Abstract
Background and objectives
Monitoring N-terminal pro–B-type natriuretic peptide (NT-proBNP) may be useful for assessing cardiovascular risk in dialysis patients. However, its biologic variation is unknown, hindering the accurate interpretation of serial concentrations. The aims of this prospective cohort study were to estimate the within- and between-person coefficients of variation of NT-proBNP in stable dialysis patients, and derive the critical difference between measurements needed to exclude biologic and analytic variation.
Design, setting, participants, & measurements
Fifty-five prevalent hemodialysis and peritoneal dialysis patients attending two hospitals were assessed weekly for 5 weeks and then monthly for 4 months between October 2010 and April 2012. Assessments were conducted at the same time in the dialysis cycle and entailed NT-proBNP testing, clinical review, electrocardiography, and bioimpedance spectroscopy. Patients were excluded if they became unstable.
Results
This study analyzed 136 weekly and 113 monthly NT-proBNP measurements from 40 and 41 stable patients, respectively. Results showed that 22% had ischemic heart disease; 9% and 87% had left ventricular systolic and diastolic dysfunction, respectively. Respective between- and within-person coefficients of variation were 153% and 27% for weekly measurements, and 148% and 35% for monthly measurements. Within-person variation was unaffected by dialysis modality, hydration status, inflammation, or cardiac comorbidity. NT-proBNP concentrations measured at weekly intervals needed to increase by at least 46% or decrease by 84% to exclude change due to biologic and analytic variation alone with 90% certainty, whereas monthly measurements needed to increase by at least 119% or decrease by 54%.
Conclusions
The between-person variation of NT-proBNP was large and markedly greater than within-person variation, indicating that NT-proBNP testing might better be applied in the dialysis population using a relative-change strategy. Serial NT-proBNP concentrations need to double or halve to confidently exclude change due to analytic and biologic variation alone.
Keywords: N-terminal pro–B-type natriuretic peptide, variability, renal dialysis, B-type, natriuretic peptide
Introduction
The N-terminal fragment of the pro–B-type natriuretic peptide (NT-proBNP) is an inactive peptide secreted from the myocardium in response to stretch, strain, ischemia, inflammation, and sympathetic overactivity (1). Cohort studies in the dialysis population have demonstrated a direct association between NT-proBNP concentrations and the risk of cardiovascular and all-cause mortality (2–5), prompting calls to incorporate NT-proBNP testing into dialysis practice as a means of monitoring individual patients’ cardiovascular risk (6–8).
However, before these findings can be translated into clinical practice, the biologic variation of NT-proBNP in dialysis patients needs to be determined in order to avoid misinterpreting serial measurements. Biologic or within-person variation is the random fluctuation of a biomarker around a homeostatic set point in healthy individuals or those with stable disease (9), resulting in potentially large numerical changes in serial biomarker concentrations that are of no clinical significance. Failure to account for biologic variation can result in false reassurance or alarm, as well as unnecessary changes to therapy with their associated morbidity and costs (10).
This study aimed to estimate the within- and between-person variation of NT-proBNP measured at weekly and monthly intervals in stable dialysis patients and to use these estimates to calculate the percentage change between serial NT-proBNP measurements needed to exclude change due to biologic and analytic variation alone. We also sought to determine whether the within-person variation of NT-proBNP differed according to cardiac comorbidity, hydration, or inflammatory status or between dialysis modalities.
Materials and Methods
Study Design and Patient Recruitment
A prospective cohort study was conducted between October 2010 and April 2012 according to methods described by Fraser and Harris (11). The study complied with the Declaration of Helsinki and received ethics approval from the Metro-South Human Research Ethics Committee (HREC/10/QPAH/131).
Participants were recruited from the in-center hemodialysis and peritoneal dialysis units of a tertiary-care teaching hospital in Brisbane, and a secondary-care hospital in Logan, Australia. Eligible participants identified from an electronic database of all patients receiving dialysis therapy were adults (aged ≥18 years) on maintenance dialysis for ≥90 days who had a stable dialysis prescription for ≥30 days and a transthoracic echocardiogram ≤12 months before screening.
Eligibility criteria were chosen to ensure that the study cohort was physiologically and clinically stable at enrollment, and was likely to remain stable for the duration of the study while still being representative of the dialysis population. Patients were excluded if they met any of the following criteria: if they had undergone coronary and/or valvular intervention or suffered a myocardial infarction or pulmonary embolism in the 6 months before screening; had echocardiographic evidence of severe pulmonary hypertension, severe functional aortic and/or mitral valvular disease, or a left ventricular ejection fraction <30%; had been hospitalized for any indication or undergone an unscheduled dialysis for the treatment of hypertension, heart failure, or dyspnea in the 30 days before screening; had been commenced on or undergone a dose change of a diuretic, β-blocker, aldosterone receptor antagonist, angiotensin-converting enzyme inhibitor or angiotensin receptor type 1 blocker in the 30 days before screening; had experienced worsening angina, a new cardiac arrhythmia, or undergone change in associated therapies in the 30 days before screening; had a contraindication to bioimpedance measurement including a pacemaker, joint replacements, or mechanical heart valve; were pregnant; had advanced malignancy; or were unable to provide informed consent.
Patient Assessment
Patients were assessed on 10 consecutive occasions—weekly for 5 weeks and then monthly for another 4 months. All assessments were conducted between 6 and 8 a.m., before the mid-week dialysis session for hemodialysis patients, and between 8 and 10 a.m. on the same weekday for peritoneal dialysis patients. Patients avoided strenuous exercise before assessment.
