Abstract
Background and objectives
Although nonesterified fatty acids (NEFAs) are essential as energy substrate for the myocardium, an excess of circulating NEFAs can be harmful. This study aimed to assess plausible relationships between serum NEFA and mortality due to cardiovascular disease (CVD) in individuals with CKD.
Design, setting, participants, & measurements
This was a prospective cohort study from the third examination cycle of the Uppsala Longitudinal Study of Adult Men, a population-based survey of 1221 elderly men aged 70–71 years residing in Uppsala, Sweden. Data collection took place during 1991–1995. All participants had measures of kidney function; this study investigated 623 (51.7%) of these patients with manifest CKD (defined as either eGFR<60 ml/min per 1.73 m2 or urine albumin excretion rate ≥20 µg/min). Follow-up for mortality was done from examination date until death or December 31, 2007. After a median follow-up of 14 years (interquartile range, 8–16.8), associations of NEFAs with mortality (related to all causes, CVD, ischemic heart disease [IHD], or acute myocardial infarction) were ascertained.
Results
The median serum NEFA was 14.1 mg/dl (interquartile range, 11.3–17.8). No association was found with measures of kidney function. Diabetes and serum triglycerides were the only multivariate correlates of NEFA. During follow-up, 453 participants died, of which 209 deaths were due to CVD, including 88 IHD deaths, with 41 attributed to acute myocardial infarction (AMI). In fully adjusted covariates, serum NEFA was an independent risk factor for all-cause mortality (hazard ratio [HR] per log2 increase, 1.22; 95% confidence interval [95% CI], 1.00 to 1.48) and CVD-related death (HR, 1.51; 95% CI, 1.15 to 1.99), including both IHD (HR, 1.51; 95% CI, 1.00 to 2.32) and AMI mortality (HR, 2.08; 95% CI, 1.09 to 3.98).
Conclusions
Elevated serum NEFA associated with CVD mortality, and particularly with mortality due to AMI, in a homogeneous population of older men with moderate CKD.
Keywords: nonesterified fatty acid, cardiovascular disease, mortality, CKD
Introduction
Nonesterified fatty acids (NEFAs), also known as free fatty acids, are released principally from the adipose tissue through hydrolysis of triglycerides (1). Although NEFAs are essential as energy substrate for the myocardium, an elevated concentration of NEFAs with subsequent increased β-oxidation in myocardial metabolism can be harmful for heart tissues (2) and heart function (3). There is considerable mechanistic evidence to support that NEFAs may be an important link between obesity, insulin resistance, type 2 diabetes mellitus, and the risk of ischemic heart disease (IHD) (4). Whereas many large epidemiologic studies in the general population or in populations with manifest cardiovascular disease (CVD) have associated NEFAs with the incidence of cardiovascular events (including myocardial infarction and sudden cardiac death) as well as with mortality (5–10), some have not (11–13).
CKD is an independent risk factor for CVD (14), and dyslipidemia often accompanies progressive kidney dysfunction (15). Currently, clinical correlates and possible outcome associations of NEFA in patients with CKD are unknown. In this study, we hypothesized that NEFA accumulation is associated with increased CVD risk and we addressed this hypothesis in a relatively large population of elderly men with manifest CKD.
Materials and Methods
Study Population
This study was performed in the Uppsala Longitudinal Study of Adult Men (ULSAM) (http://www2.pubcare.uu.se/ULSAM/). The present analyses are based on the third ULSAM examination cycle. During this examination, participants were aged 70–71 years (examinations performed during 1991–1995; n=1221). A total of 632 individuals were identified as having CKD on the basis of cystatin C eGFR<60 ml/min per 1.73 m2 (n=537) or urine albumin excretion rate (UAER) ≥20 µg/min (16) (n=86), or both of these conditions (n=125). NEFA measurements were available in 623 (98.6%) of those patients included in the analysis. The Ethics Committee at Uppsala University approved the study and all participants gave informed consent.
Demographics and Comorbidities
Investigations were performed under standardized conditions (17). Waist circumference was measured midway between the lowest rib and the iliac crest in the supine position. Smoking status was defined as current smoking versus nonsmoking. Regular physical activity was defined as the reporting of regular or athletic leisure time exercise habits according to four physical activity categories (sedentary, moderate, regular, and athletic) (18). Previous CVD was defined as a history of any CVD as recorded in the Swedish Hospital Discharge Registry (International Classification of Diseases [ICD]-8 codes 390–458 or ICD-9 codes 390–459). BP was measured in duplicate in the right arm with the participant in the supine position at rest. Hypertension was defined as BP≥140/90 mmHg or the use of antihypertensive medications. Diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dl (7.0 mmol/L), 2-hour postload glucose levels ≥200 mg/dl (11.1 mmol/L), or use of diabetes medication (insulin or oral). The use of lipid-lowering medication (statins) was collected with a medical questionnaire according to the, list of pharmaceutical specialties available in Sweden at that time (FASS 1992/1993). Information on dietary habits was collected by a diet record by the Swedish National Food Administration during 7 consecutive days. The daily intake of energy was calculated and dietary fat intake was adjusted for total energy intake by the residual method (19).
