Abstract
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repositories, part of the National Institutes of Health (NIH), are an important resource available to researchers and the general public. The Central Repositories house samples, genetic data, phenotypic data, and study documentation from >100 NIDDK-funded clinical studies, in areas such as diabetes, digestive disease, and liver disease research. The Central Repositories also have an exceptionally rich collection of studies related to kidney disease, including the Modification of Diet in Renal Disease landmark study and recent data from the Chronic Renal Insufficiency Cohort and CKD in Children Cohort studies. The data are carefully curated and linked to the samples from the study. The NIDDK is working to make the materials and data accessible to researchers. The Data Repositories continue to improve flexible online searching tools that help researchers identify the samples or data of interest, and NIDDK has created several different paths to access the data and samples, including some funding initiatives. Over the past several years, the Central Repositories have seen steadily increasing interest and use of the stored materials. NIDDK plans to make more collections available and do more outreach and education about use of the datasets to the nephrology research community in the future to enhance the value of this resource.
Keywords: biosamples, repository, chronic kidney disease, renal disease
Introduction
In 2003, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health (NIH), established repositories to increase the impact of current and previously funded major clinical studies by making their data and biospecimens available to the broader scientific community. These NIDDK Central Repositories (http://www.niddkrepository.org) house data, genetic samples, and a variety of biologic samples. They enhance the value of the original studies by allowing additional scientists to test new hypotheses without the need for new data or biospecimen collection. The updated and curated phenotypic data in the Central Repositories can also be used in combination with corresponding Genomewide Association Study (GWAS) and sequencing data that are available from NIH’s dbGaP (http://www.ncbi.nlm.nih.gov/gap) database. Moreover, researchers can use the Central Repositories to pool phenotypic and/or genetic data across several studies to increase the power of statistical analyses.
NIDDK-supported multisite clinical studies are required to reposit both samples and data in the Central Repositories according to a timeline outlined in the NIDDK’s sharing policy (1). Currently, only NIDDK-supported studies contribute materials to the Central Repositories.
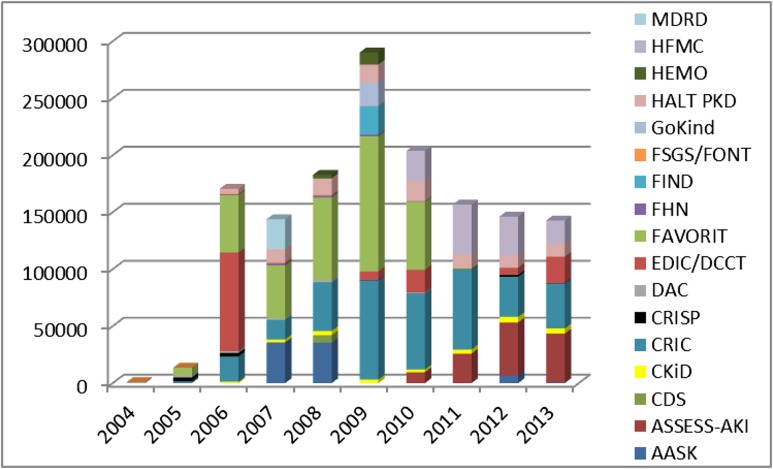
The Central Repositories have built a valuable collection of resources for nephrology studies over the past years (Table 1), with a growing collection of samples from these studies (Figure 1). Currently, data are available from 17 studies, and samples are available from 15 of those studies. The studies span a range of kidney diseases, rare and common, and include both interventional and observational protocols in pediatric and adult participants. All datasets sent to the Central Repositories are carefully curated by statisticians as a quality control measure. The curation process includes comparison of values from tables and figures in key published papers with those independently calculated from the Repositories data to help ensure the integrity of the data. In addition, all samples are matched to the phenotypic data. The Repositories perform rigorous quality control assessments of any DNA or cell lines they prepare. Quality control of other biologic samples is carried out principally by the studies, each of which has its own procedures. In addition, the Central Repositories visually inspect arriving samples, carefully monitor shipping conditions to make sure no thawing occurred, and verify that the arriving samples match those listed on the shipping manifest.
Table 1.