Several factors affect NT-proBNP concentrations, including extracellular volume (12), cardiac rhythm (13), myocardial ischemia (14,15), the dialysis prescription (16), and cardiac pharmacotherapy (17–19). These influences were assessed at every visit using a structured clinical interview, physical examination, and medical records review to ascertain interim hospitalization and changes to medication and/or the dialysis prescription. The Canadian Cardiovascular Society Angina Grading Scale (20) was used to assess change in cardiac ischemic symptoms and the Truncated Framingham Heart Failure Score (21) was used to assess for pulmonary edema. Patients underwent a standard 12-lead electrocardiogram and whole-body, multifrequency bioimpedance analysis using the Body Composition Monitor BCM (Fresenius Medical Care Asia-Pacific) at each visit to measure extracellular volume. This instrument has a detection limit for change in extracellular volume of 0.87±0.64 L (22,23).
Specimen Collection, Storage, and Analyses
NT-proBNP concentrations were measured at eight of 10 visits (baseline then at weeks 1–4 and months 2–4), providing data for 4 weekly and 4 monthly intervals. To ensure that changes in NT-proBNP concentrations during the final measurement interval did not reflect changes in subclinical risk, patients were assessed for stability at week 5 and month 5 without measurement of NT-proBNP.
Blood collected in lithium-heparin tubes was centrifuged and plasma separated within 1 hour of collection. Plasma was stored at −80°C until assayed (24).
Samples were batched and analyzed together in a single analytic run in random duplicate by a single expert operator using a single instrument and a single batch of reagent, control, and calibrators. Plasma NT-proBNP concentration (in picograms per milliliter) was measured using a twin-antibody electrochemiluminescence assay on the Elecsys 2010 instrument (Roche Diagnostics, Australia), which has a reported analytical detection range of 5–35,000 pg/ml (25). NT-proBNP was chosen in preference to brain natriuretic peptide due to its superior stability, which minimizes preanalytic variation (26), and due to the greater agreement between NT-proBNP assays from different manufacturers (27), allowing the study’s findings to be more widely generalizable.
C-reactive protein (in milligrams per liter) was measured predialysis at the baseline visit and analyzed using a turbidimetric method on the Beckman DxC800 analyzer (Beckman Coulter, CA). This method has a lower limit of detection of 2.0 mg/L, and analytic coefficients of variation of 6.3% and 3.1% at concentrations of 6.0 and 85.0 mg/L, respectively.
Statistical Analyses
Based on a ratio of analytic to within-person variation of <0.5 for NT-proBNP, we estimated that a study sample of 40 patients undergoing NT-proBNP testing on eight occasions over 4 weekly and monthly intervals would have power >0.99 to estimate the within-person coefficient of variation with a 95% confidence interval of ±3.6% (28). A sample size of 55 patients was chosen to allow for dropouts as a result of instability.
The principal assumption of biologic variation studies is that the cohort is stable with respect to physiologic, pathologic, and extrinsic factors that influence the concentration of the biomarker of interest. In this study, patients were deemed to be unstable if they experienced a change in dose of diuretic, β-blocker, aldosterone receptor antagonist, angiotensin-converting enzyme inhibitor, or angiotensin receptor blocker; a change in severity of cardiac ischemic symptoms, dose of antianginal agents, or cardiac intervention; a change in antiarrhythmic agents or new cardiac arrhythmia; a change in extracellular volume >1 L on bioimpedance analysis; a change in dialysis modality or prescription; hospitalization for any reason; or exhibited pulmonary edema defined as a score ≥2 on the Truncated Framingham Heart Failure Score. If a study participant was deemed to be unstable, the NT-proBNP concentrations from the intervals before and after the event were excluded from the statistical analysis.
Normally distributed variables are presented as the mean±SD, and non-normally distributed variables are presented as the median and interquartile range. NT-proBNP concentrations were logarithmically transformed for the variation analyses. We fitted mixed-effects models with random intercepts to calculate the between-person coefficient of variation across the cohort (CVG), the within-person coefficient of variation at weekly and monthly intervals (CVI), and the within-run analytic coefficient of variation (CVA). Outlying variances were excluded using the Reed and Cochran tests. Linear regression was used to identify and exclude participants who demonstrated a consistent increase or decrease in log NT-proBNP concentrations throughout the study, because such a trend may represent a change in future risk that may not have manifest clinically during the study.
The cohort was also divided into eight subgroups according to dialysis modality, hydration status, ischemic heart disease status, severity of left ventricular diastolic dysfunction, presence or absence of left ventricular systolic dysfunction and left ventricular hypertrophy, tertiles of C-reactive protein concentrations, and quartiles of NT-proBNP concentrations at enrollment. CVI was estimated for each subgroup and compared using Bartlett’s test. Overhydration was assessed using the ratio of absolute overhydration volume to total extracellular volume measured using bioimpedance and categorized as absent (<6.8%), moderate (6.8%–15%), or severe (>15%) (29). Ischemic heart disease was defined as any of inducible ischemia on noninvasive cardiac stress testing and/or ≥50% stenosis in ≥1 epicardial coronary artery on coronary angiography and/or a history of myocardial infarction. Left ventricular diastolic dysfunction (30) and left ventricular hypertrophy (31) were graded as absent, mild, moderate, or severe according to established algorithms using echocardiographic measurements. Left ventricular systolic dysfunction was defined as a left ventricular ejection fraction ≤50% using Simpson’s rule (30).
