Abstract
Background and objectives
Metabolically healthy obesity (MHO) is a unique obesity phenotype that apparently protects people from the metabolic complications of obesity. The association between MHO phenotype and incident CKD is unclear. Thus, this study investigated the association between MHO phenotype and incident CKD.
Design, setting, participants, & measurements
A total of 3136 Japanese participants were enrolled in an 8-year follow-up cohort study in 2001. Metabolically healthy status was assessed by common clinical markers: BP, triglycerides, HDL cholesterol, and fasting plasma glucose concentrations. Body mass index ≥25.0 kg/m2 was defined as obesity. CKD was defined by proteinuria or eGFR of <60 ml/min per 1.73 m2. To calculate the odds ratio for incident CKD, logistic regression analyses were performed.
Results
The crude incidence proportions of CKD were 2.6% (56 of 2122 participants) in participants with the metabolically healthy nonobesity phenotype, 2.6% (8 of 302) in those with the MHO phenotype, 6.7% (30 of 445) in those with the metabolically abnormal nonobesity phenotype, and 10.9% (29 of 267) in those with the metabolically abnormal obesity phenotype. Compared with metabolically healthy nonobesity phenotype, the odds ratios for incident CKD were 0.83 (95% confidence interval [95% CI], 0.36 to 1.72; P=0.64) for MHO, 1.44 (95% CI, 0.80 to 2.57; P=0.22) for metabolically abnormal nonobesity, and 2.80 (95% CI, 1.45 to 5.35; P=0.02) for metabolically abnormal obesity phenotype after adjustment for confounders, including age, sex, smoking statues, alcohol use, creatinine, uric acid, systolic BP, HDL cholesterol, and impaired fasting glucose or diabetes.
Conclusion
MHO phenotype was not associated with higher risk of incident CKD.
Keywords: obesity, epidemiology and outcomes, proteinuria, chronic kidney disease, body mass index
Introduction
Obesity (1) and metabolic syndrome (2) are major public health problems worldwide that frequently coexist and define obese people who are at risk for adverse health outcomes. Recent studies have identified a subset of obese people who have a low burden of adiposity-related metabolic abnormalities compared with at-risk obese people, the so-called metabolically healthy obesity (MHO) phenotype (3–5). MHO phenotype is characterized by high levels of insulin sensitivity, low prevalence of hypertension, and a favorable fasting glucose, lipid, and inflammation profile (6,7).
CKD is an important and increasingly prevalent health concern worldwide (8,9). It is associated with ESRD, as well as cardiovascular morbidity and mortality (10–12).
Recent studies reported that metabolic syndrome (13,14) and obesity (15–18) were risk factors for incident CKD, but the association between MHO phenotype and incident CKD remains to be elucidated. Therefore, we aimed to investigate whether MHO phenotype was associated with higher risk of incident CKD in this cohort study.
Materials and Methods
Study Participants and Study Design
The Oike Health Survey is an ongoing cohort investigation of risk factors for chronic diseases, including hypertension, diabetes, and CKD. The Oike Clinic (Kyoto, Japan) provides regular health checkups for the employees of various companies. In Japan, yearly routine examination for employees is legally mandated, and all or most of the costs for the health checkup are usually paid by their employers.
In this retrospective cohort study, we enrolled 4127 participants without malignant disease, liver cirrhosis, or hematologic disease who had health checkup examinations at the Oike Clinic in 2001 and 2009. We excluded 709 participants who had CKD at the baseline examination, which was performed in 2001. Furthermore, we excluded 282 participants with missing data on covariates. Thus, 3136 participants were eligible for this analysis.
The Ethical Committee of the Oike Clinic approved this study, and the study was conducted in accordance with the Declaration of Helsinki. Each participant provided informed consent.
Date Collection and Measurements
All participants provided demographic details. Smoking was defined as current tobacco use. Alcohol use was defined as daily alcohol consumption. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. After a brief period of rest, sitting BP was measured in either arm. BP was measured once in most participants, but up to three measurements at 1- to 2-minute intervals were made in participants who had hypertensive or prehypertensive BP values. The lowest reading was used in the analysis that assessed the incidence of hypertension. After an overnight fast, venous blood was collected for the measurement of the levels of various factors, including fasting plasma glucose, total cholesterol, triglycerides, HDL cholesterol, creatinine, and uric acid. Serum creatinine was measured using an enzymatic assay (Akyurasu-auto; Shino-test Corp., Kanagawa, Japan) in an autoanalyzer; the coefficient of variation was 2.0%.