Laboratory Measurements
Venous blood samples were drawn in the morning after an overnight fast and stored at −70°C until required for analyses. Serum NEFA was assessed in single measurements by an enzymatic colorimetric method with a NEFA C kit (Wako Chemicals, Neuss, Germany). The coefficient of variation of this assay is reported to be 5% (12). Serum cystatin C (N Latex Cystatin C; Dade Behring) was used to estimate GFR by the formula y=77.24×x−1.2623, which was shown to be closely correlated with iohexol clearance in our previous study (20). This equation was used in our primary analysis. Furthermore, serum creatinine was measured using Jaffe’s reaction (Reagence; Boehringer Mannheim) (Hitachi Instrument 717; Hitachi). eGFR was also calculated by the CKD Epidemiology Collaboration (CKD-EPI) creatinine and cystatin C plus creatinine equations (CKD-EPIcreatinine and CKD-EPIcystatin C+creatinine, respectively) (21), which were used in sensitivity analyses. Serum lipids and lipoproteins were detected as described previously (22). High-sensitivity C-reactive protein (CRP) was measured by a latex-enhanced reagent (Dade Behring, Deerfield, IL) using a Behring BN ProSpec analyzer (Dade Behring). Fasting plasma glucose (Gluc-DH; Merck, Darmstadt, Germany) and insulin (Actrapid Human; Novo, Copenhagen, Denmark) were measured by the glucose dehydrogenase method and enzymatic-immunologic assay, respectively. The euglycemic hyperinsulinemic clamp technique (23) was used to calculate the insulin sensitivity index (M/I ratio, mg/kg per minute per mU/L of insulin×100), which is a measure of the tissue sensitivity to insulin expressed per unit of insulin and was calculated by dividing the amount of glucose metabolized (M) by the mean insulin (I) concentration during the same period of the clamp. UAER was calculated on the amount of albumin in the urine collected during the night. The participants were instructed to void immediately before going to bed and to record the time. All samples during the night and the first sample of urine after rising were collected and used for the analysis with a radioimmunoassay kit (Albumin RIA 100; Pharmacia, Uppsala, Sweden).
Follow-Up and Mortality
Follow-up for mortality was done from examination date until death or December 31, 2007, with no loss to follow-up. The Swedish National Registry recording for date and cause of death was used to define end points. The primary outcome considered was death attributed to CVD (ICD-9 codes 390–459 or ICD-10 codes I00–I99). We included death due to IHD (ICD-9 codes 410–414 or ICD-10 codes I20–I25) or acute myocardial infarction (AMI) (ICD-9 code 410, ICD-10 code I21) as secondary outcomes. Other CVD causes were lumped together, including cerebrovascular events (ICD-9 codes 430–438 or ICD-10 codes I60–I69), acute rheumatic fever (ICD-9 codes 390–392 or ICD-10 codes I00–I02), chronic rheumatic heart diseases (ICD-9 codes 393–398, ICD-10 codes I05–I09), hypertensive diseases (ICD-9 codes 401–405, ICD-10 codes I10–I15), pulmonary circulation diseases (ICD-9 codes 415–417, ICD-10 codes I26–I28), and other forms of heart diseases (ICD-9 codes 420–429, ICD-10 codes I30–I32).
Statistical Analyses
Values are expressed as means±SD for normally distributed continuous variables, medians (interquartile ranges [IQRs]) for skewed variables, or percentages of the total for categorical variables. Log2-transformed means were used for skewed data such as NEFA, UAER, and eGFR. After verification for normal distribution in all data sets, effects were weighted by group where appropriate. Study participants were divided into three groups according to tertiles of serum NEFA. The Jonckheere–Terpstra test was used to assess linear trends across these groups (24), and P values for trend were reported. Spearman’s rank correlation analysis was used to determine relationships between NEFA and variables of interest. Multivariate regression models were fitted to study associations between NEFA and variables associated with it in univariate analysis. In addition, physical activity and dietary fat intake were forced into the models based on preceding evidence regarding determinants of NEFA. Data are expressed as adjusted coefficients of determination (R2) and regression coefficients.
Associations of NEFA (per log2-unit of NEFA increase) with all-cause and CVD mortality were investigated with Cox proportional-hazards regression in the whole cohort utilizing three sets of models (a crude model and models 1 and 2). Model 1 was adjusted for age and lifestyle parameters, including body mass index (BMI), smoking status, and physical activity. Model 2 was further adjusted for eGFR, UAER, and comorbidities, including prevalence of CVD, diabetes mellitus, hypertension and hyperlipidemia (plasma triglycerides, cholesterol and lipid medication), and CRP. Restricted cubic spline graphs were used to graphically evaluate systematic relationships between serum NEFA and CVD mortality. In this analysis, we included the fully adjusted model 2. Data are presented as hazard ratios and 95% confidence intervals. Proportional hazards assumptions were confirmed using Schoenfeld’s test (25). Several sensitivity analyses were performed to test the robustness of our findings. First, multivariable analyses were repeated using the CKD-EPIcreatinine and combined CKD-EPIcystatin C+creatinine equations as covariates. Second, and given the potential correlation between diabetes and NEFA, we repeated analyses excluding patients with diabetes (n=104). All statistical analyses were performed using STATA statistical software (version 12.0; Stata Corporation, College Station, TX). All tests were two tailed and a P value <0.05 was considered significant. Because correction for multiple comparisons was not performed, our results need to be considered as descriptive.