Kidney studies with data and/or samples in the Central Repositories
| Name | Acronym | Specimen Types | Current or Future Sharing? |
|---|---|---|---|
| A Cohort to Investigate the Value of Exercise/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD– a US Renal Data Survey (USRDS) Special Study | ACTIVE/ADIPOSE | S, WB | Future |
| Acute Renal Failure Trial Network | ATN | NA | Current |
| African-American Study of Kidney Disease and Hypertension Cohort Study | AASK Cohort | BC, P, S, U, DNA | Current |
| African-American Study of Kidney Disease and Hypertension Study (Clinical Trial) | AASK Trial | BC, P, S, U, DNA | Current |
| ASsessment, Serial Evaluation, and Subsequent Sequelae in AKI | ASSESS-AKI | P, S, U, DNA | Future |
| Autosomal Dominant Polycystic Kidney Disease (ADPKD) Genetic Modifier Study | ADPKD | DNA | Future |
| Chronic Renal Insufficiency Cohort Study | CRIC | BC, P, S, U, DNA | Current |
| The CKD in Children Cohort Study | CKiD | H, N, P, S, U, DNA | Current |
| Chronic Kidney Biomarkers Consortium | CKD Biocon | NA | Future |
| Comprehensive Dialysis Study - a US Renal Data System Special Study | CDS | NA | Current |
| Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease | CRISP | P, S, U, WB, DNA | Current |
| Diabetes Control and Complications Trial Study/Epidemiology of Diabetes Interventions and Complications | DCCT/EDIC | P, S, U, DNA | Current |
| Dialysis Access Consortium (Fistula) | FISTULA | P, S, DNA | Current |
| Dialysis Access Consortium (Graft) | GRAFT | P, S, DNA | Current |
| Family Investigation of Nephropathy and Diabetes | FIND | BC, PBMC, P, RBC, S, U, WB, DNA | Current |
| Focal Segmental Glomerulosclerosis Clinical Trial | FSGS | P, S, U, DNA | Current |
| Folic Acid for Vascular Outcome Reduction in Transplantation Trial | FAVORIT | BC, P, RBC, S, U | Current |
| Frequent Hemodialysis Network | FHN | P, S | Future |
| Hemodialysis Fistula Maturation Consortium | HFMC | P, S, T | Future |
| Hemodialysis Study | HEMO | S | Current |
| Modification of Diet in Renal Disease | MDRD | BC, P, S, U | Current |
| National Analgesic Nephropathy Study | NANS | NA | Current |
| Novel Therapies for Resistant FSGS | FONT | P, S, U, DNA | Future |
| The Genetics of Kidneys in Diabetes | GoKinD | P, S, U, DNA | Current |
| The Polycystic Kidney Disease Treatment Network | HALT PKD | P, S, U, WB, DNA | Future |
S, serum; WB, whole blood; NA, not available; BC, buffy coat; P, plasma; U, urine; H, hair; N, nails; PBMC, peripheral blood mononuclear cells; RBC, red blood cells; T, tissue.
Figure 1.
