finding needs to be carefully interpreted. In observational studies, multivariable adjustments are typically performed to remove potential confounding by covariates that may lead to spurious associations between variables of interest. In a randomized trial, the study design itself allows for directly answering the question of whether the intervention causes a change in the end point. Such an answer is primarily obtained from unadjusted analyses, unless major imbalance in important characteristics occurs at baseline between randomized trial arms. Another purpose of multivariable analyses in this context may be to assess potential correlates of the change in the end point (in this case, PWV) induced by randomized therapy. Either mechanisms (mediators) of the change or consequences of that change can be identified. The interesting observation that the between-arm difference in CF-PWV was not significantly different after adjustment for systolic BP needs to be interpreted while taking physiologic considerations into account. It is therefore useful to review basic principles about what determines PWV and what are its interrelationships with BP.
Stiffness is the resistance offered by an elastic body to deformation (strain) when a force (stress) acts on that body, and can be expressed as the elastic modulus, which is the ratio of stress/strain. This ratio is higher (and stiffness greater) when there is little deformation relative to the force applied. Some materials demonstrate Hookean behavior, defined by a constant elastic modulus at different degrees of stretch. Given its diverse molecular and mechanical composition, the arterial wall is not Hookean, but demonstrates an increasingly stiff behavior as it is progressively stretched. In vivo, the “distending pressure” for the arterial wall is the MAP. MAP is, from a hemodynamic perspective, independent of large arterial properties, but is dependent purely on cardiac output and peripheral resistance (which arises almost exclusively in the microvasculature, because of its strong inverse exponential relationship with vessel radius) (9). Yet arterial stiffness at any given MAP has a direct effect on pressure fluctuations around that MAP (and thus on pulse pressure). To the degree that increased pulse pressure is a consequence of arterial stiffness, changes in aortic stiffness may lead to (rather than result from) changes in systolic BP. The fact that adjustment for systolic BP attenuated the change in aortic PWV is therefore not surprising, and this should not detract from the fact that atenolol therapy was superior to lisinopril in reducing aortic PWV. Adjustments for MAP obtained during PWV measurements would be useful to infer whether atenolol therapy modified the intrinsic material properties of the arterial wall (as opposed to simply the operating stiffness by reducing the distending pressure), and should be pursued in future analyses. Similarly, formal causal modeling could be applied to disentangle relationships between BP and arterial stiffness.
The method to measure aortic PWV used by Georgianos and Agarwal (25) deserves mention. PWV can be measured using recordings of the temporal pulse profile at any two locations. Pressure and blood flow changes at a given site are attributable to the same perturbation (i.e., the arterial pulse) traveling through the artery. Therefore, travel time can be assessed from either of these signals. Aortic PWV is most commonly estimated over the carotid-femoral pathway using arterial tonometry. Although this method is widely used, ultrasonography can also be utilized for aortic PWV measurements. Ultrasonography techniques have the advantage of allowing for measurements of PWV between the aortic arch and the abdominal aorta, thus interrogating a segment that does not include muscular arteries (in contrast with CF-PWV, which includes femoral and carotid arterial segments). Although ultrasonography is well suited to measure aortic stiffness in clinical studies, strict technical considerations are required. The pulse transit time needs to be measured as the time delay between the feet of the wave at the two locations (the so-called foot-to-foot method) because this early part of the wave is not prominently “contaminated” by wave reflections, thus indicating the true wave speed. Flow interrogations in the aorta are best performed using two-dimensional imaging–guided pulsed-wave Doppler recordings at high temporal resolutions (i.e., high sweep speeds). Time-delay estimations are best made using automated algorithms (e.g., the intersecting tangent method, derivative-based methods, or cross-correlation methods). Such algorithms are well developed for tonometric signals, but similar approaches can be applied to digitized flow waveforms obtained from Doppler spectral tracings. These approaches enhance ultrasonography-derived PWV measurements and should be implemented whenever possible. Doppler ultrasonography represents a useful, underutilized, and widely available tool to measure aortic PWV in clinical studies.
Some limitations about this study should be considered. Long-term intervention studies in hemodialysis patients are notoriously difficult undertakings. The authors randomized 179 patients with adequate PWV data at baseline and truncated the duration of randomized therapy from 12 to 6 months for these analyses, with only 109 participants completing the 6-month visit. An important strength of the study was the use of interdialytic ABPM to monitor 44-hour BP, which increases the confidence regarding changes in BP in both arms.
