Abstract
Background and objectives
Vascular calcification (VC) is common in CKD, but little is known about its prognostic effect on patients with nondialysis CKD. The prevalence of VC and its ability to predict death, time to hospitalization, and renal progression were assessed.
Design, setting, participants, & measurements
The Study of Mineral and Bone Disorders in CKD in Spain is a prospective, observational, 3-year follow-up study of 742 patients with nondialysis CKD stages 3–5 from 39 centers in Spain from April to May 2009. VC was assessed using Adragao (AS; x-ray pelvis and hands) and Kauppila (KS; x-ray lateral lumbar spine) scores from 572 and 568 patients, respectively. The primary end point was death. Secondary outcomes were hospital admissions and appearance of a combined renal end point (beginning of dialysis or drop >30% in eGFR). Factors related to VC were assessed by logistic regression analysis. Survival analysis was assessed by Cox proportional models.
Results
VC was present in 79% of patients and prominent in 47% (AS≥3 or KS>6). Age (odds ratio [OR], 1.05; 95% confidence interval [95% CI], 1.02 to 1.07; P<0.001), phosphorous (OR, 1.68; 95% CI, 1.28 to 2.20; P<0.001), and diabetes (OR, 2.11; 95% CI, 1.32 to 3.35; P=0.002) were independently related to AS≥3. After a median follow-up of 35 months (interquartile range=17–36), there were 70 deaths (10%). After multivariate adjustment for age, smoking, diabetes, comorbidity, renal function, and level of phosphorous, AS≥3 but not KS>6 was independently associated with all-cause (hazard ratio [HR], 2.07; 95% CI, 1.07 to 4.01; P=0.03) and cardiovascular (HR, 3.46; 95% CI, 1.27 to 9.45; P=0.02) mortality as well as a shorter hospitalization event–free period (HR, 1.14; 95% CI, 1.06 to 1.22; P<0.001). VC did not predict renal progression.
Conclusions
VC is highly prevalent in patients with CKD. VC assessment using AS independently predicts death and time to hospitalization. Therefore, it could be a useful index to identify patients with CKD at high risk of death and morbidity as previously reported in patients on dialysis.
Keywords: vascular calcification, chronic renal disease, cardiovascular disease, mineral, metabolism
Introduction
Several studies have reported on the high prevalence of vascular calcification (VC) in patients with nondialysis CKD (1–9). It has been postulated that the early diagnosis of VC and the treatment of the factors that promote it could reduce the high rate of cardiovascular mortality observed in patients with CKD (10–14). Although VC can be assessed by various methods, such as ultrasonography, tomography, and arteriography, simple radiology has the advantages of being simple, inexpensive, and commonly applicable in daily clinical practice. Therefore, current guidelines recommend simple radiology for evaluating the presence of VC in patients with CKD (15,16).
Various studies have validated the use of the plain radiograph of the abdominal aorta (Kauppila score [KS]) or the hands and pelvis (simple VC score or Adragao score [AS]) as simpler and more affordable methods than tomography for showing the presence of VC, with a good correlation in patients on dialysis between coronary calcification (CC) and VC in other fields territories (14,17,18). In the dialysis population, the negative effect of VC on survival is well established in both the coronary territory and other locations (14,19,20). However, in patients with nondialysis CKD, only the long-term effect of CC is known (3). The aim of the Study of Mineral and Bone Disorders in CKD in Spain (OSERCE-2) was to evaluate in patients with nondialysis CKD the prevalence of VC, its correlation with parameters of bone mineral metabolism, and its effect on mortality, hospitalization, and progression of renal failure after a follow-up period of 3 years.
Material and Methods
Study Design
The OSERCE-2 is an observational, prospective, multicenter study of a cohort of patients with nondialysis CKD at stages 3–5 enrolled from 39 nephrology centers belonging to the Spanish National Health System with a follow-up period of 3 years. The Dr. Peset Hospital Research Ethics Committee approved this study, and all patients signed consent forms, consistent with the Declaration of Helsinki.
Patients
All patients included were age 18 years or over, were in nondialysis CKD stages 3–5 (eGFR<60 ml/min per 1.73 m2), and provided informed consent. The exclusion criteria were acute renal failure, serious illness that presupposed a life expectancy of <12 months, and hospital admission during the month before inclusion. In each center, researchers carried out consecutive recruitment by including the first 20 patients. All patients received a visit at baseline, and a laboratory blood sampling, a study of VC by x-ray of the abdomen, pelvis, and hands, and an ankle-brachial index (ABI) were undertaken after a clinical assessment, which included a record of past medical history and current medication. ABI was performed using continuous Doppler (Smartdop30EX; 8 MHz) as previously described (21).
VCs
VCs of iliac, femoral, radial, and digital arteries were assessed by x-ray of the pelvis and hands according to the AS (Supplemental Figure 1) (14). To analyze the prognostic effect of the location of VC, a separate analysis of VC of the radial and cubital arteries (AS-hands) was performed, because they are muscular arteries with a greater tendency to the calcification of the media (14). Aortic calcifications were evaluated by lateral abdominal x-ray, which included from vertebra T-10 to the first two vertebrae of the sacrum according to the KS (Supplemental Figure 2) (17). VC was classified as prominent at AS≥3 or KS>6 as previously reported (14,17). Assessment of the images was performed centrally by two experts in radiology (M.J.C. and R.V.) who did not have access to the clinical data of the patients. To validate the x-ray assessment, T.A. undertook an external analysis of a randomized and representative sample (27%) of the x-rays, which showed an excellent correlation with the data obtained by the radiologists of the study. The concordance was 85.5% in the final score (0–8) and 90.5% in the classification by groups (AS=0, =1–3, and >3). The interrater agreement (κ) was 0.78±0.36 (P<0.001) for the final score (0–8) and 0.84±0.03 (P<0.001) for the classification by groups.
Laboratory Tests
At baseline, blood tests were undertaken on all patients and then sent to a central laboratory.
Blood samples were analyzed for creatinine, total protein, albumin, calcium, phosphorus, intact parathyroid hormone (PTH), 25(OH) vitamin D, 1,25(OH)2 vitamin D, and high-sensitivity C-reactive protein. Intact PTH levels were determined by chemiluminescence (Immulite 2000); 25(OH) vitamin D and 1,25(OH)2 vitamin D were determined by radioimmunoassay (Biosource). In line with previously reported studies (22,23), the levels of 25(OH) vitamin D were transformed to the usual method of reference (DiaSorin Liaison chemiluminescent radioimmunoassay) to improve the comparability of the results of the study. To do this, we measured 25(OH) vitamin D concentration using both assays on 390 study participants (53%), and a regression analysis was performed to define the relationship between the DiaSorin method mean and the Biosource method mean (x). Given that the relationship was nonlinear, we fitted different regression models to the data and checked the validity of assumptions and the goodness of fit of each model. The best model was obtained with a power curve estimation regression model. The result was as follows: DiaSorin =x0.777 (adjusted R2=0.97; P<0.001).
