Abstract
Background and objectives
Congenital anomalies of the kidney and urinary tract and genetic disorders cause most cases of CKD in children. This study evaluated the relationships between baseline proteinuria and BP and longitudinal changes in GFR in children with these nonglomerular causes of CKD.
Design, setting, participants, & measurements
Urine protein-to-creatinine ratio, casual systolic and diastolic BP (normalized for age, sex, and height), and GFR decline were assessed in the prospective CKD in Children cohort study.
Results
A total of 522 children, median age 10 years (interquartile range, 7, 14 years) with nonglomerular CKD were followed for a median of 4.4 years. The mean baseline GFR in the cohort was 52 ml/min per 1.73 m2 (95% confidence interval [95% CI], 50 to 54) and declined 1.3 ml/min per 1.73 m2 per year on average (95%CI, 1.6 to 1.1). A 2-fold higher baseline urine protein-to-creatinine ratio was associated with an accelerated GFR decline of 0.3 ml/min per 1.73 m2 per year (95% CI, 0.4 to 0.1). A 1-unit higher baseline systolic BP z-score was associated with an additional GFR decline of 0.4 ml/min per 1.73 m2 per year (95% CI, 0.7 to 0.1). Among normotensive children, larger GFR declines were associated with larger baseline urine protein-to-creatinine ratios; eGFR declines of 0.8 and 1.8 ml/min per 1.73 m2 per year were associated with urine protein-to-creatinine ratio <0.5 and ≥0.5 mg/mg, respectively. Among children with elevated BP, average GFR declines were evident but were not larger in children with higher levels of proteinuria.
Conclusions
Baseline proteinuria and systolic BP levels are independently associated with CKD progression in children with nonglomerular CKD.
Keywords: chronic kidney disease, proteinuria, hypertension, renal progression
Introduction
Proteinuria, an early marker of kidney damage, is an important independent risk factor for kidney disease progression (1–4). Many adult and a few pediatric studies have demonstrated that higher levels of proteinuria are an independent risk factor for progressive decline in kidney function (5–8). Because nonglomerular (NG) causes, including congenital anomalies of the kidney and urinary tract (CAKUT) and genetic disorders, account for most cases of CKD in children (9,10), a better understanding of the contribution of proteinuria to progression in NG CKD might yield new insights on the treatment and prognosis of kidney disease in this population.
Elevated BP is also common in children with CKD. Data from the CKD in Children (CKiD) study have demonstrated that 54% of children in the cohort had hypertension at study entry (11). Hypertension is an independent predictor of renal disease progression in adult patients (12–14). In children with both glomerular and NG CKD, several studies have reported an association between BP and more rapid progression of CKD (15,16).
Given the importance of hypertension and proteinuria as risk factors for CKD progression, we analyzed longitudinal data from the CKiD cohort study to investigate the joint contribution of baseline BP and proteinuria levels on GFR decline in children with NG CKD.
Materials and Methods
Study Population
The prospective, observational CKiD study cohort is composed of children with mild to moderate CKD, recruited from 48 North American pediatric nephrology centers (17). An observational study monitoring board appointed by the National Institutes of Health and institutional review boards at each participating center approved the study design and conduct. Parental consent and participant assent/consent were obtained according to local requirements.
Details of the study design have been previously published (17). Briefly, the initial CKiD enrollment (2005–2009) involved 586 children age 1–16 years with a Schwartz-eGFR (18) of 30–90 ml/min per 1.73 m2. Beginning in 2011, an additional 305 children age 1–16 years with an eGFR of 45–90 ml/min per 1.73 m2 were enrolled. For the current analysis, we included 522 children with NG diagnoses: 411 from the first enrollment period and 111 from the second enrollment period.
Measurements and Data Collection
Children enrolled in the CKiD study attend annual clinical visits. Serum and urine specimens collected at these visits are processed at the CKiD central biochemistry laboratory (University of Rochester, Rochester, NY). Urinary protein and creatinine measurements were obtained from first-morning urine samples collected for the study visit either at home by the participant or upon arrival at the clinical center. Urine total protein and urine creatinine concentrations were measured using the Bayer Advia 2400 analyzer (19). Proteinuria was defined by the urine protein-to-creatinine ratio (Up/c) as normal/minimal (Up/c < 0.5 mg/mg) or elevated (Up/c ≥ 0.5 mg/mg).