Results
Baseline Characteristics
The median serum NEFA was 14.1 mg/dl (IQR, 11.3–17.8; range, 3.39–61.9), eGFR was 53.4 ml/min per 1.73 m2 (IQR, 47.1–57.7; range, 10.5–109), and UAER was 6.9 µg/min (IQR, 3.6–27.1; range, 0.6–1346). The numbers of individuals with CKD stages 1–5 were as follows: six (1%), 80 (12.8%), 523 (84%), 13 (2%), and one (0.2%), respectively. Clinical and biochemical characteristics of included participants are shown in Table 1 as stratified by tertiles of NEFA distribution. Across increasing NEFA tertiles, participants with CKD had a higher BMI, and a trend toward higher prevalence of diabetes, hypertension and hyperlipidemia (higher triglycerides), fasting glucose, and insulin, as well as lower insulin sensitivity among nonpatients with diabetes. NEFA did not correlate with eGFR (Spearman’s rho=0.05, P=0.22) nor did eGFR differ across NEFA tertiles (Ptrend=0.25). A similar lack of statistically significant correlations was observed between NEFA and eGFR as calculated by the CKD-EPIcreatinine (Spearman’s rho=0.08, P=0.06) or the CKD-EPIcreatinine+cystatin C (Spearman’s rho=0.07, P=0.08) equations.
Table 1.
Baseline characteristics according to tertiles of serum NEFA in 623 older men with CKD
| Parameter | Tertile of NEFA, mg/dl | P for Trend | ||
|---|---|---|---|---|
| Tertile 1 (≤18.4) | Tertile 2 (>18.4, 21.8) | Tertile 3 (>21.8) | ||
| No. of participants | 214 | 198 | 211 | |
| Age, yr | 71.1±0.6 | 71.0±0.6 | 71.0±0.7 | 0.47 |
| BMI, kg/m2 | 26.1±3.2 | 26.7±3.6 | 27.0±3.8 | 0.03 |
| Waist circumference, cm | 94 (89–100) | 95 (89–102) | 96 (89–102) | 0.39 |
| Smokers, % | 27 | 22.5 | 19 | 0.17 |
| Physical activity | 0.32 | |||
| Sedentary | 8 (4.1) | 6 (3.4) | 14 (7.5) | |
| Moderate | 64 (32.6) | 66 (37.3) | 70 (37.4) | |
| Regular | 113 (57.7) | 96 (54.2) | 98 (52.4) | |
| Athletic | 11 (5.6) | 9 (5.1) | 5 (2.7) | |
| Energy intake, kJ/d | 7266 (6066–8252) | 7359 (5888–8441) | 6702 (5776–7847) | 0.09 |
| Dietary fat, g/d | 68.2±9.3 | 70.2±10.7 | 69.1±11.8 | 0.18 |
| eGFR, ml/min per 1.73 m2 | ||||
| eGFR | 53.6 (46.3–57.7) | 53.4 (47.5–58.3) | 53.9 (48.3–57.1) | 0.25 |
| eGFR CKD-EPIcreatine | 66.9 (60.9–77.3) | 69.8 (60.7–76.5) | 68.1 (61.3–76.4) | 0.12 |
| eGFR CKD-EPIcreatine+cystatin C | 57.8 (51.7–63.6) | 58.6 (51.9–64.4) | 58.5 (52.7–63.8) | 0.23 |
| UAER, µg/min | 5.5 (3.3–21.2) | 6.7 (3.7–28.5) | 8.3 (3.8–28.1) | 0.10 |
| CVD | 79 (36.9) | 60 (30.3) | 83 (39.3) | 0.14 |
| Diabetes mellitus | 12 (5.6) | 28 (14.1) | 64 (30.3) | <0.001 |
| Glucose, mg/dl | 97.0±14.5 | 101.9±25.0 | 113.4±33.8 | <0.001 |
| Insulin, µg/L | 0.24 (0.17–0.35) | 0.26 (0.17–0.36) | 0.32 (0.21–0.47) | <0.001 |
| M/I, 100 × mg kg−1 min−1 per mU/L (n=519)a | 5.51±2.31 | 5.03±2.12 | 4.64±2.24 | <0.001 |
| Hypertension | 164 (76.6) | 155 (78.3) | 181 (85.8) | 0.04 |
| Hyperlipemia | 72 (33.6) | 64 (32.3) | 93 (44.1) | 0.02 |
| Triglycerides, mg/dl | 107.6 (78.0–142.2) | 120.5 (85.5–155.1) | 129.4 (89.5–188.7) | <0.001 |
| Cholesterol, mg/dl | ||||
| Total | 220±40.1 | 224±35.7 | 224±40.9 | 0.46 |
| LDL | 148±35.9 | 149±33.0 | 148±36.5 | 0.88 |
| HDL | 46.8 (39.7–56.1) | 46.0 (39.4–55.3) | 46.4 (38.3–55.7) | 0.83 |
| Lipid medication | 24 (13) | 13 (6) | 27 (14) | 0.72 |
| High-sensitivity CRP, mg/L | 2.09 (1.07–4.87) | 2.06 (1.09–4.34) | 2.55 (1.36–4.93) | 0.23 |
Data are expressed as mean±SD, n (%), or median (interquartile range), unless otherwise specified. NEFA, nonesterified fatty acid; BMI, body mass index; CKD-EPI, CKD Epidemiology Collaboration; UAER, urinary albumin excretion rate; CVD, cardiovascular disease; M/I, insulin sensitivity index; CRP, C-reactive protein.