Definition of CKD
GFR was estimated using the Japanese Society of Nephrology equation (19):
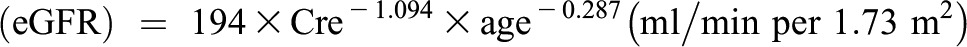 |
For women, the eGFR was multiplied by a correction factor of 0.739. Proteinuria was determined using dipstick testing (Yurifuret-S; Arkray, Kyoto, Japan) in fasting morning urine (positive: ≥1+) (20). CKD was defined as proteinuria or an eGFR<60 ml/min per 1.73 m2.
Definitions of Metabolic Phenotypes
BMI≥25.0 kg/m2, which has been proposed as a cutoff for the diagnosis of obesity in Asian people (21), was defined as obesity; BMI<25.0 kg/m2 was defined as nonobesity. The validity of this definition was confirmed previously (22,23). We used four metabolic factors (impaired fasting glucose or diabetes, hypertension, hypertriglyceridemia, and low HDL cholesterol concentration), defined by International Diabetes Federation (24), to determine whether the participant was metabolically healthy or metabolically abnormal. Data on waist circumference, visceral fat, fasting insulin, and C-reactive protein concentrations were not available for study participants, although we acknowledge that these markers can be used to define metabolic phenotypes (7,25). Participants with a systolic BP≥130 mmHg and/or a diastolic BP≥85 mmHg or who were under medical treatment were considered to have hypertension. Elevated triglyceride level was indicated by ≥150 mg/dl or treatment of hyperlipidemia, and reduced HDL cholesterol level was indicated by <40 mg/dl in men and <50 mg/dl in women. Participants with fasting plasma glucose ≥100 mg/dl or who were under medical treatment were considered to have impaired fasting glucose or diabetes. A metabolically healthy state was considered if none or one of the metabolic factors based on the International Diabetes Federation definition was present, and a metabolically abnormal state was declared if two or more metabolic factors were present (24). Then, participants were categorized at the baseline examination into four phenotypes: (1) metabolically healthy nonobesity (MHNO), (2) MHO, (3) metabolically abnormal nonobesity (MANO), or (4) metabolically abnormal obesity (MAO). This definition of metabolic phenotypes has often been used in a Japanese population (26). We also analyzed data with obesity defined as BMI≥27.5 kg/m2, which has also been proposed as a cutoff for the diagnosis of obesity in Asian people (27).
Statistical Analyses
Continuous variables were expressed as mean±SD, and categorical variables were expressed as percentage (number). The analyses of continuous and categorical variables to assess differences among four phenotypes were determined by one-way ANOVA or the chi-squared test. We performed logistic regression analyses to assess the association of metabolic phenotypes with incident CKD, adjusting for covariates that included age, sex, smoking statues, alcohol use, creatinine, uric acid, systolic BP, HDL cholesterol, and impaired fasting glucose or diabetes. In addition, we performed logistic regression analyses to assess the association of metabolic phenotypes with incident proteinuria adjusting for covariates, including age, sex, smoking statues, alcohol use, creatinine, uric acid, systolic BP, HDL cholesterol, and impaired fasting glucose or diabetes. The variance inflation factor was used for detecting the co-linearity; a variance inflation factor ≥10 indicates a colinearity problem. In addition, we conducted a second analysis when we defined obesity as a BMI≥27.5 kg/m2. The statistical analyses were performed using JMP software, version 10.0 (SAS Institute Inc., Cary, NC). A P value <0.05 was considered to represent a statistically significant difference.
Results
The baseline characteristics are shown in Table 1. The prevalence of MHNO, MHO, MANO, and MAO was 67.7% (n=2122), 9.6% (n=302), 14.2% (n=445), and 8.5% (n=267), respectively. At the follow-up examination, which was performed 8 years after baseline examination, 123 participants had developed CKD. The crude incidence proportions of CKD were 2.6% (56 of 2122) for the MHNO phenotype, 2.6% (eight of 302) for the MHO phenotype, 6.7% (30 of 445) for the MANO phenotype, and 10.9% (29 of 267) for the MAO phenotype. The crude incidence proportions of proteinuria were 0.5% (11 of 2122) for the MHNO phenotype, 1.0% (three of 302) for the MHO phenotype, 1.6% (seven of 445) for the MANO phenotype, and 5.6% (15 of 267) for the MAO phenotype.