GFR was measured by the plasma disappearance of iohexol (Ominipaque; GE Healthcare, Princeton, NJ) at baseline, at the first annual follow-up visit, and every other year thereafter. Details of the GFR assessment methods have been published previously (20). At study visits when iohexol GFR was not measured, GFR was estimated as a function of sex, height, serum creatinine, cystatin C, and/or BUN from CKiD-developed formulae (21). Going forward, the term "GFR" will be used to refer to the combined iohexol measured and eGFR measurements for any individual over follow-up.
At each study visit, casual BPs were determined as the average of three measurements obtained by auscultation using an aneroid sphygmomanometer. Details of the standardized BP measurement technique have been published (11). Casual systolic BP (SBP) and diastolic BP (DBP) measurements were standardized (using both z-scores and percentiles) for age, sex, and height according to the "National High Blood Pressure Education Program Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children And Adolescents" (22). Elevated BP was defined as casual SBP or DBP≥90th percentile for age, sex, and height at the baseline visit.
Nonlaboratory data were obtained at the baseline visit using standardized forms. Variables collected and analyzed in the current report include age, sex, race, ethnicity, height, weight, clinical BP, reported intake of renin-angiotensin-system (RAS) antagonists during the past 30 days, primary CKD diagnosis, and age at which the parent or child was first aware of the child’s CKD. RAS antagonists were classified as angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs).
Participants’ CKD diagnoses were adjudicated by the members of the CKiD Steering Committee and categorized as glomerular or NG. NG diagnoses included aplastic/hypoplastic/dysplastic kidneys, cystinosis, medullary cystic disease/juvenile nephronophthisis, obstructive uropathy, oxalosis, autosomal dominant and recessive polycystic kidney disease, pyelonephritis/interstitial nephritis, reflux nephropathy, renal infarct, syndrome of agenesis of abdominal musculature, and Wilms tumor. The duration of a participant’s CKD was determined as the time between baseline study visit and initial awareness of CKD diagnosis. Of primary interest was the relationship between baseline proteinuria and BP and subsequent change in GFR in children with NG CKD. As such, analysis was restricted to this group of children with available Up/c and BP at baseline. All visits with missing GFR determinations were dropped from the analysis.
Statistical Analyses
For analysis purposes, Up/c measurements were log-transformed; casual BP measurements were analyzed as age-, sex-, and height-standardized scores (z-scores). We used linear mixed models—univariate and multivariate with random intercept and slope—to model GFR as a function of time since baseline, baseline Up/c, and baseline BP. Interaction terms between time from baseline and baseline Up/c, SBP z-score, and DBP z-score, respectively, were included to estimate the association between these exposures and change in GFR over follow-up. Participant sex, race, baseline age, current body mass index (BMI) z-score, and current ACEI/ARB use were included as main effect covariates.
In analyses examining the association of proteinuria to GFR change as stratified by BP status, baseline Up/c was categorized as normal/minimal (Up/c<0.5 mg/mg) or elevated (Up/c≥0.5 mg/mg), and baseline casual BP was categorized as normotensive (SBP and DBP< 90th percentile) or elevated (SBP or DBP≥90th percentile). Change in GFR was estimated for each proteinuria-BP category (four total) using a multivariate linear mixed model that included an interaction between proteinuria and BP and adjusted for participant sex, race, baseline age, current BMI z-score, and current ACEI/ARB use.
All analyses were performed using SAS statistical software, version 9.3 (SAS Institute, Cary, NC).