Other determinations taken at baseline in each center were full blood count, blood glucose concentration, cholesterol and fractions, triglycerides, proteinuria (grams per 24 hours), albuminuria (milligrams per gram), and ferritin. To evaluate the progression of kidney function, creatinine levels were determined at baseline and months 12, 24, and 36. eGFR was calculated using the Modification of Diet in Renal Disease formula (24).
Survival, Hospitalization, and Beginning of RRT
The primary outcome measure was incidence of death from the recruitment period (April and May of 2009) to completion of follow-up (May of 2012). Secondary outcomes were hospital admissions and the appearance of a combined renal end point (beginning of dialysis, transplant, or drop of >30% in eGFR) over the follow-up.
Statistical Analyses
According to the information obtained from the literature, annual mortality in patients with nondialysis CKD is around 3% in stage 3, 7% in stage 4, and 9% in stage 5 (25). Approximately 75% of this population is estimated to show relevant VC (4). Although there are no studies that have analyzed the influence of VC on the mortality of patients with nondialysis CKD, studies undertaken on the hemodialysis population show a 3-fold increase in mortality in the group with VC compared with the group without VC (14). With a minimum follow-up of 3 years (assuming losses of 20% and considering an error of β=0.8), it is estimated that the initial inclusion of ≥568 patients (426 and 142 patients in the cohort with and without VC, respectively) is required to find significant differences between both groups.
The results of the continuous variables were expressed as the means±SDs or medians (interquartile ranges) as appropriate. Given that both the KS and the AS did not present a normal distribution, both VC scores were grouped together in dichotomous variables according to the presence or absence of prominent VC. The univariate analysis was undertaken using the t, Mann–Whitney U, or chi-squared test depending on the variables compared. Factors independently related to VC were assessed by logistic regression analysis, including those variables that were significant (P<0.05) in a univariate analysis: age, sex, diabetes mellitus, comorbidity, etiology, smoking, diastolic BP, eGFR, serum calcium, phosphorous, PTH, 1,25(OH)2 vitamin D, 25-hydroxivitamin D, hemoglobin, LDL-cholesterol, albumin, high-sensitivity C-reactive protein (log), glucose levels, and treatment with diuretics, vitamin D, statins, phosphate binders, and anticoagulants. The same covariates were included in all of the regression analysis. The correlation studies were carried out using Spearman’s rank correlation coefficient. The crude analysis of overall survival in each patient group depending on the degree of VC was estimated using the Kaplan–Meier method. The log-rank test was used to compare the survival curve. The univariate and multivariate analyses were conducted by means of the Cox proportional hazards model of death as a function of the degree of VC. For the multivariate model building, we first included one VC score and those variables related to VC, mortality, and morbidity according to the current literature (age, smoking, and phosphorous levels), and therefore, these four variables were included in all of the final models. Then, we generated different models by entering the other predictors significantly associated with death in the univariate analysis (comorbidity, diabetes, overweight, diastolic BP, eGFR, serum 25(OH) vitamin D, 1,25(OH)2 vitamin D, and albumin and hemoglobin levels). As previously recommended (26) and because of the limited number of deaths (70 events), we avoided including more than seven variables in each model. For the same reason, in the analysis of mortality from vascular cause (25 events), we included only VC scores and age, phosphorous levels, or smoking as covariates. Likewise, an analysis was undertaken for hospitalization and combined renal end point. The variables included in the multivariate analysis for hospitalization were age, diabetes, comorbidity, smoking, BP, ABI, AS, KS, eGFR, serum phosphorous, and albumin, hemoglobin, and cholesterol levels. The variables included in the multivariate analysis for CKD progression were age, sex, diabetes, smoking, BP, ABI, eGFR, proteinuria, and serum phosphorous, calcium, PTH, vitamin D, albumin, and hemoglobin levels. To avoid colinearity, we did not include AS and AS-hands in the same model. A P value <0.05 was considered significant.
Results
From 742 patients enrolled at baseline, complete radiographic data for assessment of VC using the AS and the KS were available for 572 (77%) and 568 (77%) patients, respectively, which were included in the final analysis. The baseline characteristics by the VC score group are summarized in Table 1.
Table 1.
Baseline patient characteristics and laboratory values as a function of vascular calcification scores
| Characteristics | All (n=742) | KS≤6 (n=392; 69%) | KS>6 (n=176; 31%) | P Value | AS<3 (n=399; 70%) | AS≥3 (n=173; 30%) | P Value |
|---|---|---|---|---|---|---|---|
| Age (yr) | 66.6±13.0 | 63.5±13.9 | 71.5±8.7 | <0.001 | 63.8±14.0 | 69.9±9.7 | <0.001 |