A variety of kidney studies have reposited samples with the National Institute of Diabetes and Digestive and Kidney Disease Biosample Repository over the past 10 years. AASK, African-American Study of Kidney Disease and Hypertension; ASSESS-AKI, ASsessment, Serial Evaluation, and Subsequent Sequelae in AKI; CDS, Comprehensive Dialysis Study; CKiD, The CKD in Children Cohort Study; CRIC, Chronic Renal Insufficiency Cohort Study; CRISP, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease; DAC, Dialysis Access Consortium; EDIC/DCCT, Epidemiology of Diabetes Interventions and Complications/Diabetes Control and Complications Trial Study; FAVORIT, Folic Acid for Vascular Outcome Reduction in Transplantation Trial; FHN, Frequent Hemodialysis Network; FIND, Family Investigation of Nephropathy and Diabetes; FSGS/FONT, Focal Segmental Glomerulosclerosis Clinical Trial/Novel Therapies for Resistant FSGS; GoKinD, The Genetics of Kidneys in Diabetes; HALT PKD, The Polycystic Kidney Disease Treatment Network; HEMO, Hemodialysis Study; HFMC, Hemodialysis Fistula Maturation Consortium; MDRD, Modification of Diet in Renal Disease
Some of the studies represent unique resources. For example, the Chronic Renal Insufficiency Cohort (CRIC) is one of the largest ongoing longitudinal studies of patients with CKD from a broad spectrum of causes, who are assessed annually for many clinical, physiologic, quality of life, and behavioral variables (2). Most of the data collected through the first 7 years of CRIC, along with associated samples and GWAS data, are available from the Central Repositories. The CKD in Children Cohort Study (CKiD) is a parallel observational study of pediatric patients with CKD that annually collects extensive data on clinical, psychosocial, neurocognitive, and growth measures (3). Most of the data from the first 5 years of CKiD, along with associated samples and GWAS data, are also available from the Repositories. Similarly, data and samples from the landmark Modification of Diet in Renal Disease and African-American Study on Kidney Disease and Hypertension have been available for several years. This article explains how to request access to these valuable collections, how the collections have been used in recent years, and future plans for educating researchers about specific datasets in order to enhance the utility of the Central Repositories for nephrology research.
Data and Sample Sharing
According to NIDDK policy, studies are expected to make datasets from major publications available within 6 months of publication, provide baseline data within 2 years after enrollment is completed, and send the entire study dataset 2 years after the data are locked for analysis at the completion of an intervention. For observational studies, incremental datasets are expected to be delivered within 2 years after each renewal of the study. NIDDK works closely with researchers to help them comply with this policy; 18 new or updated datasets have been added since January 2014.
Samples are typically sent at regular intervals during the study to the Central Repositories and are generally made available at the same time as the corresponding data. In some cases, the samples may not be available at the same time as the accompanying data because of regulatory considerations or because the study group itself is not yet permitted to use the samples, as in studies that are still blinded.
The NIDDK recognizes the need to balance data sharing with the compelling need to achieve the goals of the study and advance clinical science; the policy seeks to find that balance. The sharing timetable is tied to relevant milestones in studies that represent standard points at which a study will create a data freeze. Studies create data sharing plans in consultation with the NIDDK to facilitate planning and appropriate allocation of resources.
Accessing Collections of the Central Repositories
The Central Repositories house both renewable resources, such as data and DNA from immortalized cell lines, as well as nonrenewable resources, such as biosamples (e.g., serum, plasma, stool, urine, red blood cells, and tissue). The Central Repositories have built tools to facilitate searching among the studies so that researchers can readily identify those of interest. For each study, the public website contains a brief description, study forms, protocols, and a summary of the curation process, as well as the number and types of samples. In addition, a list of approved users and summaries of the approved projects are available (4), which allows potential requesters to see the studies being carried out.
There is no restriction on who may request resources from the Repositories, and requesters from industry are welcome. All external users are required to submit results from assays of samples within 1 year after being granted access. The timeline from application to fulfillment of request for the various types of requests is shown in Table 2.
Table 2.
Time of processing of requests for materials from the Repositories
| Variable | Type of Request | ||
|---|---|---|---|
| Data | Ancillary | External | |
| Total no. of requests | 104 | 88 | 12 |
| Average time to approval (d) | 37 | 15 | 69 |
| Average time to fulfillment (d) | 47 | 74 | 176 |
Information is shown for three types of requests. Data requests are reviewed administratively. Requests for samples for ancillary studies to ongoing parent studies are submitted by the parent study and are approved once all the paperwork is in place and a sample list has been finalized by the parent study. External requests are submitted following a favorable peer review of an application for access and are approved once all the paperwork is in place and a sample list has been finalized by the Central Repositories. Very few requests for renewable samples (DNA from cell lines) have been received, so information for those requests is not shown.
Renewable Resources
Access to renewable resources, specifically data and DNA from immortalized cell lines, has been simplified. Using a Web-based interface (http://www.niddkrepository.org), requesters describe their proposed study briefly, submit proof of institutional review board review, and a signed materials transfer agreement. NIDDK staff reviews the request for scientific appropriateness (but does not evaluate it for merit) and for ensuring that the proposed research falls within the scope of the informed consent for the study. There is no charge for application, although requesters are required to pay for shipping.