Only in nondiabetes.
Correlates of NEFA
In univariate analysis, BMI, UAER, diabetes, hypertension, high-sensitivity CRP, and serum triglycerides, fasting glucose, and insulin were positively associated with NEFA, whereas energy intake and insulin sensitivity correlated negatively with NEFA. No association was found with measures of kidney function. Diabetes, insulin, and serum triglycerides were the multivariate correlates of NEFA (Table 2). Among nonpatients with diabetes, insulin sensitivity remained as the strongest NEFA correlate.
Table 2.
Univariate and multivariate correlates of NEFA (log2, mg/dl)
| Variable | Univariate Rho | P Value | Multivariate Coefficient (95% CI) | |
|---|---|---|---|---|
| All Individuals (n=623) | Nonpatients with Diabetes (n=519) | |||
| BMI, kg/m2 | 0.12 | 0.003 | 0.01 (−0.02 to 0.03) | 0.01 (−0.01 to 0.05) |
| Waist circumference, cm | 0.09 | 0.03 | — | — |
| Physical activity, n (%) | −0.08 | 0.06 | −0.05 (−0.12 to 0.02) | −0.05 (−0.13 to 0.04) |
| UAER, µg/min | 0.14 | 0.001 | 0.01 (−0.03 to 0.05) | 0.02 (−0.02 to 0.06) |
| eGFR, ml/min per 1.73 m2 | 0.05 | 0.22 | 0.20 (−0.03 to 0.42) | 0.19 (−0.07 to 0.44) |
| CVD | 0.02 | (0.62 | — | — |
| Diabetes mellitus | 0.30 | <0.001 | 0.28 (0.09 to 0.46) a | — |
| Hypertension | 0.11 | 0.01 | 0.06 (−0.07 to 0.19) | 0.02 (−0.11 to 0.16) |
| Triglyceride, mg/dl | 0.18 | <0.001 | 0.07 (0.01 to 0.14) a | 0.04 (−0.05 to 0.13) |
| Total cholesterol, mg/dl | 0.07 | 0.08 | — | — |
| HDL, mg/dl | −0.02 | 0.67 | — | — |
| LDL, mg/dl | 0.02 | 0.61 | — | — |
| Lipid medication | −0.01 | 0.88 | — | — |
| High-sensitivity CRP, mg/L | 0.08 | 0.04 | 0.03 (−0.02 to 0.09) | 0.05 (−0.10 to 0.10) |
| Glucose, mg/dl | 0.27 | <0.001 | 0.001 (−0.002 to 0.003) | 0.002 (−0.004 to 0.01) |
| Insulin, µg/L | 0.20 | <0.001 | 0.07 (0.003 to 0.15)a | |
| M/I, 100×mg kg−1 min−1 per mU/L | −0.30 | <0.001 | — | −0.04 (−0.07 to −0.01)a |
| Dietary energy intake, kJ/d | −0.11 | 0.01 | −0.10 (−0.28 to 0.08) | −0.05 (−0.26 to 0.16) |
| Dietary fat, g/d | 0.05 | 0.21 | 0.001 (−0.003 to 0.01) | 0.000 (−0.01 to 0.004) |
Dashes indicate not included. 95% CI, 95% confidence interval.
Statistically significant association (P<0.05).
Cox Regression Models
During a median of 14 years (range, 0.1–19.4 years), 453 participants died (incidence rate, 5.92/100 person-years at risk), of which 209 deaths were due to CVD (incidence rate, 2.73/100 person-years at risk). These included 88 individuals who died from IHD (incidence rate, 1.15/100 person-years at risk), which included 41 deaths attributed to AMI (incidence rate, 0.54/100 person-years at risk). In addition, 121 deaths were attributed to other CVD causes.