Results
Of the 891 children enrolled in the CKiD cohort at the time of analysis, 689 had baseline Up/c and BP available; 522 (76%) had an NG cause of CKD. The median values of the baseline GFR and Up/c were 48 ml/min per 1.73 m2 (interquartile range [IQR], 36, 64) and 0.29 (IQR, 0.12, 0.82), respectively (Table 1). Median follow-up time from baseline was 4.4 years (IQR, 1.7, 6.0). Sixty-six percent (n=342) of participants had four or more longitudinal GFR measurements. The most common CKD diagnoses were related to CAKUT (69%): specifically, obstructive uropathy (25%; n=131), aplastic/hypoplastic/dysplastic kidneys (21%; n=112), reflux nephropathy (19%; n=100), and other CAKUT (4%; n=19).
Table 1.
Baseline clinical and demographic characteristics for 522 nonglomerular CKD Children
| Baseline Characteristic | Value |
|---|---|
| Age (yr) | 10 (7, 14) |
| Male, % (n) | 65 (340) |
| African American race, % (n) | 19 (101) |
| Duration of CKD (yr) | 10 (6, 13) |
| GFR (ml/min per 1.73 m2) | 48 (36, 64) |
| Urine protein-to-creatinine ratio (mg/mg) | 0.29 (0.12, 0.82) |
| Casual BP (percentile) | |
| Systolic | 66 (39, 86) |
| Diastolic | 70 (47, 88) |
| Renin-angiotensin-system antagonist use, % (n) | 45 (237) |
| Other antihypertensive therapy | 9 (47) |
| Duration of follow-up (yr) | 4.4 (1.7, 6.0) |
Values are presented as median (interquartile range) for continuous variables, unless otherwise indicated, and percentage (frequency) for categorical variables.
Relationship between Baseline Proteinuria, Casual BP, and GFR Decline
Overall, the mean baseline GFR was 52 ml/min per 1.73 m2 (95% confidence interval [95% CI], 50 to 54) and the mean GFR decline was 1.3 ml/min per 1.73 m2 per year (95% CI, 1.6 to 1.1) (Table 2). Without adjustment for other factors, each 2-fold higher level of baseline Up/c was associated with a more rapid GFR decline of 0.3 ml/min per 1.73 m2 per year (95% CI, 0.5 to 0.1) (Table 2). Thus, among children with NG disease, baseline Up/c levels of 0.25, 0.5, 1.0, and 2.0 were associated with average GFR declines of 1.3 (95% CI, 1.0 to 1.6), 1.6 (95% CI, 1.3 to 1.9), 1.8 (95% CI, 1.5 to 2.2), and 2.1 (95% CI, 1.6 to 2.6) ml/min per 1.73 m2 per year, respectively.
Table 2.
Results of univariable and multivariable mixed-effects linear models of GFR on baseline exposures of interest (n=522 children with nonglomerular CKD; 2101 person-visits)
| Parameter | Estimate (95% CI) | ||||
|---|---|---|---|---|---|
| Univariate | Baseline Up/c Only | Baseline SBP z-Score Only | Baseline DBP z-Score Only | Multivariablea | |
| Expected baseline GFR (ml/min per 1.73 m2) | |||||
| Interceptb | 52.1 (50.4 to 53.8) | 54.0 (52.5 to 55.5) | 52.0 (50.2 to 53.8) | 51.8 (49.8 to 53.9) | 53.3 (50.7 to 56.0) |
| Per 2-fold larger baseline Up/c | −5.5 (−6.3 to −4.7) | −5.5 (−6.3 to −4.7) | |||
| Per 1-unit larger baseline SBP z-score | 0.3 (−1.3 to 1.8) | −0.2 (−1.8 to 1.4) | |||
| Per 1-unit larger baseline DBP z-score | 0.5 (−1.4 to 2.3) | 0.8 (−1.1 to 2.8) | |||
| Expected GFR change per yr (ml/min per 1.73 m2 per yr) | |||||
| Reference groupc | −1.3 (−1.6 to −1.1) | −1.3 (−1.6 to −1.0) | −1.2 (−1.5 to −0.9) | −1.2 (−1.5 to −0.8) | −1.1 (−1.5 to −0.8) |
| Per 2-fold larger baseline Up/c | −0.3 (−0.5 to −0.1) | −0.3 (−0.4 to −0.1) | |||
| Per 1-unit larger baseline SBP z-score | −0.4 (−0.7 to −0.1) | −0.4 (−0.7 to −0.1) | |||
| Per 1-unit larger baseline DBP z-score | −0.3 (−0.6 to 0.0) | 0.0 (−0.3 to 0.4) | |||
CI, confidence interval; Up/c, urinary protein-to-creatinine ratio; SBP, systolic BP; DBP, diastolic BP.