| Sex (%) | |||||||
| Men | 469 (65%) | 244 (62%) | 110 (63%) | 0.97 | 236 (59%) | 130 (75%) | <0.001 |
| Women | 253 (35%) | 148 (38%) | 66 (37%) | 163 (41%) | 43 (25%) | ||
| CKD stage (%)a | |||||||
| 3 | 277 (40%) | 164 (42%) | 80 (45%) | 0.63 | 177 (40%) | 67 (40%) | 0.34 |
| 4 | 324 (46%) | 177 (45%) | 71 (40%) | 173 (46%) | 79 (46%) | ||
| 5 | 99 (14%) | 51 (13%) | 25 (15%) | 49 (14%) | 27 (14%) | ||
| Ethnicity (%) | |||||||
| Caucasian | 726 (99%) | 385 (98%) | 170 (97%) | 0.32 | 390 (98%) | 170 (98%) | 0.66 |
| Other | 16 (1%) | 7 (2%) | 6 (3%) | 9 (2%) | 3 (2%) | ||
| Cause of CKD (%) | |||||||
| Nephrosclerosis | 179 (24%) | 83 (21%) | 49 (28%) | <0.001 | 93 (23%) | 47 (27%) | <0.001 |
| Diabetic nephropathy | 159 (21%) | 62 (16%) | 53 (30%) | 56 (14%) | 61 (35%) | ||
| Intersticial | 103 (14%) | 63 (16%) | 26 (15%) | 68 (17%) | 18 (10%) | ||
| Glomerular | 76 (10%) | 52 (13%) | 8 (4%) | 57 (15%) | 7 (4%) | ||
| Polycystic kidney disease | 37 (5%) | 25 (6%) | 3 (2%) | 21 (5%) | 7 (4%) | ||
| Other causes | 66 (9%) | 38 (10%) | 7 (4%) | 33 (8%) | 8 (5%) | ||
| Not specified | 122 (16%) | 69 (18%) | 30 (17%) | 71 (18%) | 25 (15%) | ||
| Comorbidities (%) | |||||||
| Hypertension | 697 (94%) | 368 (94%) | 171 (97%) | 0.18 | 375 (94%) | 166 (96%) | 0.52 |
| Dyslipidemia | 491 (66%) | 241 (61%) | 134 (76%) | 0.001 | 262 (66%) | 121 (70%) | 0.30 |
| Diabetes mellitus | 274 (37%) | 108 (28%) | 90 (51%) | <0.001 | 110 (28%) | 95 (55%) | <0.001 |
| Coronary artery disease | 150 (20%) | 59 (15%) | 59 (34%) | <0.001 | 63 (16%) | 52 (30%) | <0.001 |
| Chronic heart failure | 62 (8%) | 23 (6%) | 29 (17%) | <0.001 | 27 (7%) | 25 (15%) | <0.01 |
| Cerebrovascular disease | 79 (11%) | 31 (8%) | 31 (18%) | <0.001 | 31 (8%) | 32 (19%) | <0.001 |
| Peripheral vascular disease | 151 (20%) | 51 (13%) | 59 (34%) | <0.001 | 56 (14%) | 58 (34%) | <0.001 |
| Smoking (%) | |||||||
| Ex-smoker | 227 (31%) | 108 (28%) | 69 (39%) | 0.02 | 113 (28%) | 69 (40%) | 0.01 |
| Active | 85 (11%) | 49 (13%) | 14 (8%) | 50 (13%) | 12 (7%) | ||
| BMI (kg/m2) | 28.6±5.0 | 28.7±5.3 | 29.0±4.7 | 0.46 | 28.7±5.3 | 29.0±4.7 | 0.53 |
| BP (mmHg) | |||||||
| Systolic | 142.1±21.8 | 141.5±22.1 | 143.2±21.9 | 0.41 | 141.4±21.8 | 143.3±21.7 | 0.34 |
| Diastolic | 76.6±11.3 | 78.1±10.9 | 73.6±10.5 | <0.001 | 77.8±10.9 | 74.8±10.8 | 0.003 |
| Ankle-brachial pressure index <0.9 or >1.3 (%) | 301 (41%) | 138 (35%) | 92 (52%) | <0.001 | 143 (36%) | 92 (53%) | <0.001 |
| Creatinine (mg/dl) | 2.8±1.3 | 2.8±1.4 | 2.7±1.3 | 0.41 | 2.7±1.4 | 2.9±1.3 | 0.04 |
| eGFR (MDRD; ml/min per 1.73 m2) | 27.3±11.8 | 27.4±11.7 | 27.3±11.3 | 0.93 | 27.9±11.7 | 26.5±11.5 | 0.18 |
| 24-h Urine proteinuria (g/24 h)b,c | 0.66 (0.23–1.80) | 0.76 (0.28–1.76) | 0.50 (0.21–2.00) | 0.24 | 0.76 (0.25–2.01) | 0.50 (0.22–1.61) | 0.16 |
| Urine Alb/Cr ratio (mg/g)b,c | 106 (13–563) | 148 (24–699) | 85 (25–468) | 0.24 | 114 (20–564) | 113 (30–587) | 0.95 |
| Caalb (mg/dl) | 9.6±0.8 | 9.6±0.8 | 9.6±0.8 | 0.24 | 9.6±0.8 | 9.6±0.8 | 0.64 |
| P (mg/dl) | 3.5±0.9 | 3.4±0.9 | 3.7±0.9 | 0.004 | 3.5±0.8 | 3.7±1.0 | 0.02 |
| CaxP (mg2/dl2) | 33.7±8.4 | 33.2±8.2 | 35.0±7.9 | 0.02 | 33.4±8.3 | 35.0±8.8 | 0.04 |
| iPTH (pg/ml)b | 101 (60–164) | 99 (60–162) | 101 (59–168) | 0.61 | 99 (61–164) | 108 (61–165) | 0.84 |
| 25(OH)D (ng/ml) | 20.3±8.5 | 18.4±7.3 | 18.3±7.9 | 0.95 | 18.7±7.6 | 17.7±7.2 | 0.17 |
| 1,25(OH)2D (pg/ml) | 38.8±10.6 | 39.6±9.7 | 40.0±10.7 | 0.67 | 39.9±10.5 | 39.8±10.3 | 0.98 |
| hsCRP (mg/dl)b | 2.0 (2.0–7.4) | 2.0 (2.0–6.5) | 2.0 (2.0–7.1) | 0.55 | 2.0 (2.0–6.5) | 3.1 (2.0–7.7) | 0.11 |
| Albumin (g/dl) | 4.0±0.5 | 4.0±0.5 | 3.9±0.5 | 0.03 | 4.0±0.5 | 4.0±0.5 | 0.17 |
| Total proteins | 7.7±1.2 | 7.7±1.1 | 7.6±1.2 | 0.33 | 7.6±1.1 | 7.8±1.3 | 0.12 |
| Total cholesterol (mg/dl) | 180.4±36.8 | 184.4±39.3 | 174.8±44.4 | 0.01 | 184.5±39.3 | 176.5±45.0 | 0.04 |
| HDL-cholesterol (mg/dl) | 48.7±11.0 | 49.1±13.0 | 48.5±13.6 | 0.60 | 49.5±13.2 | 46.5±13.1 | 0.03 |
| LDL-cholesterol (mg/dl) | 100.1±30.4 | 107.0±32.9 | 98.5±31.3 | <0.01 | 106.6±32.8 | 99.7±32.5 | 0.02 |
| Hemoglobin (g/L) | 12.8±1.8 | 13.1±1.7 | 12.6±1.6 | <0.01 | 13.0±1.6 | 12.7±1.7 | 0.04 |
| Ferritin (ng/ml)b | 108 (56–202) | 109 (58–191) | 99 (50–206) | 0.44 | 105 (55–189) | 107 (56–200) | 0.78 |
| Transferrin (mg/dl) | 24.0±9.9 | 24.4±9.8 | 22.8±8.9 | 0.09 | 24.7±9.9 | 22.8±9.2 | 0.04 |
| Glucose (mg/dl) | 113±40 | 108±32 | 119±50 | <0.01 | 110±37 | 120±48 | 0.01 |
| Antihypertensive drugs (%) | |||||||
| Any drugs | 697 (94%) | 368 (94%) | 171 (97%) | 0.18 | 375 (94%) | 166 (96%) | 0.52 |
| RAAS inhibition | 563 (76%) | 300 (77%) | 132 (75%) | 0.92 | 301 (75%) | 131 (76%) | 0.56 |
| Diuretic | 439 (59%) | 232 (59%) | 116 (66%) | 0.30 | 230 (59%) | 119 (69%) | 0.03 |
| Phosphate binders (%) | |||||||
| Any binders | 156 (21%) | 85 (22%) | 31 (18%) | 0.34 | 83 (21%) | 40 (23%) | 0.11 |
| Calcium carbonate | 81 (11%) | 51 (13%) | 14 (8%) | 0.17 | 45 (11%) | 22 (13%) | 0.72 |
| Calcium acetate | 43 (6%) | 27 (7%) | 10 (6%) | 0.09 | 24 (6%) | 12 (7%) | 0.76 |
| Lantanum carbonate | 18 (2%) | 8 (2%) | 5 (3%) | 0.67 | 9 (2%) | 6 (3%) | 0.57 |
| Sevelamer | 14 (2%) | 5 (1%) | 2 (1%) | 0.79 | 8 (2%) | 1 (1%) | 0.36 |