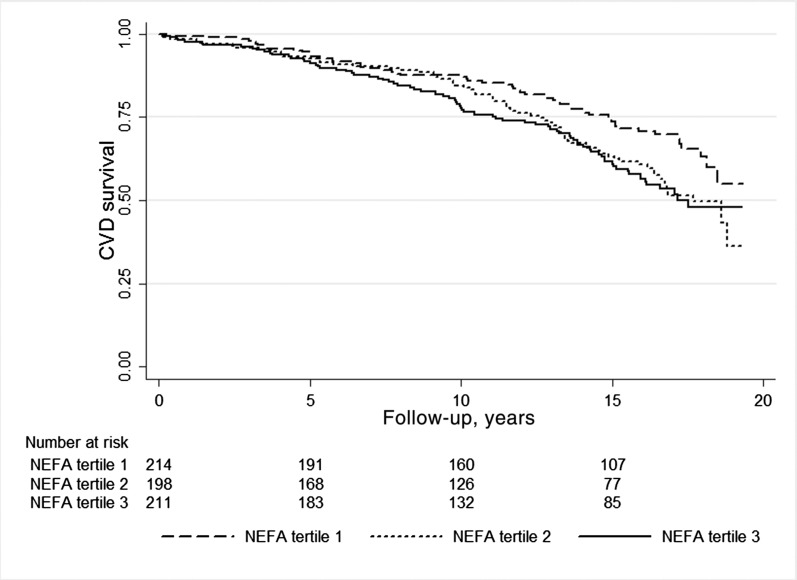
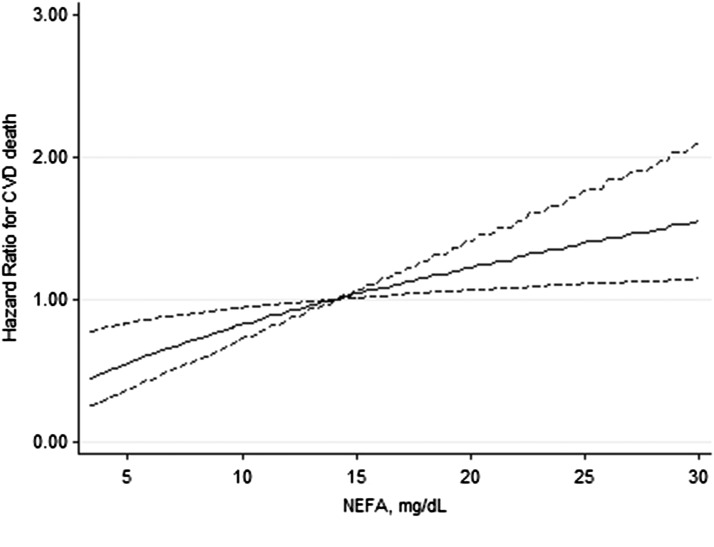
Kaplan–Meier curves show an association between NEFA and CVD mortality (log rank× 2=8.83, P=0.01; Figure 1). Table 3 presents analyses of the association between NEFA as a continuous variable and outcomes. Despite multivariable adjustment, every log2 mg/dl higher in NEFA (thus, every doubling in NEFA concentration) associated with higher hazards for mortality, and more strongly for CVD mortality. The magnitude of the hazards increased when addressing the secondary outcomes of IHD and AMI mortality. A spline curve analysis based on hazard ratios of the fully adjusted model for CVD mortality associated with serum NEFA showed a significant association of NEFA levels with mortality risk (Figure 2). Table 4 shows the final and fully adjusted model for prediction of CVD mortality in this population of elderly men with CKD. NEFA, CVD history, smoking, eGFR, and UAER were found to be independent predictors for CVD mortality. Sensitivity analysis using alternative eGFR equations (not shown) or excluding patients with diabetes (Supplemental Table 1), or including insulin sensitivity as a covariate (Supplemental Table 2) showed similar results.
Figure 1.
Kaplan–Meier curves and individuals at risk of cardiovascular mortality according to tertiles of serum NEFA distribution. CVD, cardiovascular disease; NEFA, nonesterified fatty acid.
Table 3.
HRs for all-cause and CVD-specific mortality according to NEFA (per log2 mg/dl higher)
| Outcome | No. of Events/At Risk | Crude | Adjusted Model 1 | Adjusted Model 2 | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| All-cause mortality | 453/623 | 1.19 (1.01 to 1.41) | 0.04 | 1.21 (1.01 to 1.44) | 0.04 | 1.22 (1.00 to 1.48) | 0.05 |
| CVD mortality | 209/623 | 1.42 (1.12 to 1.80) | 0.004 | 1.49 (1.16 to 1.91) | 0.002 | 1.51 (1.15 to 1.99)a | 0.004 |
| IHD mortality | 88/623 | 1.56 (1.08 to 2.24) | 0.02 | 1.50 (1.02 to 2.22) | 0.04 | 1.51 (1.00 to 2.32) | 0.05 |
| AMI mortality | 41/623 | 2.06 (1.24 to 3.40) | 0.01 | 2.17 (1.30 to 3.62) | 0.003 | 2.08 (1.09 to 3.98) | 0.03 |
| Other CVD mortality | 121/623 | 1.32 (0.96 to 1.82) | 0.09 | 1.48 (1.07 to 2.05) | 0.02 | 1.53 (1.06 to 2.21) | 0.02 |
Model 1 was adjusted for age, BMI, smoking status, and physical activity. Model 2 was adjusted for the variables in model 1 plus CVD history, hypertension, diabetes, plasma triglycerides, cholesterol and lipid medication, CRP, eGFR, and UAER. HR, hazard ratio; IHD, ischemic heart disease; AMI, acute myocardial infarction.