The index of individuality (IOI) was calculated as CVI/CVG. This ratio gives an indication of whether a biomarker is best used within a relative-change monitoring strategy (IOI <0.6) or a reference interval strategy (IOI>0.6). The bidirectional reference change value (RCV) was calculated according to the method described by Fokkema et al. (32) for logarithmically transformed data as = exp(–Z×√2×σ) to exp(+Z×√2×σ), where σ=√ln(CVi2+1) and Z is the Z score of a standard normal distribution corresponding to a given probability.
Results
Patient Characteristics
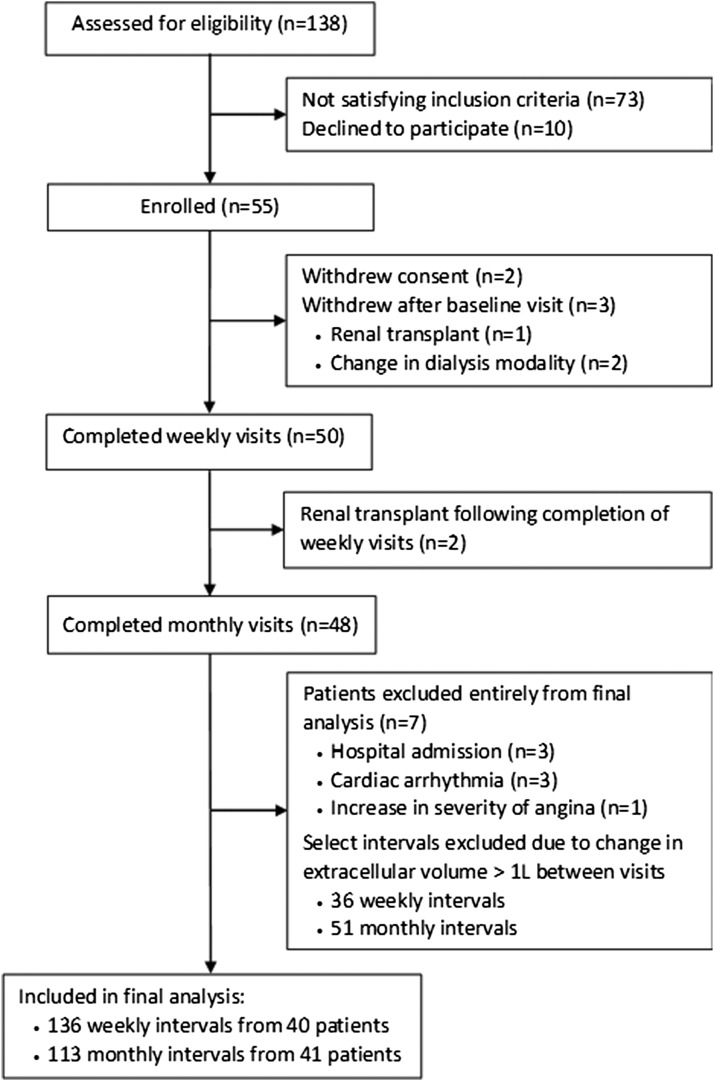
Details of the number of patients assessed, enrolled, and included in the final analysis are shown in Figure 1. Fifty-five patients were recruited from the hemodialysis (n=28) and peritoneal dialysis (n=27) units of the participating institutions and their baseline characteristics are summarized in Table 1. Cardiovascular risk factors, including hypertension (100%), diabetes mellitus (40%), and current or former smoking (51%) were highly prevalent. A substantial proportion of the cohort also had evidence of established cardiovascular disease including ischemic heart disease (22%), left ventricular hypertrophy (44%), diastolic (87%) and/or systolic (9%) dysfunction, and peripheral vascular disease and/or cerebrovascular disease (9%). Baseline NT-proBNP concentrations demonstrated a right-skewed frequency distribution with a median of 1698 pg/ml (interquartile range, 718–3742). Ninety-three percent of the study cohort had a NT-proBNP concentration >300 pg/ml, the threshold used to exclude acute decompensated heart failure in the general population (33).
Figure 1.
Flow diagram of patients assessed for eligibility, enrolled, and analyzed in the study.
Table 1.