In summary, the authors should be congratulated for their important contribution to our understanding of the effects of atenolol and lisinopril on aortic stiffness in patients with ESRD. Measurements of aortic PWV as an end point are important in trials such as HDPAL, because the relevance of large artery stiffening in human health goes well beyond its effects on systolic BP. As we continue to learn about mechanisms that lead to arterial stiffening in CKD, the quick translation of this knowledge into clinical trials that test novel interventions for attenuating or reversing arterial stiffness in this population should remain a high-priority goal for future research.
Disclosures
J.A.C. is a consultant for OPCO HealthCare, Bristol Myers Squibb, and Fukuda Denshi, and receives research funding from the National Institutes of Health, US Department of Veterans Affairs, American College of Radiology Network, and Fukuda Denshi. R.R.T. is a consultant for Medtronic, Janssen, and Novartis and has a National Institutes of Health grant in a relevant area.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Effect of Lisinopril and Atenolol on Aortic Stiffness in Patients on Hemodialysis,” on pages 639–645.
References
- 1.Mitchell GF: Arterial stiffness and hypertension: Chicken or egg? Hypertension 64: 210–214, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols WW, O’Rourke MF, Vlachopoulos C, editors: McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles, 6th Ed, Boca Raton, FL, CRC Press, 2011 [Google Scholar]
- 3.Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA: Inflammation and arterial stiffness in humans. Atherosclerosis 237: 381–390, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Prenner SB, Chirinos JA: Arterial stiffness in diabetes mellitus. Atherosclerosis 238: 370–379, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Townsend RR: Arterial stiffness and chronic kidney disease: Lessons from the Chronic Renal Insufficiency Cohort study. Curr Opin Nephrol Hypertens 24: 47–53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briet M, Boutouyrie P, Laurent S, London GM: Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int 82: 388–400, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Segers P, Verdonck P: Principles of vascular physiology. In: Pan Vascular Medicine: Integrated Clinical Management, edited by Lanzer P, Topol E, Berlin, Springer, 2002 [Google Scholar]
- 8.Chirinos JA, Segers P: Noninvasive evaluation of left ventricular afterload: Part 1: Pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension 56: 555–562, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Chirinos JA, Segers P: Noninvasive evaluation of left ventricular afterload: Part 2: Arterial pressure-flow and pressure-volume relations in humans. Hypertension 56: 563–570, 2010 [DOI] [PubMed] [Google Scholar]
- 10.O’Rourke MF, Safar ME: Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 46: 200–204, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Vermeersch SJ, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, Van Bortel LM, Gillebert TC, Verdonck PR, Segers P: Determining carotid artery pressure from scaled diameter waveforms: Comparison and validation of calibration techniques in 2026 subjects. Physiol Meas 29: 1267–1280, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Triantafyllou A, Anyfanti P, Gavriilaki E, Zabulis X, Gkaliagkousi E, Petidis K, Triantafyllou G, Gkolias V, Pyrpasopoulou A, Douma S: Association between retinal vessel caliber and arterial stiffness in a population comprised of normotensive to early-stage hypertensive individuals. Am J Hypertens 27: 1472–1478, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Chirinos JA: Arterial stiffness: Basic concepts and measurement techniques. J Cardiovasc Transl Res 5: 243–255, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Dahan M, Paillole C, Ferreira B, Gourgon R: Doppler echocardiographic study of the consequences of aging and hypertension on the left ventricle and aorta. Eur Heart J 11[Suppl G]: 39–45, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Roy J, Pan Q, Go AS, Townsend RR; CRIC Study Investigators: Arterial stiffness, central pressures, and incident hospitalized heart failure in the Chronic Renal Insufficiency Cohort study. Circ Heart Fail 7: 709–716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlachopoulos C, Aznaouridis K, Stefanadis C: Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S: Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension 39: 10–15, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC: Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation 113: 657–663, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM: Stiffness of capacitive and conduit arteries: Prognostic significance for end-stage renal disease patients. Hypertension 45: 592–596, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, Ishimura E, Tabata T, Nishizawa Y: Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 12: 2117–2124, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Zoungas S, Cameron JD, Kerr PG, Wolfe R, Muske C, McNeil JJ, McGrath BP: Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis 50: 622–630, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Verbeke F, Van Biesen W, Honkanen E, Wikström B, Jensen PB, Krzesinski JM, Rasmussen M, Vanholder R, Rensma PL; CORD Study Investigators: Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: Outcome of the Calcification Outcome in Renal Disease (CORD) study. Clin J Am Soc Nephrol 6: 153–159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 103: 987–992, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Taal MW: Arterial stiffness in chronic kidney disease: An update. Curr Opin Nephrol Hypertens 23: 169–173, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Georgianos PI, Agarwal R: Effect of lisinopril and atenolol on aortic stiffness in patients on hemodialysis. Clin J Am Soc Nephrol 10: 639–645, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]