| Aluminum based | 5 (1%) | 1 (1%) | 0 (0%) | 0.50 | 1 (1%) | 0 (0%) | 0.70 |
| Vitamin D (%) | |||||||
| Native vitamin D | 79 (11%) | 36 (9%) | 25 (14%) | 0.08 | 46 (12%) | 18 (10%) | 0.79 |
| Active vitamin D | 197 (27%) | 100 (26%) | 47 (27%) | 0.70 | 103 (26%) | 39 (23%) | 0.53 |
| Other treatments (%) | |||||||
| Cinacalcet | 12 (2%) | 9 (2%) | 1 (1%) | 0.27 | 7 (2%) | 2 (1%) | 0.89 |
| Bisphosphonate | 18 (2%) | 10 (3%) | 5 (3%) | 0.74 | 10 (3%) | 6 (4%) | 0.72 |
| Statins | 491 (62%) | 241 (61%) | 134 (76%) | 0.001 | 262 (66%) | 121 (70%) | 0.30 |
| Anticoagulant | 114 (15%) | 49 (13%) | 38 (22%) | 0.02 | 54 (14%) | 38 (22%) | 0.04 |
Complete radiographic data for assessment of vascular calcification were available in 572 (77%) and 568 (77%) patients in the cases of the AS and the KS, respectively; 525 (71%) patients were available for both vascular calcification scores. If not indicated otherwise, results are presented as means±SDs. BMI, body mass index; MDRD, Modification of Diet in Renal Disease; Alb/Cr, albumin/creatinine; Caalb, calcium adjusted for albumin levels; P, phosphorus; CaxP, calcium-phosphorus product; iPTH, intact parathyroid hormone; 25(OH)D, 25-hydroxivitamin D; 1,25(OH)2D, 1,25(OH)2 vitamin D; hsCRP, high–sensitivity C–reactive protein; RAAS, renin-angiotensin-aldosterone system; KS, Kauppila score; AS, Adragao score.
Centralized creatinine levels obtained in 700 (94%) patients.
Skewed values are presented as medians (interquartile ranges).
For the proteinuria analysis, 24-hour urine samples were obtained from 386 (52%) patients, whereas the albumin/creatinine quotient was determined in 236 (32%) patients.
VC Scores
Of all of the patients included in the study, the x-rays of 9% of patients were not performed because of logistic problems. In 14% of patients, although x-rays were performed, the images were too low in quality to be accurately assessed because of technical problems (i.e., low resolution of the image sent to the central radiology department, areas of fecal matter, and other technical difficulties in reading the x-rays appropriately), and the information was not included in the analysis; 525 (71%) patients were available for both VC scores assessments. The proportion of patients with VC was 78%, and in 47% of these patients, VC was prominent (AS≥3, 30%; KS>6, 31%); 24% of patients had VC on radial or cubital arteries (AS-hands ≥1).
Factors Associated with High VC Scores
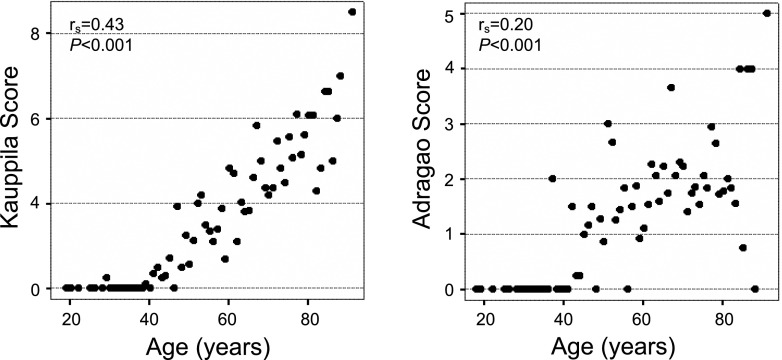
The proportion of patients with a KS>6 and an AS≥3 did not vary with the stage of CKD, whereas the proportion of patients with AS-hands ≥1 was higher among patients with lower eGFR (Figure 1). Although both KS and AS showed a positive and significant correlation with age, the correlation was stronger with KS than AS (Figure 2). Other factors that showed a significant and positive correlation with VC were phosphorus, diastolic BP, pulse pressure, and waist circumference (Supplemental Table 1). Only AS-hands showed a significant correlation with PTH levels and renal function. The independent determinant predictors of significant VC according to KS, AS, and AS-hands after multivariate analysis are shown in Table 2.
Figure 1.
Proportion of patients with Kauppila score >6, Adragao score ≥3, and vascular calcification of the radial and cubital arteries (Adragao Index-hands) ≥1 according to the stages of CKD.
Figure 2.
Vascular calcification scores in individual patients in relation to their age.
Table 2.
Multiple logistic regression analysis for independent predictors of significant vascular calcification according to Kauppila score >6, Adragao score ≥3, and Adragao score (hands only) ≥1
| Covariates | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Multiple logistic regression analysis for Kauppila score >6 | ||
| Age (yr) | 1.08 (1.05 to 1.10) | <0.001 |
| Diabetes mellitus | 1.68 (1.07 to 2.62) | 0.02 |
| Phosphorous (mg/dl) | 1.84 (1.39 to 2.44) | <0.001 |
| Peripheral vascular disease | 2.00 (1.20 to 3.35) | 0.01 |
| Smoking (active) | 1.67 (1.04 to 2.69) | 0.03 |
| Statins treatment | 1.91 (1.17 to 3.11) | 0.01 |
| Multiple logistic regression analysis for Adragao score ≥3 | ||
| Age (yr) | 1.05 (1.02 to 1.07) | <0.001 |
| Diabetes mellitus | 2.11 (1.32 to 3.35) | 0.002 |
| Phosphorous (mg/dl) | 1.68 (1.28 to 2.20) | <0.001 |
| Peripheral vascular disease | 1.71 (1.00 to 2.92) | 0.05 |
| Sex (women) | 0.34 (0.21 to 0.56) | <0.001 |
| Multiple logistic regression analysis for Adragao score (hands only) ≥1 | ||
| Age (yr) | 1.03 (1.01 to 1.05) | 0.003 |
| Diabetes mellitus | 2.70 (1.71 to 4.27) | <0.001 |
| Phosphorous (mg/dl) | 1.68 (1.28 to 2.20) | <0.001 |
| Pathologic ankle-brachial index | 1.76 (1.12 to 2.79) | 0.02 |
| Anticoagulant treatment | 2.02 (1.13 to 3.60) | 0.02 |
The same covariates were included in all of the regression analyses: age, sex, diabetes mellitus, comorbidity, etiology, smoking, diastolic BP, eGFR, serum calcium, phosphorous, parathyroid hormone, 1,25(OH)2 vitamin D, 25-hydroxivitamin D, hemoglobin, LDL-cholesterol, albumin, high-sensitivity C-reactive protein (log), glucose levels, and treatment with diuretics, vitamin D, statins, phosphate binders, and anticoagulants. CI, confidence interval.