This model, primary outcome of our analysis, is shown in full in Table 4.
Figure 2.
Restricted cubic spline curve showing adjusted hazard ratios (solid line) and 95% confidence intervals (95% CIs) (dashed lines) for cardiovascular-related mortality associated with serum NEFA concentration. Covariates include age, body mass index, smoking status, physical activity, cardiovascular history, hypertension, diabetes mellitus, plasma triglycerides, cholesterol and lipid medication, C-reactive protein, eGFR, and urinary albumin excretion rate.
Table 4.
Multivariate associations (fully adjusted model) between NEFA (per log2 mg/dl higher) and CVD mortality
| Variable | HR (95% CI) | P Value |
|---|---|---|
| NEFA, per log2 mg/dl | 1.51 (1.15 to 1.99) | 0.004 |
| Age, per yr | 1.15 (0.88 to 1.49) | 0.31 |
| BMI, per kg/m2 | 0.99 (0.94 to 1.04) | 0.61 |
| Physical activity, active | 0.91 (0.71 to 1.17) | 0.47 |
| Current smoker | 1.41 (1.00 to 1.99) | 0.05 |
| UAER, per log2 µg/min | 1.20 (1.09 to 1.33) | <0.001 |
| eGFR, per log2 ml/min per 1.73 m2 | 0.30 (0.16 to 0.56) | <0.001 |
| CVD, presence | 1.82 (1.35 to 2.47) | <0.001 |
| Diabetes mellitus, presence | 1.01 (0.64 to 1.46) | 0.86 |
| Hypertension, presence | 1.39 (0.88 to 2.19) | 0.16 |
| High-sensitivity CRP, per log2 mg/L | 1.16 (0.98 to 1.37) | 0.09 |
| Triglycerides, per log2 mg/dl | 1.06 (0.84 to 1.34) | 0.62 |
| Cholesterol, mg/dl | 1.00 (0.99 to 1.01) | 0.31 |
| Lipid medication | 1.08 (0.69 to 1.68) | 0.75 |
Discussion
To our knowledge, this is the first prospective cohort study to address serum NEFA concentrations in relation to CVD mortality in individuals with CKD. We show that NEFAs are independent predictors for mortality, but more specifically so for CVD mortality, including IHD and AMI mortality as secondary outcomes.
Our cohort included older community-dwelling men with manifest CKD, the majority of which had moderately reduced kidney function. In this setting, we failed to observe an association between NEFA and renal function. We acknowledge that our study design, however, does not allow us to address this question. First, we lack a control group. Second, the homogeneity of our population biases this potential link. Further studies including a broader CKD spectrum should follow that test this hypothesis. Physiologically, NEFA serves as an important energy substrate for the heart, because it is released during the hydrolysis of triglycerides in adipose tissue (1) and is regulated according to the energy demands of the body (2). Conditions such as increased waist circumference, BMI, presence of diabetes, hypertension, and dyslipidemia have been associated with NEFA in non-CKD studies (26) as well as in our analysis, and are considered traditional risk factors for CVD events (26). Nevertheless, in agreement with community reports (26,27), our multivariate analysis showed that the presence of diabetes mellitus (or insulin resistance) and the concentrations of triglycerides were the main independent determinants of NEFA variance in our CKD population. Both diabetes and hypertriglyceridemia have been associated with increased CVD risk in patients with CKD (28–30), but the association between insulin resistance and CVD risk in nondiabetic CKD is less conclusive (31,32).
Here we show that NEFA levels predict fatal cardiovascular events in a moderately large population of elderly patients with CKD. This is in line with previous case-control studies in individuals with diabetes (33), linking NEFA concentrations with IHD risk. In another study of patients with IHD, increased NEFA concentrations predicted all-cause and CVD mortality (6). Finally, at a community level, NEFA associated with AMI incidence (5,10) and the risk of sudden cardiac death (7–9). However, not all observational studies agree, such as the Paris Prospective Study (9), which failed to observe an association between NEFA and fatal AMI.
NEFA as an energy source for myocardial metabolism has been described as “the lost child of cardiology” (34). Several mechanisms may explain our findings and suggest a potentially causal link between NEFA and CVD death. Elevated concentrations of NEFA with subsequent increased β-oxidation in the cardiomyocytes can be harmful for the heart via increased oxidative stress (35) and activation of apoptosis of endothelial cells (27). The heart toxicity of NEFA has been demonstrated to cause abnormalities in mitochondrial function (which may further impair myocardial function), and leads to damage in plasma membranes, including disturbances of the cardiomyocytes’ ion channels (2), which have been considered to exert proarrhythmic effects (36–38). Finally, levocarnitine deficiency often appears with progressive kidney dysfunction (39,40) and has been suggested to affect cardiac function in this patient population (41). Interestingly, levocarnitine plays a crucial role in counteracting the toxic effect of NEFA (42), and we speculate that alterations at this level may, at least in part, provide an additional link between NEFA and CVD risk in patients with CKD that warrants further investigation.