Table 1.
Characteristics of study participants at the baseline examination
| Characteristic | MHNO | MHO | MANO | MAO | P Value |
|---|---|---|---|---|---|
| Participants (n) | 2122 | 302 | 445 | 267 | – |
| Age (yr) | 45.3±9.3 | 45.0±9.4 | 52.2±9.0 | 49.2±9.0 | <0.001 |
| Men, % (n) | 50.4 (1070) | 72.2 (218) | 73.5 (327) | 81.6 (218) | <0.001 |
| Body mass index (kg/m2) | 21.1±2.1 | 26.8±1.7 | 22.4±1.7 | 27.2±2.0 | <0.001 |
| Systolic BP (mmHg) | 111.9±14.2 | 120.1±15.5 | 130.3±16.8 | 132.0±15.7 | <0.001 |
| Diastolic BP (mmHg) | 67.2±9.5 | 72.4±10.4 | 78.8±11.0 | 79.7±9.9 | <0.001 |
| Fasting plasma glucose (mg/dl) | 88.9±8.9 | 91.6±10.3 | 100.4±20.0 | 108.5±29.4 | <0.001 |
| Total cholesterol (mg/dl) | 203.4±32.0 | 212.2±34.6 | 219.3±35.4 | 217.3±36.5 | <0.001 |
| Triglycerides (mg/dl) | 86.3±54.9 | 122.9±78.1 | 175.4±132.5 | 202.7±134.1 | <0.001 |
| HDL cholesterol (mg/dl) | 65.9±15.3 | 55.6±11.9 | 54.8±16.4 | 48.1±12.1 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 76.6±11.6 | 75.0±9.9 | 73.5±9.8 | 75.1±10.7 | <0.001 |
| Uric acid (mg/dl) | 4.9±1.3 | 5.7±1.4 | 5.7±1.3 | 6.0±1.4 | <0.001 |
| Smoking, % (n) | 15.0 (319) | 18.9 (57) | 16.9 (75) | 19.5 (52) | 0.11 |
| Alcohol use, % (n) | 33.7 (715) | 33.4 (101) | 39.8 (177) | 37.5 (100) | 0.07 |
Data are expressed as percentage (number) or mean±SD. The analyses of continuous and categorical variables to assess differences among the four groups were determined by one-way ANOVA or the chi-square test. Smoking was defined as current tobacco use. Alcohol use was defined as daily alcohol consumption. MHNO, metabolically healthy nonobesity; MHO, metabolically healthy obesity; MANO, metabolically abnormal nonobesity; MAO, metabolically abnormal obesity.
Logistic regression analyses were performed to investigate the association between each metabolic phenotype and incident CKD (Table 2). No colinearity was found between variables. The MHO phenotype was not associated with higher risk of incident CKD. On the other hand, MAO phenotype was associated with significantly higher risk of incident CKD (multivariate-adjusted odds ratio [OR], 2.80; 95% confidence interval [95% CI], 1.45 to 5.35; P=0.02).
Table 2.
Odds ratios for incident CKD at 8 years after the baseline examination according to metabolic phenotypes
| Variable | MHNO | MHO | MANO | MAO |
|---|---|---|---|---|
| Incidence of CKD (n/n) | 56/2122 | 8/302 | 30/445 | 29/267 |
| Model 1a | 1.00 (Reference) | 1.00 (0.44 to 2.01) | 2.67 (1.67 to 4.18)b | 4.50 (2.78 to 7.12)b |
| Model 2c | 1.00 (Reference) | 0.97 (0.42 to 1.95) | 1.83 (1.12 to 2.93)d | 3.52 (2.14 to 5.69)b |
| Model 3e | 1.00 (Reference) | 0.97 (0.42 to 1.95) | 1.83 (1.12 to 2.93)d | 3.51 (2.13 to 5.68)b |
| Model 4f | 1.00 (Reference) | 0.83 (0.36 to 1.72) | 1.44 (0.80 to 2.57) | 2.80 (1.45 to 5.35)d |
Unless otherwise noted, values are expressed as odds ratio (95% confidence interval).
Model 1 was unadjusted.
P<0.001 versus MHNO phenotype.
Model 2 adjusted for age and sex.
P<0.05 versus MHNO phenotype.
Model 3 adjusted for model 2 plus smoking status and alcohol use.