Multivariable model adjusted for variables shown in table, as well as sex, race, baseline age, current body mass index z-score, and current angiotensin-enzyme inhibitor/angiotensin-receptor blocker use.
For models including Up/c, this is the expected baseline GFR for children with Up/c of 0.25 mg/mg; for models with SBP z-score and DBP z-score, this is the expected baseline GFR for children with SBP and DBP, respectively, at the 50th percentile for age, sex, and height.
For models including Up/c, this is the expected 1-year change in GFR for children with Up/c of 0.25 mg/mg; for models with SBP z-score and DBP z-score, this is the expected 1-year change in GFR for children with SBP and DBP, respectively, at the 50th percentile for age, sex, and height.
Higher baseline standardized SBP measurements (z-scores) were also associated with sharper declines in GFR over follow-up in the univariate analysis. For every 1-unit higher SBP z-score, GFR declined faster by 0.4 ml/min per 1.73 m2 per year (95% CI, 0.7 to 0.1). For children at the 50th percentile for SBP, GFR declined on average by 1.2 ml/min per 1.73 m2 per year (95% CI, 0.9 to 1.5); children at the 90th percentile had average GFR declines of 1.7 ml/min per 1.73 m2 per year (95% CI, 1.3 to 2.0). Higher baseline standardized diastolic BP measurements were also associated with sharper declines in GFR over follow-up, although this relationship was not statistically significant.
After adjustment for SBP z-score, DBP z-score, Up/c, sex, race, baseline age, current BMI z-score, and current use of an RAS antagonist (ACEI or ARB), baseline Up/c and SBP z-score remained significantly associated with GFR decline over follow-up. On average, a 2-fold higher level of baseline Up/c was associated with a 0.3–ml/min per 1.73 m2 per year (95% CI, 0.4 to 0.1) faster rate of GFR decline. Each 1-unit higher level in baseline SBP z-score was associated with a 0.4–ml/min per 1.73 m2 per year (95% CI, 0.7 to 0.1) faster rate of GFR decline. Baseline DBP z-score was not associated with subsequent GFR change.
Association between Baseline Proteinuria and GFR Decline by BP Status
We sought to determine whether the association between higher baseline Up/c category and faster rate of GFR decline differed according to baseline BP status: normotensive or elevated BP. Among normotensive children, the presence of elevated baseline Up/c was associated with expected GFR declines >2.2 times as large as those in children with normal protein excretion (P=0.01 for difference) (Table 3). In contrast, we did not detect an association between Up/c level and GFR decline among children with elevated BP: Children with normal and elevated Up/c levels had similar GFR declines of 1.9 (95% CI, 2.5 to 1.2) and 1.7 (95% CI, 2.4 to 1.0) ml/min per 1.73 m2 per year, respectively (P=0.77 for difference).
Table 3.
Estimated declines in GFR by combined baseline urinary protein-to-creatinine ratio and BP status
| Baseline Up/c | Normotensive (BP < 90th Percentile) (n=358) | Elevated BP (BP ≥90th Percentile) (n=164) | ||||
|---|---|---|---|---|---|---|
| Patients (n) | Median (IQR) Baseline GFR (ml/min per 1.73 m2) | Mean Change in GFR (ml/min per 1.73 m2 per yr) (95% CI)a | Patients (n) | Median (IQR) Baseline GFR (ml/min per 1.73 m2) | Mean Change in GFR (ml/min per 1.73 m2 per yr) (95% CI)a | |
| <0.5 | 229 | 53 (42, 69) | −0.8 (−1.2 to −0.4) | 94 | 58 (44, 71) | −1.9 (−2.5 to −1.2) |
| ≥0.5 | 129 | 39 (29, 51) | −1.8 (−2.4 to −1.2) | 70 | 40 (30, 49) | −1.7 (−2.4 to −1.0) |
| P value for difference | <0.001b | 0.01a | <0.001b | 0.77a | ||
Test for heterogeneity of association between baselineUp/c and GFR change by BP status: P=0.08.95% CI.