Mortality
After a median follow-up of 35 months (25th percentile, 17 months; 75th percentile, 36 months), there were 70 deaths. The causes of death were cardiovascular (n=25; 36%), infections (n=13; 19%), tumors (n=7; 10%), sudden death (n=3; 4%), and others (n=8; 11%). In 14 patients (20%), the cause of death was unknown. The crude analysis of survival using the Kaplan–Meier method showed that the presence of significant VC estimated by any method predicted all-cause and cardiovascular mortality (Figure 3).
Figure 3.
Curves of overall survival and cardiovascular death of patients with CKD according to the presence of Kauppila score (KS) ≤6 or >6 ([A] overall survival; [B] cardiovascular survival), Adragao score (AS)<3 or ≥3 ([C] overall survival; [D] cardiovascular survival), and AS (only hands; AS-hands) =0 or ≥1 ([E] overall survival; [F] cardiovascular survival).
In the age-adjusted analysis of the factors associated with mortality (Supplemental Table 2), the group AS≥3 showed more than double the risk of all-cause mortality than the AS<3 group, whereas the risk was increased 5-fold when VC was evaluated using AS-hands. In contrast, VC assessed by KS did not predict mortality. Table 3 shows the predictive power of VC scores for death when adjusted for multiple covariates. In the multivariate analysis, AS≥3 but not KS>6 independently predicted all-cause and cardiovascular mortality (Table 3). AS-hands ≥1 was also an independent predictor of cardiovascular mortality.
Table 3.
Cox regression models of multivariate analysis for the factors associated with all-cause mortality and cardiovascular cause
| Model: Variables Included | HR (95% CI) for Kauppila Score >6 | P Value | HR (95% CI) for Adragao Score ≥3 | P Value | HR (95% CI) for Adragao Score (Only Hands) ≥1 | P Value |
|---|---|---|---|---|---|---|
| All-cause mortality (70 events) | ||||||
| Model 0: Unadjusted | 2.05 (1.19 to 3.51) | <0.01 | 3.41 (1.95 to 5.95) | <0.001 | 2.33 (1.36 to 3.99) | 0.002 |
| Model 1: VC score, age (yr), smoking (active), phosphorous (mg/dl) | 0.93 (0.50 to 1.75) | 0.83 | 2.33 (1.23 to 4.36) | <0.01 | 1.50 (0.81 to 2.79) | 0.20 |
| Model 2: VC score, age (yr), smoking (active), phosphorous (mg/dl), diabetes mellitus, comorbidity,a eGFR (ml/min per 1.73 m2) | 0.81 (0.42 to 1.57) | 0.53 | 2.07 (1.07 to 4.01) | 0.03 | 1.46 (0.77 to 2.77) | 0.25 |
| Model 3: VC score, age (yr), smoking (active), phosphorous (mg/dl), 1,25(OH)2D (pg/ml), diastolic BP (mmHg), hemoglobin (g/L) | 0.82 (0.43 to 1.56) | 0.55 | 2.31 (1.23 to 4.34) | 0.01 | 1.69 (0.91 to 3.15) | 0.10 |
| Model 4: VC score, age (yr), smoking (active), phosphorous (mg/dl), 25(OH)D (ng/ml), albumin (g/dl), overweight (BMI=25.0–29.9) | 0.90 (0.48 to 1.70) | 0.74 | 2.32 (1.23 to 4.37) | <0.01 | 1.53 (0.81 to 2.87) | 0.19 |
| Cardiovascular mortality (25 events) | ||||||
| Model 0: Unadjusted | 2.58 (0.99 to 6.68) | 0.05 | 4.39 (1.62 to 11.86) | 0.004 | 5.50 (2.13 to 14.18) | <0.001 |
| Model 1: VC score, age (yr) | 1.78 (0.67 to 4.68) | 0.25 | 3.46 (1.27 to 9.45) | 0.02 | 4.41 (1.70 to 11.46) | 0.002 |
| Model 2: VC score, smoking (active) | 1.38 (0.45 to 4.21) | 0.57 | 5.17 (1.59 to 16.81) | <0.01 | 6.26 (2.06 to 19.08) | 0.001 |
| Model 3: VC score, phosphorous (mg/dl) | 2.03 (0.71 to 5.88) | 0.19 | 3.63 (1.20 to 11.05) | 0.02 | 4.51 (1.58 to 12.82) | <0.01 |
HR, hazard ratio; VC, vascular calcification; 1,25(OH)2D, 1,25(OH)2 vitamin D; 25(OH)D, 25-hydroxivitamin D.
Includes background of coronary disease, congestive cardiac insufficiency, cerebrovascular disease, or peripheral vascular disease.
Hospitalization
There were 297 hospital admissions for 174 (24%) patients. The main pathologies that prompted hospitalization were cardiovascular (n=129; 43%) and infections (n=52; 18%). In the Kaplan–Meier analysis, the presence of significant VC had a reduced hospitalization-free survival caused by either all-cause or cardiovascular disease (Figure 4). In multivariate models, AS≥3 but not KS>6 independently predicted a shorter all-cause and cardiovascular hospitalization event–free period (Table 4). AS-hands ≥1 was also an independent predictor of hospitalization-free survival when introduced into the models for all-cause (hazard ratio [HR], 1.29; 95% confidence interval [95% CI], 1.13 to 1.49; P<0.001) and cardiovascular-related (HR, 1.30; 95% CI, 1.07 to 1.57; P<0.01) hospitalization.
Figure 4.
All-cause and cardiovascular hospitalization event–free periods in patients with CKD according to the presence of KS ≤6 or >6 ([A] all–cause hospitalization event–free period; [B] cardiovascular hospitalization event–free period), AS <3 or ≥3 ([C] all-cause hospitalization event–free period; [D] cardiovascular hospitalization event–free period), and AS (only hands; AS-hands) =0 or ≥1 ([E] all-cause hospitalization event–free period; [F] cardiovascular hospitalization event–free period).
Table 4.