Model 4 adjusted for model 3 plus creatinine, uric acid, systolic BP, HDL cholesterol, and impaired fasting glucose or diabetes.
Logistic regression analyses were also performed to investigate the association between each metabolic phenotype and incident proteinuria (Table 3). The MHO phenotype was not associated with higher risk of incident proteinuria. On the other hand, MAO phenotype was associated with a significantly higher risk of incident proteinuria (multivariate-adjusted OR, 6.29; 95% CI, 2.05 to 19.6; P<0.01).
Table 3.
Odds ratios for incident proteinuria at 8 years after the baseline examination according to metabolic phenotypes
| Variable | MHNO | MHO | MANO | MAO |
|---|---|---|---|---|
| Incidence of proteinuria (n/n) | 11/2122 | 3/302 | 7/445 | 15/267 |
| Model 1a | 1.00 (Reference) | 1.93 (0.43 to 6.21) | 3.07 (1.12 to 7.83)b | 11.4 (5.22 to 25.8)c |
| Model 2d | 1.00 (Reference) | 1.84 (0.41 to 5.99) | 2.05 (0.73 to 5.43) | 8.68 (3.83 to 20.3)c |
| Model 3e | 1.00 (Reference) | 1.88 (0.42 to 6.13) | 2.06 (0.73 to 5.47) | 8.85 (3.89 to 20.8)c |
| Model 4f | 1.00 (Reference) | 1.65 (0.36 to 5.57) | 1.62 (0.50 to 5.00) | 6.29 (2.05 to 19.6)g |
Unless otherwise noted, values are expressed as odds ratio (95% confidence interval).
Model 1 was unadjusted.
P<0.05 versus MHNO phenotype.
P<0.001 versus MHNO phenotype.
Model 2 adjusted for age and sex.
Model 3 adjusted for model 2 plus smoking status and alcohol use.
Model 4 adjusted for model 3 plus creatinine, uric acid, systolic BP, HDL cholesterol, and impaired fasting glucose or diabetes.
P<0.01 versus MHNO phenotype.
Results of the Second Analyses
The prevalence of MHNO, MHO, MANO or MAO in the analysis that defined obesity as a BMI≥27.5 kg/m2 (and in which participants were considered as being in a metabolically healthy or abnormal state) was 74.7% (n=2344), 2.6% (n=80), 19.8% (n=622), and 2.9% (n=90). Crude incidence proportions of CKD were 2.6% (61 of 2344) for the MHNO phenotype, 3.8% (three of 80) for the MHO phenotype, 7.2% (45 of 622) for the MANO phenotype, and 15.6% (14 of 90) for the MAO phenotype. Crude incidence proportions of proteinuria were 0.5% (12 of 2344) for the MHNO phenotype, 2.5% (two of 80) for the MHO phenotype, 2.1% (13 of 622) for the MANO phenotype, and 10.0% (nine of 90) for the MAO phenotype. The MHO phenotype was not associated with a higher risk of incident CKD (multivariate-adjusted OR, 1.35; 95% CI, 0.32 to 3.93; P=0.64). On the other hand, MAO phenotype was associated with a significantly higher risk of incident CKD (multivariate-adjusted OR, 5.34; 95% CI, 2.35 to 11.7; P<0.001). In addition, the MHO phenotype was not associated with a higher risk of incident proteinuria (multivariate-adjusted OR, 5.06; 95% CI, 0.85 to 6.53; P=0.09). On the other hand, MAO phenotype was associated with a significantly higher risk of incident proteinuria (multivariate-adjusted OR, 14.0; 95% CI, 4.01 to 48.5; P<0.001).
Discussion
The major finding of our study is that MHO phenotype was not associated with higher risk of incident CKD. On the other hand, MAO phenotype was associated with significantly higher risk of incident CKD. In addition, we also showed that MHO phenotype was not associated with higher risk of incident proteinuria. On the other hand, MAO phenotype was associated with a significantly higher risk of incident proteinuria.
People with the MHO phenotype are apparently protected from the metabolic complications of obesity; at least, the risk appears to be considerably lower than expected for the given level of obesity (4,5). To our knowledge, ours is the first study to investigate the association between MHO phenotype and incident CKD.