Baseline characteristics of the study cohort
| Characteristic | Value (n=55) |
|---|---|
| Men (%) | 45 |
| Age | |
| Mean±SD (yr) | 59±15 |
| Age distribution (%) | |
| 18–49 | 33 |
| 50–69 | 40 |
| 70–79 | 22 |
| 80–90 | 5 |
| Hemodialysis (%) | 51 |
| Dialysis sessions per week (n) | 3 |
| Duration of dialysis session (h) | 5.1±0.7 |
| Single pool Kt/V | 1.73±0.38 |
| Interdialytic weight gain (kg) | 1.7 (1.5–2.3) |
| Interdialytic weight gain relative to estimated dry weight (%) | 2.3±1 |
| Peritoneal dialysis (%) | 49 |
| Volume of peritoneal dialysis solution exchanged per 24 h (L) | 8 (8–10) |
| 4-h D/P creatinine | 0.72±0.52 |
| Weekly Kt/V | 2.21 (1.95–2.49) |
| Time on dialysis (mo) | 35 (16–58) |
| Body mass index (kg/m2) | 30.2 (28.5–34.6) |
| Systolic BP (mmHg) | 130±15 |
| Diastolic BP (mmHg) | 74±12 |
| Diabetes mellitus (%) | 40 |
| Current or former smoker (%) | 51 |
| Ischemic heart disease (%) | 22 |
| Peripheral vascular disease and/or cerebrovascular disease (%) | 9 |
| Hydration status | |
| Ratio of extracellular to total body water | 0.48±0.04 |
| Overhydration volume relative to extracellular volume (%) | 3±9 |
| Normohydrated (%) | 69 |
| Moderately overhydrated (%) | 21 |
| Severely overhydrated (%) | 10 |
| Left ventricular structure and function | |
| Left ventricular hypertrophy (%) | |
| Nil | 56 |
| Mild | 33 |
| Moderate | 11 |
| Ejection fraction (%) | 60±7 |
| Left ventricular systolic dysfunction (%) | 9 |
| Diastolic dysfunction (%) | |
| Nil | 13 |
| Mild | 24 |
| Moderate | 45 |
| Severe | 18 |
| Antihypertensive agents | |
| Median number of antihypertensive agents | 1 (1–2) |
| Proportion taking β-blockers (%) | 42 |
| Proportion taking ACEIs or ARBs (%) | 38 |
| C-reactive protein (mg/L) | 5.0 (2.1–12) |
| NT-proBNP (pg/ml) | |
| Median (interquartile range) | 1698 (718–3742) |
| Distribution (%) | |
| <300 | 7 |
| 300–899 | 20 |
| 900–4999 | 51 |
| 5000–19,999 | 11 |
| ≥20,000 | 11 |
D/P creatinine, ratio of creatinine concentration in dialysate to plasma; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin type 1 receptor blocker; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
Weekly and Monthly Variation of NT-proBNP
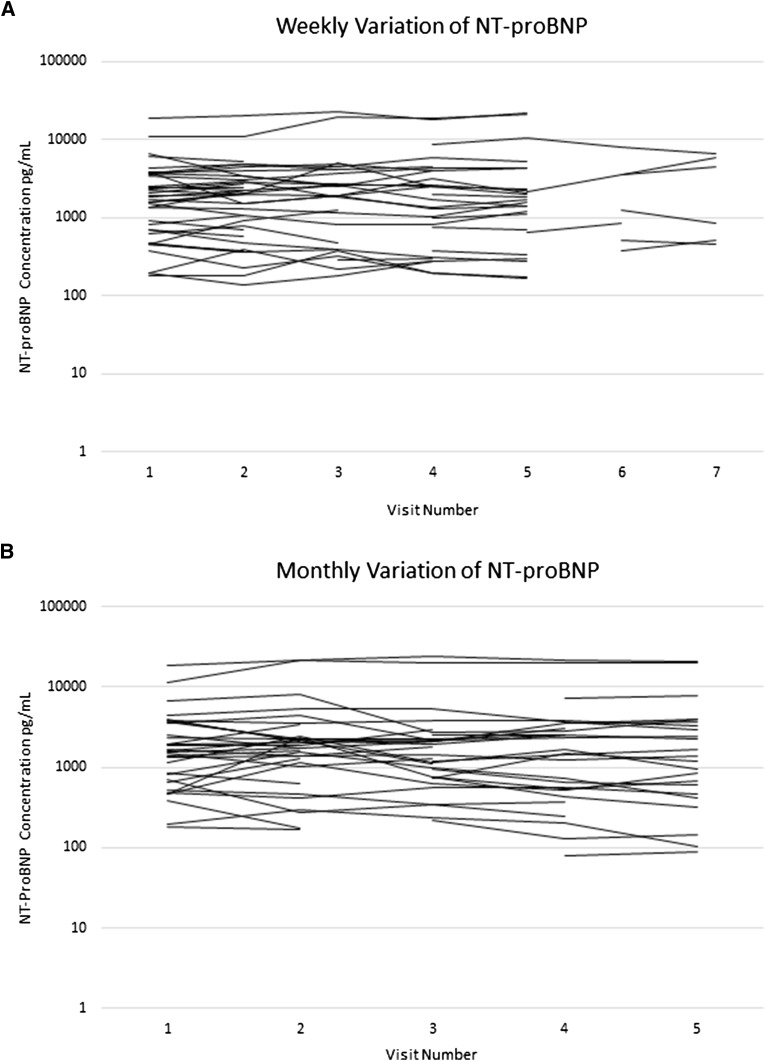
Seven patients and their corresponding NT-proBNP measurements were excluded due to hospital admission (n=3), paroxysmal atrial fibrillation (n=3), and escalating anginal symptoms (n=1). In addition, 36 weekly and 51 monthly NT-proBNP sample pairs were excluded due to a change in extracellular volume of >1 L between consecutive visits. None of the participants were excluded on the basis of outlying variances or the linear regression analysis. NT-proBNP measurements made over 136 weekly intervals from 40 patients and 113 monthly intervals from 41 patients were included in the final analysis. These data are shown in Figure 2 with excluded measurements represented by gaps.
Figure 2.
Variation of N-terminal pro–B-type natriuretic peptide (NT-proBNP) concentrations measured from each of the stable participants over the course of the study. (A) Weekly and (B) monthly follow-up phases. Gaps represent measurements excluded due to a change in extracellular volume >1L between consecutive visits.