Multivariate analysis of the factors associated with hospitalization-free survival with either all-cause or cardiovascular disease
| Factor | All-Cause Hospitalization (297 Admissions) | Cardiovascular Hospitalization (129 Admissions) | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value | |
| Kauppila score >6 | 1.00 | 0.96 to 1.04 | 0.94 | 1.06 | 1.01 to 1.11 | 0.02 |
| Adragao score ≥3 | 1.14 | 1.06 to 1.22 | <0.001 | 1.14 | 1.02 to 1.26 | 0.02 |
| Age (yr) | 1.02 | 1.00 to 1.03 | 0.10 | 1.01 | 0.98 to 1.03 | 0.62 |
| Smoking (active) | 1.06 | 0.59 to 1.90 | 0.85 | 0.82 | 0.31 to 2.15 | 0.68 |
| Diabetes mellitus | 1.41 | 0.95 to 2.09 | 0.08 | 1.19 | 0.68 to 2.08 | 0.54 |
| Comorbiditya | 1.03 | 0.69 to 1.52 | 0.90 | 2.06 | 1.18 to 3.62 | 0.01 |
| Systolic BP | 0.99 | 0.98 to 1.00 | 0.09 | 0.99 | 0.97 to 0.99 | 0.02 |
| Diastolic BP | 1.00 | 0.99 to 1.02 | 0.71 | 0.99 | 0.97 to 1.02 | 0.61 |
| Pathologic ankle-brachial index | 1.05 | 0.71 to 1.53 | 0.82 | 1.21 | 0.69 to 2.10 | 0.51 |
| Phosphorous (mg/dl) | 1.25 | 1.02 to 1.54 | 0.03 | 1.08 | 0.74 to 1.60 | 0.68 |
| eGFR (ml/min per 1.73 m2) | 0.99 | 0.97 to 1.00 | 0.12 | 1.01 | 0.98 to 1.03 | 0.66 |
| Hemoglobin (g/L) | 1.02 | 0.90 to 1.15 | 0.78 | 0.99 | 0.83 to 1.19 | 0.95 |
| Albumin (g/dl) | 0.66 | 0.47 to 0.93 | 0.02 | 0.74 | 0.43 to 1.28 | 0.28 |
| Total cholesterol | 1.00 | 0.99 to 1.00 | 0.15 | 1.00 | 0.99 to 1.00 | 0.15 |
Includes background of coronary disease, congestive cardiac insufficiency, cerebrovascular disease, or peripheral vascular disease.
CKD Progression and Initiation of RRT (Composite Renal End Point)
After 3 years of follow-up, 271 (38%) patients had a decrease in eGFR>30% or started RRT. Table 5 shows the Cox regression analysis adjusted for baseline eGFR of factors associated with CKD progression. VC by either method did not predict the composite renal end point, even when we stratify by CKD stages (data not shown). The multivariate analysis showed eGFR (HR, 0.94; 95% CI, 0.92 to 0.95; P<0.001), age (HR, 0.99; 95% CI, 0.98 to 0.99; P=0.04), PTH levels (HR, 1.14; 95% CI, 1.02 to 1.26; P=0.02), proteinuria (HR, 1.75; 95% CI, 1.21 to 1.25; P=0.003), active tobacco smoking (HR, 1.63; 95% CI, 1.11 to 2.39; P=0.01), and hemoglobin levels (HR, 0.88; 95% CI, 0.79 to 0.98; P=0.02) to be independent predictors of CKD progression.
Table 5.
Cox regression analysis adjusted for baseline eGFR of factors associated with the renal end point (beginning of dialysis, transplant, or reduction of eGFR≥30% over evolution)
| Factor | HR | 95% CI | P Value |
|---|---|---|---|
| eGFR (MDRD, unadjusted) | 0.92 | 0.90 to 0.93 | <0.001 |
| Age (yr) | 0.99 | 0.98 to 1.00 | 0.09 |
| Sex (women) | 0.71 | 0.54 to 0.92 | 0.01 |
| Comorbiditya | 1.13 | 0.88 to 1.45 | 0.33 |
| Smoking (active) | 1.76 | 1.26 to 2.45 | <0.001 |
| Diabetes mellitus | 1.27 | 0.99 to 1.63 | 0.06 |
| Etiology (diabetic nephropathy) | 1.31 | 0.85 to 2.03 | 0.22 |
| Overweight (BMI=25.0–29.9)b | 0.97 | 0.71 to 1.31 | 0.82 |
| Obesity (BMI>29.9) | 0.96 | 0.70 to 1.32 | 0.80 |
| Pathologic waistc | 0.90 | 0.67 to 1.21 | 0.46 |
| Systolic BP | 1.01 | 1.00 to 1.01 | <0.001 |
| Diastolic BP | 1.01 | 1.00 to 1.02 | 0.07 |
| Adragao score ≥3 | 0.81 | 0.60 to 1.10 | 0.18 |
| Adragao (numerical) | 0.99 | 0.93 to 1.05 | 0.68 |
| Adragao (only hands; ≥1) | 0.88 | 0.63 to 1.21 | 0.42 |
| Adragao (only hands; numerical) | 1.01 | 0.89 to 1.15 | 0.84 |
| Kauppila score >6 | 1.12 | 0.83 to 1.52 | 0.45 |
| Kauppila (numerical) | 1.01 | 0.98 to 1.03 | 0.72 |
| ABI<0.9 or >1.3 | 0.96 | 0.74 to 1.23 | 0.72 |
| Proteinuria (g/24 h; log)d | 2.17 | 1.57 to 3.00 | <0.001 |
| Albuminuria (mg/g; log)e | 1.05 | 0.96 to 1.16 | 0.30 |
| Calcium | 0.76 | 0.66 to 0.89 | <0.001 |
| Phosphorus | 1.12 | 0.96 to 1.31 | 0.14 |
| iPTH (log) | 1.22 | 1.10 to 1.34 | <0.001 |
| 25-OH vitamin D | 0.99 | 0.98 to 0.99 | 0.001 |
| 1,25(OH)2 vitamin D | 1.01 | 0.99 to 1.02 | 0.28 |
| Albumin | 0.71 | 0.56 to 0.91 | <0.01 |
| hsCRP (log) | 1.03 | 0.89 to 1.19 | 0.70 |
| Transferrin | 1.00 | 0.99 to 1.01 | 0.93 |
| Total cholesterol | 1.00 | 1.00 to 1.00 | 0.46 |
| LDL-cholesterol | 1.00 | 0.99 to 1.00 | 0.45 |
| Glucose | 1.00 | 1.00 to 1.01 | 0.06 |
| Hemoglobin | 0.90 | 0.82 to 0.97 | <0.01 |
| Ferritin (log) | 1.03 | 0.89 to 1.19 | 0.73 |
ABI, ankle-brachial index.
Includes background of coronary disease, congestive cardiac insufficiency, cerebrovascular disease, or peripheral vascular disease.
There was only one malnourished patient (BMI<17.0), and the patient was excluded from the analysis.
Waist >94 cm for men and >80 cm for women.
Available in 596 patients.
Available in 233 patients.
Discussion
This study is the largest and longest prospective study evaluating the power of VC in predicting outcomes of patients with CKD stages 3–5 before RRT. We found that the presence of VC assessed by radiographs of the hand and pelvis is an independent and robust predictor of all-cause and cardiovascular death and reduced hospitalization-free survival. Although the negative effects of CC have been previously reported in patients with nondialysis CKD (3), our study shows, for the first time, the negative effect of VC in locations other than the coronary territory.
This association between VC and the risk of mortality and morbidity is a common finding in patients on dialysis (14,19,20). Our data showed that this association begins at earlier stages of CKD, strengthening the Kidney Disease Improving Global Outcomes (KDIGO) guidelines that recommend the use of simple radiology for screening VC in patients with CKD (15).