Strengths of this study include its prospective nature and ahomogenous, community-based cohort with a complete long follow-up period and detailed phenotypic characterization (43). Limitations include the selection of participants as per the cohort design, which consisted of Swedish Caucasian men aged 70–71 years. This limits the generalizability of our results to women, other ethnic groups, or younger adults. We did not assess GFR, but instead estimated it from cystatin C, and also utilized assessment of CKD based on UAER. Although this is common practice in the vast majority of clinical and epidemiologic studies of the kind, any potential bias by CKD misclassification or variations in UAER and GFR levels would most likely represent a conservative bias of our risk estimates. NEFA was measured only once, and we do not know whether the studied values are predictive of NEFA levels over the follow-up period. We lack detailed information on smoking (e.g., smoking history or number of cigarettes per day). In addition, participants were not prohibited from smoking during the day of examination, something that may have influenced our measured NEFA concentrations (44). Finally, we have no information on platelet levels, which are also shown to be affected by NEFA variation (45).
In conclusion, serum NEFA was associated with CVD mortality in elderly men with CKD. These findings, if confirmed in subsequent studies, offer interesting perspectives regarding therapeutic strategies focusing on improved myocardial metabolism via decreased NEFA levels (26).
Disclosures
B.L. is affiliated with Baxter Healthcare.
Supplementary Material
Acknowledgments
We acknowledge research grants from the Swedish Research Council (K2012-99X-21949-01-3) and the National Science Foundation for Young Scholars of China (81402737). H.X. is partially supported by the Karolinska Institute faculty (KID grant for postgraduate funding). Baxter Novum is the result of a grant from Baxter Healthcare Corporation to Karolinska Institute.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08830914/-/DCSupplemental.
References
- 1.Stich V, Berlan M: Physiological regulation of NEFA availability: Lipolysis pathway. Proc Nutr Soc 63: 369–374, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Pilz S, März W: Free fatty acids as a cardiovascular risk factor. Clin Chem Lab Med 46: 429–434, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Opie LH, Knuuti J: The adrenergic-fatty acid load in heart failure. J Am Coll Cardiol 54: 1637–1646, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Boden G: Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46: 3–10, 1997 [PubMed] [Google Scholar]
- 5.Roy VK, Kumar A, Joshi P, Arora J, Ahanger AM: Plasma free fatty acid concentrations as a marker for acute myocardial infarction. J Clin Diagn Res 7: 2432–2434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilz S, Scharnagl H, Tiran B, Seelhorst U, Wellnitz B, Boehm BO, Schaefer JR, März W: Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab 91: 2542–2547, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Pilz S, Scharnagl H, Tiran B, Wellnitz B, Seelhorst U, Boehm BO, März W: Elevated plasma free fatty acids predict sudden cardiac death: A 6.85-year follow-up of 3315 patients after coronary angiography. Eur Heart J 28: 2763–2769, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Havmoeller R, Reinier K, Teodorescu C, Ahmadi N, Kwok D, Uy-Evanado A, Chen YD, Rotter JI, Gunson K, Jui J, Chugh SS: Elevated plasma free fatty acids are associated with sudden death: a prospective community-based evaluation at the time of cardiac arrest. Heart Rhythm 11: 691–696, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jouven X, Charles MA, Desnos M, Ducimetière P: Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation 104: 756–761, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Huber AH, Kampf JP, Kwan T, Zhu B, Adams J, 3rd, Kleinfeld AM: Usefulness of serum unbound free fatty acid levels to predict death early in patients with ST-segment elevation myocardial infarction (from the Thrombolysis In Myocardial Infarction [TIMI] II trial). Am J Cardiol 113: 279–284, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charles MA, Fontbonne A, Thibult N, Claude JR, Warnet JM, Rosselin G, Ducimetière P, Eschwège E: High plasma nonesterified fatty acids are predictive of cancer mortality but not of coronary heart disease mortality: Results from the Paris Prospective Study. Am J Epidemiol 153: 292–298, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Djoussé L, Biggs ML, Ix JH, Kizer JR, Lemaitre RN, Sotoodehnia N, Zieman SJ, Mozaffarian D, Tracy RP, Mukamal KJ, Siscovick DS: Nonesterified fatty acids and risk of sudden cardiac death in older adults. Circ Arrhythm Electrophysiol 5: 273–278, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirro M, Mauriège P, Tchernof A, Cantin B, Dagenais GR, Després JP, Lamarche B: Plasma free fatty acid levels and the risk of ischemic heart disease in men: Prospective results from the Québec Cardiovascular Study. Atherosclerosis 160: 377–384, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Stompór T: Coronary artery calcification in chronic kidney disease: An update. World J Cardiol 6: 115–129, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaziri ND: Dyslipidemia of chronic renal failure: The nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 290: F262–F272, 2006 [DOI] [PubMed] [Google Scholar]