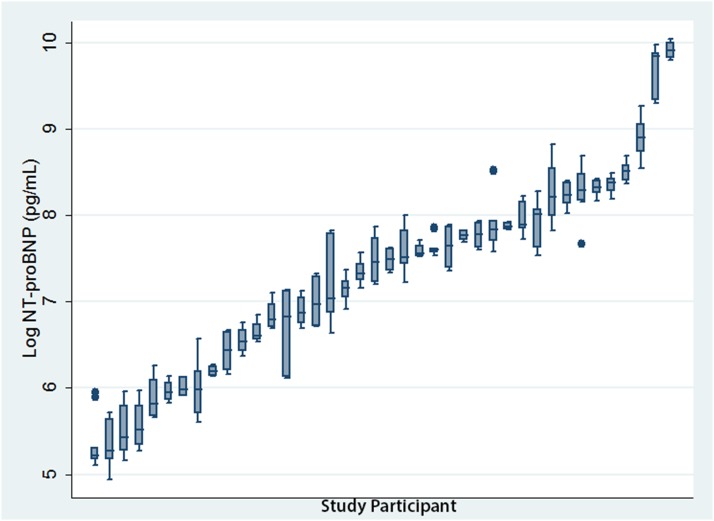
The respective analytic, within-person, and between-person coefficients of variation of NT-proBNP were 1.9%, 27%, and 153% for weekly intervals, and 1.6%, 35%, and 148% for monthly intervals (Table 2). Between-person variation was much greater than within-person variation (Figure 3), yielding low indices of individuality of 0.18 and 0.24 for the weekly and monthly intervals, respectively (Table 2).
Table 2.
Estimates of variance components of NT-proBNP, bidirectional reference change values for stated degrees of statistical confidence, and index of individuality over weekly and monthly intervals for stable study participants
| Interval | Coefficient of Variation (%) | Reference Change Value | Index of Individuality | ||||
|---|---|---|---|---|---|---|---|
| Analytic (CVA) | Between-Person (CVG) | Within-Person (CVI) | 90% | 80% | 70% | CVI:CVG | |
| Weekly | 1.9 | 153 | 27 | −46% and +84% | −38% and +61% | −32% and +47% | 0.18 |
| Monthly | 1.6 | 148 | 35 | −54% and +119% | −46% and +84% | −39% and +64% | 0.24 |
CVA, analytic coefficient of vatiation; CVG, between-person coefficient of variation; CVI, within-person coefficient of variation.
Figure 3.
Box plot of NT-proBNP concentrations for stable participants during the weekly follow-up phase plotted in ascending order of median NT-proBNP concentrations. Each box plot depicts five logarithmically transformed NT-proBNP concentrations taken from a single stable participant over the first 5 weeks of the study. The whiskers delimit the range and the middle, lower, and upper lines of the box represent the median, upper, and lower quartiles respectively. Between-person variation is reflected in the variation between median NT-proBNP concentrations across the entire cohort and was large. By comparison, within-person variation depicted by the box plots was much smaller and largely uniform between patients.
Weekly and monthly RCVs for the 70%, 80%, and 90% degrees of statistical confidence are shown in Table 2. Thus, NT-proBNP concentrations measured at weekly intervals needed to increase by 84% or decrease by 46% to ensure with 90% confidence that the observed change exceeded analytic and biologic variation alone. Monthly RCVs were slightly larger than weekly values for a given degree of statistical confidence.
The within-person coefficient of variation did not differ significantly between dialysis modalities; by ischemic heart disease, hydration, or inflammatory status; by severity of diastolic dysfunction; by presence or absence of left ventricular hypertrophy; or across quartiles of NT-proBNP concentration (Table 3). The effect of left ventricular systolic dysfunction on within-person variation was unable to be meaningfully analyzed because only one such patient was retained in the final analysis after the exclusion of unstable patients.
Table 3.
Within-person coefficients of variation of NT-proBNP by subgroups of dialysis modality, ischemic heart disease status, left ventricular hypertrophy, cardiac diastolic function, hydration status, tertiles of C-reactive protein concentration, and quartiles of NT-proBNP concentration
| Subgroup | Weekly Within-Person Coefficient of Variation, CVI (%) | P Value |
|---|---|---|
| Dialysis modality | ||
| Peritoneal dialysis | 24.5 (20.5 to 29.2) | 0.45 |
| Hemodialysis | 29.3 (24.5 to 35.2) | |
| Coronary artery disease | ||
| Absent | 29.3 (25.3 to 34.0) | 0.15 |
| Present | 18.8 (14.7 to 24.1) | |
| Left ventricular hypertrophy | ||
| Absent | 28.6 (24.3 to 33.7) | 0.52 |
| Present | 24.3 (19,9 to 29.7) | |
| Diastolic dysfunction | ||
| Nil/mild | 27.3 (22.8 to 32.8) | 0.98 |
| Moderate/severe | 27.1 (22.7 to 32.5) | |
| Hydration status | ||
| Normohydration | 28.4 (24.7 to 32.7) | 0.28 |
| Moderate/severe overhydration | 22.3 (15.9 to 31.3) | |
| C-reactive protein concentration (mg/L) | ||
| First tertile (2–4) | 29.6 (23.9 to 36.7) | 0.43 |
| Second tertile (5–13) | 28.8 (23.3 to 35.7) | |
| Third tertile (14–59) | 20.6 (16.3 to 26.0) | |
| NT-proBNP concentration (pg/ml) | ||
| First quartile (180–718) | 29.4 (22.6 to 38.5) | 0.89 |
| Second quartile (719–1698) | 29.7 (22.6 to 39.0) | |
| Third quartile (1699–3742) | 25.1 (19.6 to 32.1) | |
| Fourth quartile (3743–44,091) | 23.9 (19.0 to 30.2) |
Data are presented as mean (95% confidence interval).
Discussion
This study demonstrated that NT-proBNP concentrations vary considerably across the dialysis population with a between-person coefficient of variation of 158%, markedly greater than that reported for healthy individuals (36%–70%) (34,35). This marked variability likely reflects the broad range of hydration status, residual renal function, dialysis regimens, pharmacotherapies, and the numerous types and severities of cardiac pathologies present in the dialysis population. By contrast, the within-person coefficient of variation of NT-proBNP among stable dialysis patients in this study was markedly smaller, equating to 27% and 35% for the weekly and monthly measurement intervals, respectively.