Samples for Ancillary Studies to Ongoing Studies
The Central Repositories have also tried to facilitate access to stored resources for approved ancillary studies. Ancillary studies are research projects carried out using the resources of one of the original contributing clinical studies (“parent study”) storing samples in the Central Repositories but are not part of the parent study protocol. Ancillary studies must be approved by the parent study group. Once the parent study has approved the ancillary study, the requester needs only to provide a signed materials transfer agreement and an approval letter from the parent study. The request is reviewed administratively to ensure that all the submitted materials are appropriate; there is no additional scientific review. Requesters must pay for shipping.
The NIDDK has created several funding opportunities for researchers to support ancillary studies to ongoing parent projects. An ongoing program announcement with special review solicits R01 applications for ancillary studies (5) , which are reviewed in a dedicated study section.
Nonrenewable Resources
Requests by external requesters for nonrenewable resources must undergo scientific peer review to ensure that the samples, which are finite, are distributed equitably to scientifically meritorious projects that take advantage of the design of the original study. Requesters can propose to remeasure specific analytes or repeat assays carried out by the parent study, but the project must be well justified and considered meritorious by a peer-review panel.
Requesters can apply for access only (no funds) using a X01 application (6) or for funding to use the samples via a program announcement with special review for R01s (7). In addition, there have been periodic Requests for Applications soliciting applications specifically to conduct studies with Central Repository samples related to type 1 diabetes. Several clinical studies focused on type 1 diabetes, including the Diabetes Control and Complications Trial–Epidemiology of Diabetes Interventions and Complications (EDIC) (8) and The Genetics of Kidneys in Diabetes (GoKinD) (9), have extensive data on diabetic kidney disease.
For external requests, there is no requirement to interact with the original study group, nor do the original investigators participate in the review. However, because the resources are finite, the process for applying for access can be time-consuming. Applications for X01s are peer reviewed an average of 65 days after receipt (data not shown) and administrative review takes place shortly thereafter. If the application is not approved, the applicant can resubmit. However, grant applications for funding as well as access take considerably longer and follow a more typical NIH application timeline of approximately 10 months from application to award (10).
After peer review, it takes an average of 6 months to completely fulfill approved requests (see Table 2). Much of this delay results from the extended dialogue between the requester and the Repositories that is needed to identify the appropriate samples.
Use of the Central Repositories
Over the years, the number of requests to the Central Repositories has increased steadily (Table 3). Most samples are used by ancillary studies, suggesting that it is easiest to understand and take advantage of the original study by working with the investigators who best know the study. In addition, some studies have created special opportunities to compete for funding and access to data and collections. For example, The Environmental Determinants of Diabetes in the Young (11) study has issued several requests for proposals to support work on gene expression, proteomics, dietary biomarkers, and metabolomics using study resources.
Table 3.
Total approved requests to the Central Repositories for all studies
| Year | No. of Sample Requests | No. of Data Requests | ||||
|---|---|---|---|---|---|---|
| Internal | Ancillary | External Renewable | External Nonrenewable | Total External | ||
| 2003 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2004 | 1 | 0 | 0 | 0 | 0 | 0 |
| 2005 | 4 | 1 | 0 | 0 | 0 | 4 |
| 2006 | 41 | 9 | 0 | 1 | 1 | 5 |
| 2007 | 32 | 10 | 0 | 2 | 2 | 15 |
| 2008 | 30 | 26 | 0 | 0 | 0 | 16 |
| 2009 | 90 | 15 | 0 | 10 | 10 | 33 |
| 2010 | 125 | 21 | 1 | 4 | 5 | 29 |
| 2011 | 110 | 31 | 3 | 3 | 6 | 58 |
| 2012 | 82 | 26 | 3 | 2 | 5 | 94 |
| 2013 | 101 | 34 | 2 | 3 | 5 | 55 |
Information about successful applications for resources is shown. There are multiple types of requests, as discussed in the text. Internal requests provide materials back to the original contributing study. Ancillary requests are for studies collaborating with the original contributing study. External requests are not associated with the original study.