Most studies reported that obesity is a risk factor for incident CKD (15–18) and proteinuria (28); however, a study showed that obesity was not associated with higher risk of incident CKD after adjusting for known cardiovascular risk factors, including diabetes, systolic BP, and HDL cholesterol (29). Our study also suggested that MAO phenotype, not MHO phenotype, was associated with higher risk of incident CKD in the obese people. Thus, we demonstrated that the association between obesity and CKD may be mediated by metabolic abnormalities. The association between obesity and CKD might be mediated through multiple biologic mechanisms, including hormonal factors, inflammation, oxidative stress, and endothelial dysfunction (30,31). The expansion of visceral adipose tissue (i.e., a target for infiltration by immune cells) is involved in these mechanisms (32). Excess visceral adipose tissue can lead to the activation of the sympathetic nervous and renin-angiotensin systems, as well as lipid deposition, hyperfiltration, and increased sodium absorption in the kidneys, resulting in a feedback loop where obesity-induced declines in kidney function lead to the development of hypertension, which results in further damage to the kidneys (33). Previous studies have demonstrated that hypertension mediates the association between obesity and incident CKD (34,35). Moreover, the decrease in adiponectin concentration is relevant: It is associated with reduced whole body insulin sensitivity and possibly causes increased proinflammatory signaling in the kidney as well (36). On this point, studies of obese people suggested that the MHO phenotype had a more favorable distribution of low visceral fat, although the total fat mass was similar between MHO phenotype and MAO phenotype (3,37). Taking these findings together, not MHO phenotype but MAO phenotype is associated with higher risk of incident CKD.
Strengths of our study include the large number of participants both at baseline and at follow-up. However, this study has some limitations that require consideration. First, because we could not assess changes in waist circumference, insulin resistance, or insulin secretion, some metabolically healthy participants might have isolated insulin resistance or visceral adiposity without the major common metabolic abnormalities. Thus, we cannot deny the possibility of misclassification of participants. However, the four metabolic factors (impaired fasting glucose or diabetes, hypertension, hypertriglyceridemia, and low HDL cholesterol concentration) used in this study are commonly available in clinical settings, and the validity of this definition was confirmed previously (26,38).
Second, this is a relatively long follow-up study, but duration of follow-up may have been insufficient to allow us to evaluate the risk of incident CKD. Recent studies revealed the possibility that MHO phenotype was also a risk factor for different clinical characteristics, including diabetes (26,38,39), cardiovascular diseases (40–42), and hypertension (43). On this point, some clinical outcomes occurred only after a long-term follow-up (40,43). Thus, further long-term follow-up study is needed.
Third, the study population consisted of Japanese men and women; therefore, it is uncertain whether these findings can be generalized to other ethnic groups.
Fourth, we defined proteinuria by dipstick testing and thus did not quantitate the proteinuria. However, a dipstick test is a useful tool. Most patients with a 1+ or 2+ dipstick test result have microalbuminuria instead of macroalbuminuria, whereas patients with 3+ proteinuria mostly have macroalbuminuria (20). In addition, proteinuria by dipstick testing was also useful to determine development of ESRD (44,45).
Finally, our study participants underwent a health examination; thus, some participants might have made lifestyle changes based on results of the health examination to prevent the development of metabolic abnormalities.
In conclusion, our study showed that MAO phenotype, not MHO phenotype, was associated with higher risk of incident CKD.
Disclosures
None.