Estimates of change in GFR and reported P values derived from multivariable linear mixed-effects model adjusting for baseline Up/c, baseline BP status, baseline age, sex, race, current BMI z-score, and current angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker use.
Derived from Wilcoxon rank-sum test.
On average, participants with both normal Up/c and BP exhibited lower rates of GFR decline (0.8 versus 1.9, 1.8, and 1.7 ml/min per 1.73 m2 per year) (Table 3) compared with children with elevated Up/c, elevated BP, or both. The 70 children with both elevated Up/c and elevated BP had GFR declines similar to those of children who possessed only one of these two risk factors. Only control of both Up/c and BP levels appeared to be associated with noticeably slower progression (0.8 versus 1.9, 1.8, and 1.7 ml/min per 1.73 m2 per year). These results suggest that elevated Up/c and elevated BP do not express additive effects on GFR decline, although the test of heterogeneity of effect was borderline nonsignificant (P=0.08).
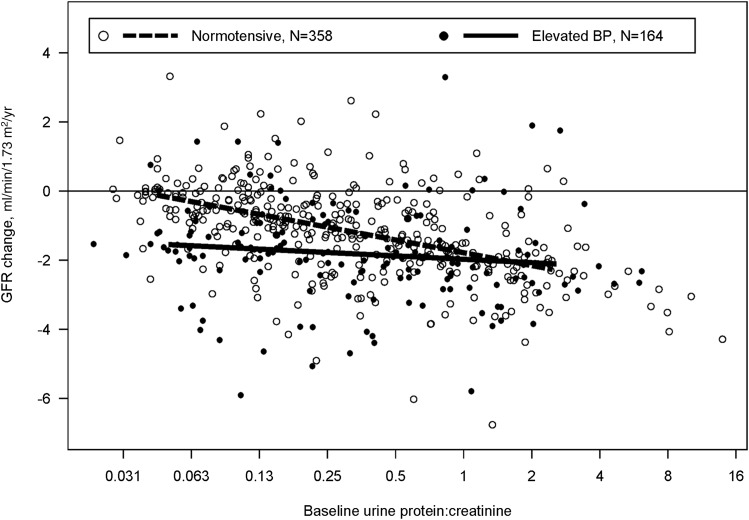
The association between proteinuria and GFR decline in children with normal and elevated BP, respectively, is also displayed in Figure 1, which presents proteinuria as a continuous variable. Normotensive children with a baseline Up/c of 0.25 (overall median, 0.29) had an expected GFR decline of 1.0 ml/min per 1.73 m2 per year (95% CI, 0.7 to 1.4). For every 2-fold higher baseline Up/c, expected GFR decline was 0.4 ml/min per 1.73 m2 per year greater (95% CI, 0.2 to 0.6; P<0.001). Among children with elevated BP and a baseline Up/c of 0.25, expected GFR decline was 1.8 ml/min per 1.73 m2 per year (95% CI, 1.3 to 2.3). In children with elevated BP, we could not detect an association between higher levels of baseline proteinuria and larger GFR declines over follow-up (P=0.53).
Figure 1.
Changes in GFR (ml/min per 1.73 m2 per year) by baseline urine protein-to-creatinine ratio (Up/c) and casual BP status for 522 children with nonglomerular CKD. Individual empirical Bayes estimates are shown as dots: black for children with elevated BP (>90th percentile for age, sex, and height) and white for children with normotensive BP (≤90th percentile). BP status–specific expected changes derived from linear mixed-effects models of GFR on baseline Up/c are shown as a solid line (elevated BP children) and a dashed line (normotensive BP children).