Working with study groups has distinct advantages because those researchers are most familiar with the data and samples. Requesters accessing archival collections after the study group has disbanded can find it difficult to select the correct vials; in addition, the selected vials may have a lower volume than expected. This is reflected in the length of time it takes to process requests (Table 2). When the parent study group selects samples for an ancillary study, requests are processed much more quickly than when the Repositories have to do the selection for external requests.
Nevertheless, in the past 3 years external researchers have submitted applications for access to samples. The success rate for these programs is good. For example, 38% of X01 applications are granted access.
Utility of the Central Repositories
More than 100 reports based on the NIDDK study materials distributed by the Central Repositories and dbGaP have been published. Many requesters have used the GWAS data in dbGaP along with the phenotypic datasets from the Central Repositories to identify genetic variants of interest. For example, Pezzolesi et al. used GWAS (12,13) data from EDIC to identify susceptibility loci for diabetic nephropathy. Grassi et al. (14) analyzed the GWAS data from EDIC and GoKinD to identify several putative loci for severe diabetic retinopathy. Central Repositories resources have also been used to support major genetic studies, including a GWAS of pediatric CKD (R01-DK082394 and R01-DK080099) and a GWAS to identify modifiers of autosomal dominant polycystic kidney disease (U01-DK079856).
Other investigators have studied samples to characterize biomarkers. In a study of CKiD participants, Scialla et al. (15) found that participants with the largest increases in levels of fibroblast growth factor-23, parathyroid hormone, and phosphate exhibited faster rates of GFR decline. The CKD Biomarkers Consortium (16) has aggregated samples from many studies, including several in the Repositories, to rigorously evaluate new biomarkers for CKD onset and progression.
Secondary analysis of datasets in the Central Repositories has also yielded interesting results. For example, Argyropoulos et al. (17) and Sattar et al. (18) have reanalyzed data from the Hemodialysis Study. This reanalysis led them to propose new models for studying outcomes in dialysis patients and to conclude that the risk of death associated with diabetes in ESRD increases over time. Afshinnia et al. (19) reanalyzed the AKI Trial Network dataset and concluded that severe hypocalcemia with ionized calcium <1 mmol/L independently predicted mortality in patients with AKI requiring dialysis. These data showed that a subgroup analysis of a “negative” study can provide important insights into pathophysiology or suggest further lines of clinical investigation.
Allowing others to access study data and samples has potential pitfalls. The researcher may not be sufficiently familiar with the details of the study and, potentially, may draw erroneous conclusions. However, the NIDDK considers that the advantages of having fresh approaches to the large datasets outweigh the risks of an occasional publication with inaccuracies.
Looking Forward
While the ultimate value of the Central Repositories program must be judged by the scientific research it makes possible, it is important to understand that its operating costs exceed $4 million annually. NIDDK must work within that budget while assuring excellent customer services, controlling costs, and maintaining the quality of stored materials. NIDDK has used competitive bidding processes, external advisors, and feedback from users to help address these issues. Furthermore, NIDDK is continually evaluating the collection to balance the desire to archive all study materials for unknown future research with realistic assessments of the potential future demand for the samples. Recently, for example, the Central Repositories deaccessioned a large fraction of the samples from two studies, removing samples that could not be distributed because of consent issues or samples that were excessively redundant. In both cases, most of the samples were returned to the original investigators.
In parallel, the NIDDK is also working to increase interest in Central Repositories resources by acquiring new materials and creating an outreach program. In the coming year, several more datasets will be made available, including an interventional study in autosomal dominant polycystic kidney disease (HALT-PKD), a study of antibiotic prophylaxis in children with vesicoureteral reflux (RIVUR), and the Frequent Hemodialysis Network (FHN). In addition, a new program aimed at junior investigators will create opportunities to learn about the CRIC and CKiD datasets through workshops so they can develop collaborations with the investigators on these important kidney studies. This program is designed to reduce the considerable burden associated with learning to use an unfamiliar large dataset and the samples associated with it.