By assessing VC through the combination of KS and AS, we could extrapolate where VC predominated: whether in the tunica intima or media of the vessel. This is because the KS evaluates VC in an elastic artery, such as the aorta, and therefore, is more susceptible to calcification of the intima. By contrast, AS evaluates VC in muscular (radial and digital) or predominantly muscular (iliac and femoral) arteries, which are more susceptible to the calcification of the media (14,27). Although age, diabetes, and phosphorus levels seemed to be common elements associated with both pathologic KS and AS, this study shows the different prognostic value of both indexes, which suggests that the location of calcification seems to have prognostic significance in patients not on dialysis (28). Although both indexes behaved as predictors of death and hospital admission in the unadjusted analysis, only the AS is confirmed as an independent predictor in the multivariate analysis. Greater predictive power was obtained even when we analyzed the AS-hands, which suggests that, in patients with nondialysis CKD, it is the calcification of the media that presents a greater prognostic power. Although data are not available for patients with CKD, Hong et al. (29) have reported, in a dialysis population, that the calcification of digital arteries but not the abdominal aorta is a good predictor of mortality. Hong et al. (29) postulated that the high prevalence of aortic calcification and its association with age may limit its prognostic value for the survival of patients on dialysis. The same reasoning could be applied to patients with nondialysis CKD, in whom the presence of aortic calcification is age related and very prevalent, which was reported in this study and others (1–9). This discovery of the independent prognostic value that a plain radiograph of hands may have in detecting VC constitutes the most original finding of this study.
Renal function may have an important role in the onset and progression of VC. In this study, we observed that the VC of muscular arteries (radial and digital) was significantly correlated with the severity of renal dysfunction, which is in accordance with previous studies. Watanabe et al. (3) observed, in 117 patients with nondialyzed CKD, a trend toward an increase in the prevalence of severe CC according to the stage of kidney disease. Sigrist et al. (9) followed 46 patients with CKD stage 4 for 2 years and showed a correlation between reduction in eGFR and increase in CC score. However, we did not find a predictive effect of VC on eGFR reduction or initiation of RRT. This observation is of special interest, because as far as we are concerned, no study has shown the relationship between VC and kidney progression (3,30), supporting the hypothesis that the uremic milieu promotes VC but that the VC does not per se aggravate CKD as previously postulated (3,10,11).
The assessment of CKD-mineral and bone disorder as a factor for mortality and kidney progression was a secondary aim of this study. In line with previous studies (3,4,8,9), hyperphosphatemia was confirmed as an independent risk factor of VC, mortality, and hospitalization. Phosphorus could increase cardiovascular mortality by mechanisms other than VC, such as through fibroblast growth factor-23, which has been implicated in the pathogenesis of both myocardial hypertrophy and atherosclerosis (31,32). More interestingly, hyperparathyroidism was an independent predictor for kidney progression, which had not been fully shown previously (30), whereas low vitamin D levels were associated to all-cause mortality and ESRD after adjusting for age and eGFR, respectively. Lastly, we would like to draw attention to the independent association observed between the use of oral anticoagulants and VC, which has been previously reported (33–35). Although this association does not imply causation, it should be evaluated in future clinical trials designed to test anticoagulation strategies in the CKD population.
Strengths and Limitations
Apart from the centralization of the analytic parameters and the radiologic reading, the strength of this study resides in the adequate sample size and the relatively long period of follow-up. This study, however, presents limitations that should be taken into account. First, its observational design does not allow us to determine whether prevention or treatment of a risk factor like VC could lead to an improvement in patient survival. Second, as in any semiquantitative radiologic analysis, there is a dependent observer limitation. To minimize this factor, a centralized and blind reading of the radiographs by the two participating radiologists was undertaken, and also, an external analysis by T.A. was carried out. Third, the low number of deaths from cardiovascular causes limited its multivariate analysis.
Conclusions and Clinical Implications
Our data suggest that the presence of VC assessed by radiographs of the hand and pelvis is an independent and robust predictor of all-cause and cardiovascular mortality and the period of hospitalization in patients with nondialysis CKD, supporting the KDIGO guidelines on the use of simple radiology for screening VC in patients with CKD. According to our results, the screening should be directed especially at territories where muscular arteries prevail, such as the radial and digital arteries, using hand radiography. Clinical trials are warranted to evaluate strategies for preventing or delaying the appearance of VC and analyze its effect on the survival of patients with CKD, in whom life expectancy continues to be unacceptable.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank George Mattingley for his collaboration in translating this text. The authors thank the Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunidad Valenciana and Red de Investigación en Enfermedades Renales (RD12/0021/0019), Instituto de Salud Carlos III (ISCIII)-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional Foundations for the support that they provide to research projects and all of the coinvestigators of the Study of Mineral and Bone Disorders in CKD in Spain. Research activity by JFNG is supported by Programa de Intensificación de la Actividad Investigadora del ISCIII (Convenio ISCIII/Comunidad Autónoma de Canarias), Ministerio de Economía y Competitividad.
This work was supported by Abbvie and the Spanish Society of Nephrology.
A summary of this study was presented at the American Society of Nephrology Kidney Week 2013 Annual Meeting in Atlanta, Georgia held November 7–10, 2013 (Poster TH-PO166; “Impact of vascular calcifications assessed by simple radiography in the prognosis of non-dialysis chronic kidney disease patients: Results of 3-year observational OSERCE II study”).