The large discrepancy between the within- and between-person coefficients of variation has important implications for how NT-proBNP is interpreted in the dialysis population. The ratio of within- to between-person variation is termed the IOI, and in this study equated to 0.18 and 0.24 for the weekly and monthly measurement intervals, respectively. Ratios <0.6 indicate a high degree of individuality and imply that NT-proBNP testing is better applied in the dialysis population using a relative-change strategy wherein serial measurements from the same patient are compared with each other rather than comparing single values to a reference interval or a threshold value (36). This finding represents an important departure from the majority of contemporary studies in this area that have sought to derive an absolute NT-proBNP concentration, below which cardiac dysfunction and/or overhydration can be confidently excluded in a manner analogous to which a NT-proBNP concentration <300 pg/ml is used to exclude acute decompensated heart failure in the nondialysis population (33,37–41). Our findings suggest that such a strategy would be inaccurate for predicting cardiovascular risk in the dialysis population, resulting in unacceptably high false negative or false positive rates depending on the threshold value chosen, while also missing the majority of important changes in serial NT-proBNP concentrations measured from an individual dialysis patient (36). Instead, we suggest that a relative-change strategy would be of greater clinical value by accurately detecting significant changes in serial NT-proBNP concentrations irrespective of the absolute values. Significant changes in NT-proBNP concentrations would likely reflect important changes in composite cardiovascular risk caused by one or more pathophysiologic processes including volume overload, ventricular dysfunction, and/or cardiac ischemia. This hypothesis is supported by longitudinal studies that have demonstrated a direct correlation between change in NT-proBNP concentrations and the risk of fatal cardiovascular events (2–5), change in left ventricular mass (42), and change in surrogate measures of volume status (4,7).
However, not all changes in serial NT-proBNP concentrations are of clinical significance and may instead be attributed to analytic and/or biologic variation. The within-person coefficients of variation estimated in this study will play a crucial role in making this distinction in both the clinical and research settings (10,43,44). Using these estimates, we calculated a bidirectional magnitude of change between serial NT-proBNP measurements that excludes change due to biologic and analytic variation alone for a prespecified degree of statistical confidence—the RCV (32,43). The 90% RCV for NT-proBNP measured at weekly intervals was −46% and +84%, implying that serial NT-proBNP concentrations must increase by at least 84% or decrease by at least 46% to exclude change due to biologic and analytic variation alone with 90% certainty. Reducing the degree of statistical confidence reduces the magnitude of change required in either direction and increases the sensitivity but reduces the specificity for detecting a clinically important change. The degree of statistical confidence used is dictated by whether a change in biomarker levels is intended to rule in a given medical condition (high specificity required) or rule it out (high sensitivity required). Although the precise role of NT-proBNP monitoring in the dialysis population has yet to be absolutely defined, we hypothesize that it will primarily be used to identify patients at risk of major adverse cardiac events who require further evaluation to determine which pathophysiologic process is responsible. A lower degree of statistical confidence would be suitable for such a strategy because neither diagnosis nor therapy would be solely predicated on changing NT-proBNP concentrations alone. Finally, we found that the within-person coefficient of variation of NT-proBNP was not significantly different between dialysis modalities; by ischemic heart disease, inflammatory, or hydration status; by severity of diastolic dysfunction; by presence or absence of left ventricular hypertrophy; or across quartiles of baseline NT-proBNP concentrations, implying that a single decision limit can be applied across the entire dialysis population.
Our estimates of within-person variation are supported by the findings of Aakre et al. (35), who recently reported a within-person coefficient of variation of 26% for NT-proBNP measured at weekly intervals from a cohort of 17 hemodialysis patients. This study was limited by its small sample size, the exclusion of peritoneal dialysis patients, and a lack of rigor in ensuring patient stability, wherein the investigators relied either on patient report or a history of hospitalization alone to determine stability and did not measure volume status. As previously described, NT-proBNP concentrations may be affected by volume status, cardiac arrhythmias, the dialysis prescription, and changes in angina class that may not necessarily warrant admission. Ensuring stability of all of these parameters is essential to deriving accurate estimates of within-person variation.
Our study has a number of strengths that address these limitations, including the use of a rigorous approach to ensure the stability of all factors that influence NT-proBNP concentrations, the enrollment of both peritoneal dialysis and hemodialysis patients, measurement of NT-proBNP over both weekly and monthly intervals, and a larger sample size. Nevertheless, our study has several potential limitations. First, patients with the most severe cardiovascular comorbidities were excluded to maximize the likelihood that patients would remain stable for the duration of follow-up. Although this potentially limits the generalizability of the study’s findings, the baseline characteristics of our study cohort were similar to those reported for prevalent Australasian dialysis patients in the Australian and New Zealand Dialysis and Transplant Registry (45). Second, mean hemodialysis session duration in this study was considerably longer than that reported by most North American centers (46). Whether hemodialysis session length affects the within-person variation of NT-proBNP could not be investigated in this study due to the homogeneity of dialysis prescriptions and should be investigated by future studies before these results are applied to populations receiving shorter hemodialysis sessions. Third, we were unable to determine whether the within-person coefficient of variation of NT-proBNP was affected by left ventricular systolic impairment as only one such patient remained stable during the study. Finally, RCVs only exclude biologic and analytic variation, and changes in biomarker concentrations exceeding the RCV are not automatically of clinical significance.