Discussion
Our analysis of data from children with NG causes of CKD in the CKiD cohort demonstrates that both elevated proteinuria and BP are independently associated with faster CKD progression. We show that children with higher levels of proteinuria experience more rapid annual declines in GFR on average compared with those with lower levels of proteinuria. Children with elevated BP also experience more rapid declines in GFR compared with normotensive patients. In the joint model considering the association between proteinuria and GFR decline stratified by BP status, we showed that CKD progressed faster in the presence of elevated Up/c, elevated BP, or both. We could not detect that having both elevated Up/c and elevated BP was associated with a more rapid GFR decline compared with one risk factor alone. As such, children with both risk factors had expected GFR declines similar to those of children with only one of the two risk factors; only children with both nonelevated Up/c and BP levels exhibited noticeably slower GFR declines.
Our analysis was restricted to children with NG CKD diagnoses, which make up the majority of cases in pediatric patients with CKD. Children with glomerular causes of CKD have significantly higher levels of proteinuria (23) and more rapid progression; our findings confirm that proteinuria is also an important marker of CKD progression in NG CKD. In 2004, the ItalKid project reported that higher baseline proteinuria (Up/c>0.9 mg/mg) correlated with faster decline in kidney function in children with renal hypodysplasia (n=225) (6). In a retrospective and cross-sectional analysis of 92 patients (all but six of whom had NG causes of CKD), Litwin found that proteinuria after 3 years of observation was associated with children described as having progressive CKD (24). In another retrospective study of 176 children with CKD secondary to renal dysplasia and CAKUT, González Celedón et al. reported that albuminuria correlates with a faster rate of kidney function decline (7). Sanna-Cherchi et al. also observed that proteinuria in children with CAKUT is associated with a higher risk of progression to ESRD (25). Our findings expand on these previously published data and highlight the importance of proteinuria as a clinical marker associated with disease progression, even in children with NG CKD.
Consistent with prior reports on hypertension as a risk factor for CKD progression, we provide evidence that elevated BP is independently associated with accelerated CKD progression in children with NG causes of CKD. In 1997, Wingen et al. noted that SBP was an independent risk factor (apart from proteinuria) for CKD progression in children (15). González Celedón et al. found that hypertension contributed to faster deterioration of kidney function in children with CKD secondary to renal dysplasia and CAKUT (7). Moreover, the Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) trial demonstrated that intensified BP control effectively delayed the progression of renal disease among a population of children with mostly renal hypoplasia or dysplasia (16). We not only report that higher levels of SBP are associated with faster rates of decline of GFR but also demonstrate varying contributions of proteinuria to GFR decline depending on baseline BP. Among children who are normotensive, higher levels proteinuria were associated with more rapid decline in GFR. Among children with elevated baseline BP, more rapid decline was seen even among children with normal levels of proteinuria.
We acknowledge several limitations of this prospective observational study. First, the diagnoses are heterogeneous. Diagnoses provided to the study were by report of the caregiver on standardized forms. As such, there may be misclassification by a specific diagnosis or the diagnosis may be unknown to the caregiver. To minimize the impact of this problem, the CKiD steering committee conducts detailed review of participants’ diagnoses and seeks clarification from the site principal investigator when needed. Despite this effort some misclassification may remain but is likely to be minimal. Additionally, we classified proteinuria and BP based on measurements taken at one time point, and the measurements were taken 1 year apart. Moreover, although all of our patients had NG diagnoses, we did not assess for albuminuria or perform urine protein electrophoresis to determine the origin of proteinuria, or whether patients had evolved from having tubular proteinuria to glomerular proteinuria.
Despite these limitations, this study has substantial strengths, including its large sample size and long-term follow-up of >4 years. All laboratory measures were conducted centrally and BP was obtained by a standardized protocol at all sites. The prospective nature of the relationship of the baseline variables on repeated assessments of GFR provides data for robust estimates of the association between proteinuria and BP on the rate of GFR decline over time. Although more studies would be needed to determine whether intervention on these risk factors slows GFR decline, these data suggest that proteinuria and elevated BP are important risk factors in the clinical care of children with NG CKD.