Acknowledgments
We thank all of the staff members of the Oike Clinic.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) : National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 377: 557–567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults : Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, Poehlman ET: What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab 86: 1020–1025, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Sims EA: Are there persons who are obese, but metabolically healthy? Metabolism 50: 1499–1504, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET: Metabolic and body composition factors in subgroups of obesity: What do we know? J Clin Endocrinol Metab 89: 2569–2575, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Karelis AD: Metabolically healthy but obese individuals. Lancet 372: 1281–1283, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Velho S, Paccaud F, Waeber G, Vollenweider P, Marques-Vidal P: Metabolically healthy obesity: Different prevalences using different criteria. Eur J Clin Nutr 64: 1043–1051, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, Locatelli F, MacLeod A, Vanholder R, Walker R, Wang H: The burden of kidney disease: Improving global outcomes. Kidney Int 66: 1310–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Meguid El Nahas A, Bello AK: Chronic kidney disease: The global challenge. Lancet 365: 331–340, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Kurella M, Lo JC, Chertow GM: Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol 16: 2134–2140, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD: Metabolic syndrome and kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol 6: 2364–2373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D: Obesity and prevalent and incident CKD: The Hypertension Detection and Follow-Up Program. Am J Kidney Dis 46: 587–594, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM: Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 46: 871–880, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ: Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int 73: 19–33, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Konta T, Hao Z, Takasaki S, Abiko H, Ishikawa M, Takahashi T, Ikeda A, Ichikawa K, Kato T, Kawata S, Kubota I: Clinical utility of trace proteinuria for microalbuminuria screening in the general population. Clin Exp Nephrol 11: 51–55, 2007 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Western Pacific Region, International Association for the Study of Obesity/International Obesity Task Force : The Asia-Pacific Perspective: Redefining Obesity and Its Treatment, Melbourne, Australia, Health Communications Australia, 2000 [Google Scholar]
- 22.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K: The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 143: 722–728, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Wen CP, David Cheng TY, Tsai SP, Chan HT, Hsu HL, Hsu CC, Eriksen MP: Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 12: 497–506, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Alberti KG, Zimmet P, Shaw J: Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23: 469–480, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Pataky Z, Bobbioni-Harsch E, Golay A: Open questions about metabolically normal obesity. Int J Obes (Lond) 34[Suppl 2]: S18–S23, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Heianza Y, Arase Y, Tsuji H, Fujihara K, Saito K, Hsieh SD, Tanaka S, Kodama S, Hara S, Sone H: Metabolically healthy obesity, presence or absence of fatty liver, and risk of type 2 diabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 20 (TOPICS 20). J Clin Endocrinol Metab 99: 2952–2960, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Expert Consultation WHO, WHO Expert Consultation : Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Shen WW, Chen HM, Chen H, Xu F, Li LS, Liu ZH: Obesity-related glomerulopathy: Body mass index and proteinuria. Clin J Am Soc Nephrol 5: 1401–1409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, Levy D, Fox CS: Overweight, obesity, and the development of stage 3 CKD: The Framingham Heart Study. Am J Kidney Dis 52: 39–48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Jong PE, Verhave JC, Pinto-Sietsma SJ, Hillege HL, PREVEND study group : Obesity and target organ damage: The kidney. Int J Obes Relat Metab Disord 26[Suppl 4]: S21–S24, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Liu Z, Xiang Z, Zeng C, Chen Z, Ma X, Li L: Obesity-related glomerulopathy: Insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology 147: 44–50, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Odegaard JI, Chawla A: Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 339: 172–177, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aneja A, El-Atat F, McFarlane SI, Sowers JR: Hypertension and obesity. Recent Prog Horm Res 59: 169–205, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Griffin KA, Kramer H, Bidani AK: Adverse renal consequences of obesity. Am J Physiol Renal Physiol 294: F685–F696, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Griffin KA, Bidani AK: Progression of renal disease: Renoprotective specificity of renin-angiotensin system blockade. Clin J Am Soc Nephrol 1: 1054–1065, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Adamczak M, Wiecek A: The adipose tissue as an endocrine organ. Semin Nephrol 33: 2–13, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, Visser M, Houston DK, Nicklas BJ, Tylavsky FA, Satterfield S, Goodpaster BH, Ferrucci L, Harris TB, Health ABC Study : Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 18: 2354–2361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, Adams RJ, North West Adelaide Health Study Team : Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: A cohort study. Diabetes Care 36: 2388–2394, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell JA, Kivimaki M, Hamer M: Metabolically healthy obesity and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes Rev 15: 504–515, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnlöv J, Ingelsson E, Sundström J, Lind L: Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 121: 230–236, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Kramer CK, Zinman B, Retnakaran R: Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med 159: 758–769, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Roberson LL, Aneni EC, Maziak W, Agatston A, Feldman T, Rouseff M, Tran T, Blaha MJ, Santos RD, Sposito A, Al-Mallah MH, Blankstein R, Budoff MJ, Nasir K: Beyond BMI: The “Metabolically healthy obese” phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality—a systematic review. BMC Public Health 14: 14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SK, Kim SH, Cho GY, Baik I, Lim HE, Park CG, Lee JB, Kim YH, Lim SY, Kim H, Shin C: Obesity phenotype and incident hypertension: A prospective community-based cohort study. J Hypertens 31: 145–151, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Grimm RH, Jr, Svendsen KH, Kasiske B, Keane WF, Wahi MM: Proteinuria is a risk factor for mortality over 10 years of follow-up. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Kidney Int Suppl 63: S10–S14, 1997 [PubMed] [Google Scholar]
- 45.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD: Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 17: 1444–1452, 2006 [DOI] [PubMed] [Google Scholar]