The following investigators participated in the study: R. Alcazar (Hospital Infanta Leonor), A. Almoguera (Hospital Reina Sofia), A. Antolín (Hospital Francesc de Borja), J. Ampuero (Hospital Gregorio Marañón), G. Barril (Hospital de La Princesa), A. Blasco (Hospital Miguel Servet), J. Bover (Fundació Puigvert), J. Calviño (Complejo Hospitalario A Coruña, Coruña), S. Cigarran (Hospital de Burela), M.D. Del Pino (Hospital Torrecardenas), M. Dotori (Hospital Universitario de Málaga), V. Escudero (Hospital U. Dr. Peset), J.E. Fernández-Nájera (Hospital Francesc de Borja), R. Esteban (Hospital Virgen de las Nieves, Granada), M. Galicia (Hospital Vall d’Hebron), C. Garcia-Canton (Hospital Insular de Gran Canarias), G. García Erauskin (Hospital de Cruces), S. Garcia-Vinuesa (Hospital Gregorio Marañon), J. Gascó (Hospital Son Llàtzer), J.M. Gil-Cunquero (Complejo Hospitalario de Jaén), T. González Álvarez (Hospital Bellvitge), J.L. Górriz (Hospital U. Dr. Peset), E. Gruss (Fundación Hospital Alcorcón), M.A. Guerrero (Hospital Virgen del Rocio), J. Herrero (Hospital Clinico), J. Hervas (Hospital San Cecilio, Granada), M.P. Marco (Hospital Arnau de Vilanova), G. Martin-Reyes (Hospital Universitario de Málaga), A.L. Martin de Francisco (Hospital Marques de Valdecilla), A. Martinez Castelao (Hospital Bellvitge), I. Martínez Fernández (Hospital de Galdácano), E. Morales Ruiz (Hospital Doce de Octubre), P. Molina (Hospital U. Dr. Peset), R. Mouzo (Hospital del Bierzo), M.A. Munar (Hospital Son Dureta), J. Navarro-Gonzalez (H. Virgen de la Candelaria), J. Nieto (Hospital General de Ciudad Real), E. Novoa (Complejo Hospitalario de Orense), A. Osuna (H. Virgen de las Nieves), L. Orte (Hospital Ramón y Cajal), J.R. Ortiz-Vigon (Hospital Basurto), M.C. Páez (Hospital Universitario Virgen Macarena), A. Peris (Hospital Francesc de Borja), J. Pérez-Pérez (Hospital Miguel Servet), C. Piñera (Hospital Marqués de Valdecilla), M. Puerta (Hospital Infanta Leonor), B. Rivas (Hospital Universitario La Paz), R. Ruiz-Calero (Hospital Infanta Cristina), Carmen Sanchez (Hospital de La Princesa), P. Sanchez-Perez (Hospital Francesc de Borja), A. Soldevilla (Hospital La Fe), C. Solozabal (H. Virgen del Camino), S. Soriano (Hospital Infanta Sofia), N. Vega (H. U. Gran Canarias Dr. Negrin), and P. Vidau (Hospital General de Asturias).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07450714/-/DCSupplemental.
See related editorial, “Vascular Calcification in Predialysis CKD: Common and Deadly,” on pages 551–553.
References
- 1.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE: Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis 44: 1024–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Garland JS, Holden RM, Groome PA, Lam M, Nolan RL, Morton AR, Pickett W: Prevalence and associations of coronary artery calcification in patients with stages 3 to 5 CKD without cardiovascular disease. Am J Kidney Dis 52: 849–858, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Watanabe R, Lemos MM, Manfredi SR, Draibe SA, Canziani ME: Impact of cardiovascular calcification in nondialyzed patients after 24 months of follow-up. Clin J Am Soc Nephrol 5: 189–194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Canton C, Bosch E, Ramírez A, Gonzalez Y, Auyanet I, Guerra R, Perez MA, Fernández E, Toledo A, Lago M, Checa MD: Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol Dial Transplant 26: 2250–2256, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG: Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 23: 586–593, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Lee SY, Kim HY, Gu SW, Kim HJ, Yang DH: 25-hydroxyvitamin D levels and vascular calcification in predialysis and dialysis patients with chronic kidney disease. Kidney Blood Press Res 35: 349–354, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Hanada S, Ando R, Naito S, Kobayashi N, Wakabayashi M, Hata T, Sasaki S: Assessment and significance of abdominal aortic calcification in chronic kidney disease. Nephrol Dial Transplant 25: 1888–1895, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Porter CJ, Stavroulopoulos A, Roe SD, Pointon K, Cassidy MJ: Detection of coronary and peripheral artery calcification in patients with chronic kidney disease stages 3 and 4, with and without diabetes. Nephrol Dial Transplant 22: 3208–3213, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Sigrist MK, Taal MW, Bungay P, McIntyre CW: Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2: 1241–1248, 2007 [DOI] [PubMed] [Google Scholar]
- 10.London GM, Marchais SJ, Guérin AP, Métivier F: Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens 14: 525–531, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Giachelli CM: Vascular calcification mechanisms. J Am Soc Nephrol 15: 2959–2964, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9[Suppl]: S16–S23, 1998 [PubMed] [Google Scholar]
- 14.Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Gonçalves M, Negrao AP: A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant 19: 1480–1488, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Torregrosa JV, Cannata Andia J, Bover J, Caravaca F, Lorenzo V, Martín de Francisco AL, Martín-Malo A, Martínez I, González Parra E, Fernández Giráldez E, Rodríguez Portillo M, Sociedad Española de Nefrologia : SEN Guidelines. Recommendations of the Spanish Society of Nephrology for managing bone-mineral metabolic alterations in chronic renal disease patients. 28[Suppl 1]: S1–S22, 2008 [PubMed] [Google Scholar]
- 17.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW: New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis 132: 245–250, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Bellasi A, Ferramosca E, Muntner P, Ratti C, Wildman RP, Block GA, Raggi P: Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int 70: 1623–1628, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, Maekawa K, Yamakawa T, Imanishi Y, Inaba M, Nishizawa Y: Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis 49: 417–425, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Adragão T, Pires A, Birne R, Curto JD, Lucas C, Gonçalves M, Negrão AP: A plain X-ray vascular calcification score is associated with arterial stiffness and mortality in dialysis patients. Nephrol Dial Transplant 24: 997–1002, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, Strandness DE, Jr., Taylor LM: Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation 94: 3026–3049, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, Vitamin D Standardization Program (VDSP) : Vitamin D status as an international issue: National surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 243: 32–40, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Carter GD, Carter R, Jones J, Berry J: How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem 50: 2195–2197, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 25.O’Hare AM, Bertenthal D, Covinsky KE, Landefeld CS, Sen S, Mehta K, Steinman MA, Borzecki A, Walter LC: Mortality risk stratification in chronic kidney disease: One size for all ages? J Am Soc Nephrol 17: 846–853, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Peduzzi P, Concato J, Feinstein AR, Holford TR: Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48: 1503–1510, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Coll B, Betriu A, Martínez-Alonso M, Amoedo ML, Arcidiacono MV, Borras M, Valdivielso JM, Fernández E: Large artery calcification on dialysis patients is located in the intima and related to atherosclerosis. Clin J Am Soc Nephrol 6: 303–310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.London GM: Cardiovascular calcifications in uremic patients: Clinical impact on cardiovascular function. J Am Soc Nephrol 14[Suppl 4]: S305–S309, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hong D, Wu S, Pu L, Wang F, Wang J, Wang Z, Gao H, Zhang Y, Deng F, Li G, He Q, Wang L: Abdominal aortic calcification is not superior over other vascular calcification in predicting mortality in hemodialysis patients: A retrospective observational study. BMC Nephrol 14: 120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K: Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int 73: 1296–1302, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Khan AM, Chirinos JA, Litt H, Yang W, Rosas SE: FGF-23 and the progression of coronary arterial calcification in patients new to dialysis. Clin J Am Soc Nephrol 7: 2017–2022, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCabe KM, Booth SL, Fu X, Shobeiri N, Pang JJ, Adams MA, Holden RM: Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int 83: 835–844, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Chatrou ML, Winckers K, Hackeng TM, Reutelingsperger CP, Schurgers LJ: Vascular calcification: The price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev 26: 155–166, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Schurgers LJ: Vitamin K: Key vitamin in controlling vascular calcification in chronic kidney disease. Kidney Int 83: 782–784, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.