In conclusion, higher baseline proteinuria and SBP are associated with faster declines of GFR in children with NG causes of CKD. Considering the contributions of both proteinuria and BP, we showed that among normotensive children CKD progression was associated with level of proteinuria; however, among children with elevated BP, there was a clinically meaningful decline in GFR regardless of the proteinuria level.
Disclosures
None.
Supplementary Material
Acknowledgments
Data in this manuscript were collected by the CKD in children prospective cohort study (CKiD) with clinical coordinating centers (principal investigators) at Children’s Mercy Hospital and the University of Missouri–Kansas City (Bradley Warady) and Children’s Hospital of Philadelphia (Susan Furth), Central Biochemistry Laboratory (George Schwartz) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz) at the Johns Hopkins Bloomberg School of Public Health.
The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute (U01-DK66143, U01-DK66174, U01-DK082194, U01-DK66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07480714/-/DCSupplemental.
References
- 1.Schaefer B, Wühl E: Educational paper: Progression in chronic kidney disease and prevention strategies. Eur J Pediatr 171: 1579–1588, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Ishikura K, Uemura O, Hamasaki Y, Ito S, Wada N, Hattori M, Ohashi Y, Tanaka R, Nakanishi K, Kaneko T, Honda M, Pediatric CKD Study Group in Japan. Committee of Measures for Pediatric CKD of Japanese Society of Pediatric Nephrology : Progression to end-stage kidney disease in Japanese children with chronic kidney disease: results of a nationwide prospective cohort study. Nephrol Dial Transplant 29: 878–884, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, de Jong PE, de Zeeuw D, Shahinfar S, Ruggenenti P, Remuzzi G, Levey AS, AIPRD Study Group. Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease : Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 60: 1131–1140, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Taal MW, Brenner BM: Defining renal risk. Curr Opin Nephrol Hypertens 16: 554–556, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Wühl E, Mehls O, Schaefer F, ESCAPE Trial Group : Antihypertensive and antiproteinuric efficacy of ramipril in children with chronic renal failure. Kidney Int 66: 768–776, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ardissino G, Testa S, Daccò V, Viganò S, Taioli E, Claris-Appiani A, Procaccio M, Avolio L, Ciofani A, Dello Strologo L, Montini G, Ital Kid Project : Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatr Nephrol 19: 172–177, 2004 [DOI] [PubMed] [Google Scholar]
- 7.González Celedón C, Bitsori M, Tullus K: Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol 22: 1014–1020, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL: Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 123: 754–762, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Seikaly MG, Ho PL, Emmett L, Fine RN, Tejani A: Chronic renal insufficiency in children: The 2001 Annual Report of the NAPRTCS. Pediatr Nephrol 18: 796–804, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ: Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27: 363–373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA, Chronic Kidney Disease in Children Study Group : Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension 52: 631–637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iseki K, Ikemiya Y, Iseki C, Takishita S: Proteinuria and the risk of developing end-stage renal disease. Kidney Int 63: 1468–1474, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Locatelli F, Marcelli D, Comelli M, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A, Northern Italian Cooperative Study Group : Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Nephrol Dial Transplant 11: 461–467, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J: Blood pressure and end-stage renal disease in men. N Engl J Med 334: 13–18, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Wingen AM, Fabian-Bach C, Schaefer F, Mehls O, European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood : Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. Lancet 349: 1117–1123, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F, ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Wong CS, Pierce CB, Cole SR, Warady BA, Mak RH, Benador NM, Kaskel F, Furth SL, Schwartz GJ, CKiD Investigators : Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol 4: 812–819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A: Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 23.Wong H, Mylrea K, Feber J, Drukker A, Filler G: Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int 70: 585–590, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Litwin M: Risk factors for renal failure in children with non-glomerular nephropathies. Pediatr Nephrol 19: 178–186, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, Izzi C, Scolari F, Mattioli G, Allegri L, Ghiggeri GM: Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int 76: 528–533, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.