- 16.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 17.Vessby B, Tengblad S, Lithell H: Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 37: 1044–1050, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Byberg L, Zethelius B, McKeigue PM, Lithell HO: Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia 44: 2134–2139, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Howe GR, Kushi LH: Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65[Suppl]: 1220S–1228S, discussion 1229S–1231S, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Larsson A, Malm J, Grubb A, Hansson LO: Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest 64: 25–30, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Stevens PE, Levin A, Kidney Disease Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members : Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Byberg L, Siegbahn A, Berglund L, McKeigue P, Reneland R, Lithell H: Plasminogen activator inhibitor-1 activity is independently related to both insulin sensitivity and serum triglycerides in 70-year-old men. Arterioscler Thromb Vasc Biol 18: 258–264, 1998 [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Chang CH, Chin CC, Yu WW, Huang YY: Using the Bernoulli trial approaches for detecting ordered alternatives. BMC Med Res Methodol 13: 148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananth CV, Kleinbaum DG: Regression models for ordinal responses: A review of methods and applications. Int J Epidemiol 26: 1323–1333, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Mozaffarian D: Free fatty acids, cardiovascular mortality, and cardiometabolic stress. Eur Heart J 28: 2699–2700, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Boden G: Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 18: 139–143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Said S, Hernandez GT: The link between chronic kidney disease and cardiovascular disease. J Nephropathol 3: 99–104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaziri ND: Role of dyslipidemia in impairment of energy metabolism, oxidative stress, inflammation and cardiovascular disease in chronic kidney disease. Clin Exp Nephrol 18: 265–268, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Carrero JJ, de Mutsert R, Axelsson J, Dekkers OM, Jager KJ, Boeschoten EW, Krediet RT, Dekker FW, NECOSAD Study Group : Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant 26: 270–276, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Huang X, Arnlöv J, Cederholm T, Stenvinkel P, Lindholm B, Risérus U, Carrero JJ: Clinical correlates of insulin sensitivity and its association with mortality among men with CKD stages 3 and 4. Clin J Am Soc Nephrol 9: 690–697, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia T, Huang X, Qureshi AR, Xu H, Ärnlöv J, Lindholm B, Cederholm T, Stenvinkel P, Risérus U, Carrero JJ: Validation of insulin sensitivity surrogate indices and prediction of clinical outcomes in individuals with and without impaired renal function. Kidney Int 86: 383–391, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Thulaseedharan N, Augusti KT: Risk factors for coronary heart disease in noninsulin dependent diabetes mellitus (NIDDM). Indian Heart J 47: 471–476, 1995 [PubMed] [Google Scholar]
- 34.Taegtmeyer H: Metabolism—the lost child of cardiology. J Am Coll Cardiol 36: 1386–1388, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Zhou H, Wu K, Lee S, Li R, Liu X: PTEN phosphorylation and nuclear export mediate free fatty acid-induced oxidative stress. Antioxid Redox Signal 20: 1382–1395, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliver MF: Sudden cardiac death: the lost fatty acid hypothesis. QJM 99: 701–709, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Makiguchi M, Kawaguchi H, Tamura M, Yasuda H: Effect of palmitic acid and fatty acid binding protein on ventricular fibrillation threshold in the perfused rat heart. Cardiovasc Drugs Ther 5: 753–761, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Soloff LA: Arrhythmias following infusions of fatty acids. Am Heart J 80: 671–674, 1970 [DOI] [PubMed] [Google Scholar]
- 39.Belay B, Esteban-Cruciani N, Walsh CA, Kaskel FJ: The use of levo-carnitine in children with renal disease: A review and a call for future studies. Pediatr Nephrol 21: 308–317, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Calò LA, Vertolli U, Davis PA, Savica V: L-carnitine in hemodialysis patients. Hemodial Int 16: 428–434, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Molyneux R, Seymour AM, Bhandari S: Value of carnitine therapy in kidney dialysis patients and effects on cardiac function from human and animal studies. Curr Drug Targets 13: 285–293, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili A, Ceconi C: Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: A review. Ann N Y Acad Sci 1033: 79–91, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Nerpin E, Ingelsson E, Risérus U, Sundström J, Larsson A, Jobs E, Jobs M, Hallan S, Zethelius B, Berglund L, Basu S, Arnlöv J: The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrol Dial Transplant 26: 2820–2827, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Kershbaum A, Bellet S, Jimenez J, Feinberg LJ: Differences in effects of cigar and cigarette smoking on free fatty acid mobilization and catecholamine excretion. JAMA 195: 1095–1098, 1966 [PubMed] [Google Scholar]
- 45.Myrup B, Bregengaard C, Petersen LR, Winther K: Platelet aggregation and fatty acid composition of platelets in type 1 diabetes mellitus. Clin Chim Acta 204: 251–261, 1991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.