NT-proBNP holds much promise as a biomarker of cardiovascular risk in the dialysis population; however, much remains to be established before it can be adopted into clinical practice. This study represents an important step in that direction. On the basis of these findings, we suggest that NT-proBNP testing may be better applied in the dialysis population using a relative-change strategy rather than by comparing absolute values to a reference interval or threshold value, and that large changes in NT-proBNP concentrations are needed to confidently exclude change due to biologic variation alone. Moving forward, longitudinal cohort studies are needed to determine the accuracy of serial NT-proBNP testing for predicting adverse cardiovascular events in the dialysis population and potential targets for therapeutic intervention.
Disclosures
Fresenius Medical Care Asia-Pacific provided an unrestricted equipment loan and Roche Diagnostics Australia provided unrestricted reagent support for this study. They played no part in conceiving, designing, analyzing or preparing the manuscript for this study.
Acknowledgments
We acknowledge the nephrologists, nurses, and dialysis patients at the Princess Alexandra and Logan Hospitals for their support throughout the study.
This study was funded in part by a research project grant from Kidney Health Australia. M.A.F. is a recipient of a National Health and Medical Research Council of Australia Postgraduate Research Scholarship, and D.W.J. is a current recipient of a Queensland Health Research Fellowship.
This study was presented at the Young Investigator of the Year award session of the Australia and New Zealand Society of Nephrology Annual Scientific Meeting, held September 9–11, 2013, in Brisbane, Australia.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Mair J: Biochemistry of B-type natriuretic peptide—where are we now? Clin Chem Lab Med 46: 1507–1514, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Breidthardt T, Kalbermatter S, Socrates T, Noveanu M, Klima T, Mebazaa A, Mueller C, Kiss D: Increasing B-type natriuretic peptide levels predict mortality in unselected haemodialysis patients. Eur J Heart Fail 13: 860–867, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Winkler K, Wanner C, Drechsler C, Lilienthal J, März W, Krane V, German Diabetes and Dialysis Study Investigators : Change in N-terminal-pro-B-type-natriuretic-peptide and the risk of sudden death, stroke, myocardial infarction, and all-cause mortality in diabetic dialysis patients. Eur Heart J 29: 2092–2099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutiérrez OM, Tamez H, Bhan I, Zazra J, Tonelli M, Wolf M, Januzzi JL, Chang Y, Thadhani R: N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations in hemodialysis patients: Prognostic value of baseline and follow-up measurements. Clin Chem 54: 1339–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Paniagua R, Amato D, Mujais S, Vonesh E, Ramos A, Correa-Rotter R, Horl WH: Predictive value of brain natriuretic peptides in patients on peritoneal dialysis: Results from the ADEMEX trial. Clin J Am Soc Nephrol 3: 407–415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth J, Pinney J, Davenport A: N-terminal proBNP—marker of cardiac dysfunction, fluid overload, or malnutrition in hemodialysis patients? Clin J Am Soc Nephrol 5: 1036–1040, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chazot C, Vo-Van C, Zaoui E, Vanel T, Hurot JM, Lorriaux C, Mayor B, Deleaval P, Jean G: Fluid overload correction and cardiac history influence brain natriuretic peptide evolution in incident haemodialysis patients. Nephrol Dial Transplant 26: 2630–2634, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Parfrey PS: BNP in hemodialysis patients. Clin J Am Soc Nephrol 5: 954–955, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Fraser C: The nature of biological variation. In: Biological Variation: From Principles to Practice, Washington, DC, AACC Press, 2001, p 1–27 [Google Scholar]
- 10.Doust J: Qualification versus validation of biomarkers. Scand J Clin Lab Invest Suppl 242: 40–43, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Fraser CG, Harris EK: Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 27: 409–437, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Fagugli RM, Palumbo B, Ricciardi D, Pasini P, Santirosi P, Vecchi L, Pasticci F, Palumbo R: Association between brain natriuretic peptide and extracellular water in hemodialysis patients. Nephron Clin Pract 95: c60–c66, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Shin DI, Jaekel K, Schley P, Sause A, Müller M, Fueth R, Scheffold T, Guelker H, Horlitz M: Plasma levels of NT-pro-BNP in patients with atrial fibrillation before and after electrical cardioversion. Z Kardiol 94: 795–800, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA: N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA 297: 169–176, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber M, Dill T, Arnold R, Rau M, Ekinci O, Müller KD, Berkovitsch A, Mitrovic V, Hamm C: N-terminal B-type natriuretic peptide predicts extent of coronary artery disease and ischemia in patients with stable angina pectoris. Am Heart J 148: 612–620, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Sommerer C, Heckele S, Schwenger V, Katus HA, Giannitsis E, Zeier M: Cardiac biomarkers are influenced by dialysis characteristics. Clin Nephrol 68: 392–400, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Stanek B, Frey B, Hülsmann M, Berger R, Sturm B, Strametz-Juranek J, Bergler-Klein J, Moser P, Bojic A, Hartter E, Pacher R: Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol 38: 436–442, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Latini R, Masson S, Anand I, Judd D, Maggioni AP, Chiang YT, Bevilacqua M, Salio M, Cardano P, Dunselman PH, Holwerda NJ, Tognoni G, Cohn JN, Valsartan Heart Failure Trial Investigators : Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: The Valsartan Heart Failure Trial (Val-HeFT). Circulation 106: 2454–2458, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Brunner-La Rocca HP, Weilenmann D, Kiowski W, Maly FE, Candinas R, Follath F: Within-patient comparison of effects of different dosages of enalapril on functional capacity and neurohormone levels in patients with chronic heart failure. Am Heart J 138: 654–662, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Campeau L: Letter: Grading of angina pectoris. Circulation 54: 522–523, 1976 [PubMed] [Google Scholar]