The goal of the Central Repositories is to enhance the value of the original studies by allowing additional scientists to test new hypotheses without new data or specimen collection. Many researchers have taken advantage of the collections already, and the NIDDK is working to enhance interest in and access to these valuable samples.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.National Institute of Diabetes and Digestive and Kidney Diseases. Information for Potential Grant Applicants and Awardees National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Data Sharing Policy. July 2013. Available at: http://www.niddk.nih.gov/research-funding/process/human-subjects-research/Documents/PublicversionNIDDKdatasharingpolicy2013July2013.pdf. Accessed July 2, 2014
- 2.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIDDK Central Repository. List of approved users. Available at: https://www.niddkrepository.org/requests/approved/data. Accessed July 2, 2014
- 5.Department of Health and Human Services. Funding opportunity for Ancillary Studies to Major Ongoing Clinical Research Studies to Advance Areas of Scientific Interest within the Mission of the NIDDK (R01). Available at: http://grants.nih.gov/grants/guide/pa-files/PAR-12-265.html. Accessed July 2, 2014
- 6.Department of Health and Human Services. Funding opportunity for NIDDK Central Repositories Non-renewable Sample Access (X01). Available at: http://grants.nih.gov/grants/guide/pa-files/PAR-11-306.html. Accessed July 2, 2014
- 7.Department of Health and Human Services. Funding opportunity for Biomarkers for Diabetes, Digestive, Kidney and Urologic Diseases Using Biosamples from the NIDDK Repository (R01). Available at: http://grants.nih.gov/grants/guide/pa-files/PAR-13-228.html. Accessed July 2, 2014
- 8.EDIC. The Epidemiology of Diabetes Interventions and Complications. Available at: https://portal.bsc.gwu.edu/web/edic;jsessionid=C22AE8A8D8694D34053DEA633B3B317C.ajp13. Accessed July 2, 2014
- 9.NIDDK Central Repository. The genetics of kidneys in diabetes.Available at: https://www.niddkrepository.org/studies/gokind/?query=gokind. Accessed July 2, 2014
- 10.National Institutes of Health. Grants process overview. August 12, 2014. Available at: http://grants.nih.gov/grants/grants_process.htm. Accessed August 14, 2014
- 11.EDDY. The Environmental Determinants of Diabetes in the Young. Available at: http://teddy.epi.usf.edu/. Accessed July 2, 2014
- 12.Pezzolesi MG, Poznik GD, Skupien J, Smiles AM, Mychaleckyj JC, Rich SS, Warram JH, Krolewski AS: An intergenic region on chromosome 13q33.3 is associated with the susceptibility to kidney disease in type 1 and 2 diabetes. Kidney Int 80: 105–111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pezzolesi MG, Poznik GD, Mychaleckyj JC, Paterson AD, Barati MT, Klein JB, Ng DP, Placha G, Canani LH, Bochenski J, Waggott D, Merchant ML, Krolewski B, Mirea L, Wanic K, Katavetin P, Kure M, Wolkow P, Dunn JS, Smiles A, Walker WH, Boright AP, Bull SB, Doria A, Rogus JJ, Rich SS, Warram JH, Krolewski AS, DCCT/EDIC Research Group : Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 58: 1403–1410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grassi MA, Tikhomirov A, Ramalingam S, Below JE, Cox NJ, Nicolae DL: Genome-wide meta-analysis for severe diabetic retinopathy. Hum Mol Genet 20: 2472–2481, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M: Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 24: 125–135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CKD Biomarkers Consortium. Overview. Available at: http://www.ckdbiomarkersconsortium.org/. Accessed July 2, 2014
- 17.Argyropoulos C, Chang CCH, Plantinga L, Fink N, Powe N, Unruh M: Biological considerations in the statistical analysis of hemodialysis patient survival. J Am Soc Nephrol 20: 1867–1869, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattar A, Argyropoulos C, Weissfeld L, Younas N, Fried L, Kellum JA, Unruh M: All-cause and cause-specific mortality associated with diabetes in prevalent hemodialysis patients. BMC Nephrol 13: 130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afshinnia F, Belanger K, Palevsky PM, Young EW: Effect of ionized serum calcium on outcomes in acute kidney injury needing renal replacement therapy: Secondary analysis of the acute renal failure trial network study. Ren Fail 35: 1310–1318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]