- 21.Ho KK, Pinsky JL, Kannel WB, Levy D: The epidemiology of heart failure: The Framingham Study. J Am Coll Cardiol 22[Suppl A]: 6A–13A, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, Korth O, Müller MJ, Ellegård L, Malmros V, Kaitwatcharachai C, Kuhlmann MK, Zhu F, Fuller NJ: Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 27: 921–933, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kraemer M, Rode C, Wizemann V: Detection limit of methods to assess fluid status changes in dialysis patients. Kidney Int 69: 1609–1620, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Nowatzke WL, Cole TG: Stability of N-terminal pro-brain natriuretic peptide after storage frozen for one year and after multiple freeze-thaw cycles. Clin Chem 49: 1560–1562, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Sokoll LJ, Baum H, Collinson PO, Gurr E, Haass M, Luthe H, Morton JJ, Nowatzke W, Zingler C: Multicenter analytical performance evaluation of the Elecsys proBNP assay. Clin Chem Lab Med 42: 965–972, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Mueller T, Gegenhuber A, Dieplinger B, Poelz W, Haltmayer M: Long-term stability of endogenous B-type natriuretic peptide (BNP) and amino terminal proBNP (NT-proBNP) in frozen plasma samples. Clin Chem Lab Med 42: 942–944, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Clerico A, Zaninotto M, Prontera C, Giovannini S, Ndreu R, Franzini M, Zucchelli GC, Plebani M, Study Group on Cardiovascular Risk Biomarkers of the Italian Society of Clinical Biochemistry : State of the art of BNP and NT-proBNP immunoassays: The CardioOrmoCheck study. Clin Chim Acta 414: 112–119, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Røraas T, Petersen PH, Sandberg S: Confidence intervals and power calculations for within-person biological variation: Effect of analytical imprecision, number of replicates, number of samples, and number of individuals. Clin Chem 58: 1306–1313, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D: The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 24: 1574–1579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ: Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA 289: 194–202, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee. European Association of Echocardiography : Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Fokkema MR, Herrmann Z, Muskiet FA, Moecks J: Reference change values for brain natriuretic peptides revisited. Clin Chem 52: 1602–1603, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Kim HN, Januzzi JL, Jr: Natriuretic peptide testing in heart failure. Circulation 123: 2015–2019, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Wu AH, Smith A, Wieczorek S, Mather JF, Duncan B, White CM, McGill C, Katten D, Heller G: Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol 92: 628–631, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Aakre KM, Røraas T, Petersen PH, Svarstad E, Sæle K, Sandberg S: Week-to-week biological variation in the N-terminal prohormone of brain natriuretic peptide in hemodialysis patients and healthy individuals. Clin Chem 59: 1813–1814, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Iglesias N, Petersen PH, Ricós C: Power function of the reference change value in relation to cut-off points, reference intervals and index of individuality. Clin Chem Lab Med 43: 441–448, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Mallamaci F, Zoccali C, Tripepi G, Benedetto FA, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Stancanelli B, Malatino LS, CREED Investigators. The Cardiovascular Risk Extended Evaluation : Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int 59: 1559–1566, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Helal I, Belhadj R, Mohseni A, Bazdeh L, Drissa H, Elyounsi F, Abdallah TB, Abdelmoula J, Kheder A: Clinical significance of N-terminal Pro-B-type natriuretic peptide (NT-proBNP) in hemodialysis patients. Saudi J Kidney Dis Transpl 21: 262–268, 2010 [PubMed] [Google Scholar]
- 39.David S, Kümpers P, Seidler V, Biertz F, Haller H, Fliser D: Diagnostic value of N-terminal pro-B-type natriuretic peptide (NT-proBNP) for left ventricular dysfunction in patients with chronic kidney disease stage 5 on haemodialysis. Nephrol Dial Transplant 23: 1370–1377, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Wang AY-M, Lam CW-K, Wang M, Chan IH-S, Lui S-F, Zhang Y, Sanderson JE: Diagnostic potential of serum biomarkers for left ventricular abnormalities in chronic peritoneal dialysis patients. Nephrol Dial Transplant 24: 1962–1969, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Lee SW, Song JH, Kim GA, Lim HJ, Kim MJ: Plasma brain natriuretic peptide concentration on assessment of hydration status in hemodialysis patient. Am J Kidney Dis 41: 1257–1266, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Choi SY, Lee JE, Jang EH, Kim MO, Baek H, Ki CS, Park SW, Kim DJ, Huh WS, Oh HY, Kim YG: Association between changes in N-terminal pro-brain natriuretic peptide levels and changes in left ventricular mass index in stable hemodialysis patients. Nephron Clin Pract 110: c93–c100, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Fraser C: Changes in serial results. In: Biological Variation: From Principles to Practice, Washington, DC, AACC Press, 2001, p 67–90 [Google Scholar]
- 44.Barnett AG, van der Pols JC, Dobson AJ: Regression to the mean: What it is and how to deal with it. Int J Epidemiol 34: 215–220, 2005 [DOI] [PubMed] [Google Scholar]
- 45.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR: Relationship between dialysis modality and mortality. J Am Soc Nephrol 20: 155–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tentori F, Zhang J, Li Y, Karaboyas A, Kerr P, Saran R, Bommer J, Port F, Akiba T, Pisoni R, Robinson B: Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 27: 4180–4188, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]