Abstract
Alport syndrome is an inherited disease characterized by progressive renal failure, hearing loss, and ocular abnormalities. Mutations in the COL4A5 (X-linked), or COL4A3 and COL4A4 (autosomal recessive) genes result in absence of the collagen IV α3α4α5 network from the basement membranes of the cornea, lens capsule, and retina and are associated with corneal opacities, anterior lenticonus, fleck retinopathy, and temporal retinal thinning. Typically, these features do not affect vision or, in the case of lenticonus, are correctable. In contrast, the rarer ophthalmic complications of posterior polymorphous corneal dystrophy, giant macular hole, and maculopathy all produce visual loss. Many of the ocular features of Alport syndrome are common, easily recognizable, and thus, helpful diagnostically, and in identifying the likelihood of early-onset renal failure. Lenticonus and central fleck retinopathy strongly suggest the diagnosis of Alport syndrome and are associated with renal failure before the age of 30 years, in males with X-linked disease. Sometimes, ophthalmic features suggest the mode of inheritance. A peripheral retinopathy in the mother of a male with hematuria suggests X-linked inheritance, and central retinopathy or lenticonus in a female means that recessive disease is likely. Ocular examination, retinal photography, and optical coherence tomography are widely available, safe, fast, inexpensive, and acceptable to patients. Ocular examination is particularly helpful in the diagnosis of Alport syndrome when genetic testing is not readily available or the results are inconclusive. It also detects complications, such as macular hole, for which new treatments are emerging.
Keywords: Alport syndrome, genetic renal disease, extracellular matrix
Introduction
Alport syndrome is characterized by hematuria, progressive renal failure, hearing loss, and ocular abnormalities affecting the cornea, lens, and retina (1,2). Corneal scarring, temporal retinal thinning, giant macular hole, and maculopathy are recently described features that extend the ophthalmic phenotype (Figures 1–3) (3–7).
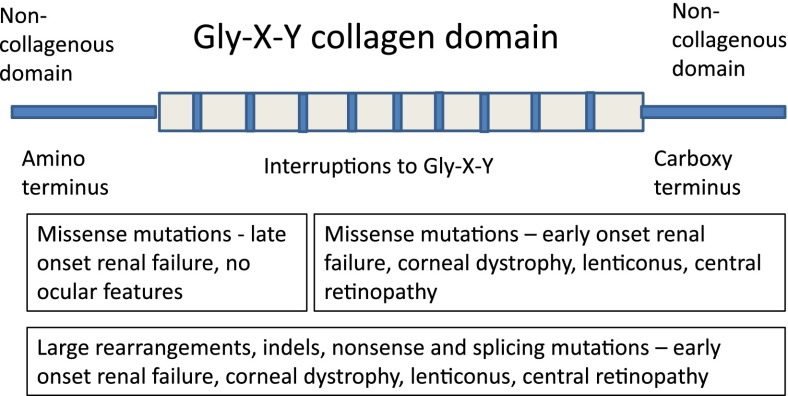
Figure 1.
Collagen IV molecule—location and nature of mutations causing X-linked Alport syndrome.
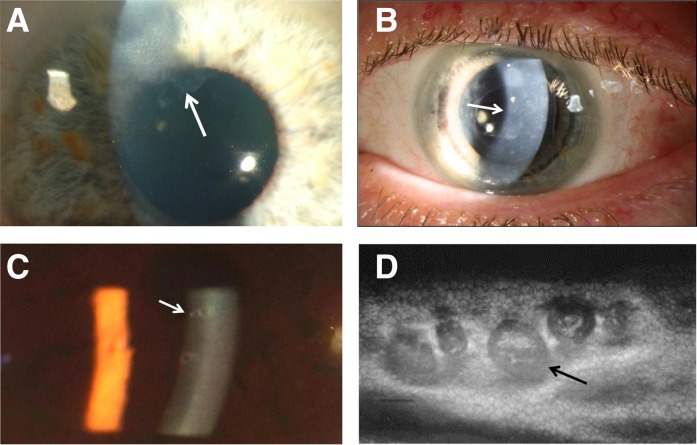
Figure 3.
Lens abnormalities. (A) Lenticonus appearance on slit-lamp examination showing an anterior dimple or oil droplet (arrow) in a man with X-linked Alport syndrome, renal impairment, and posterior polymorphous corneal dystrophy. The oil droplet is also obvious on direct ophthalmoscopy. (B) Anterior segment view showing the anterior bulging of the lens (arrows) with a Scheimpflug camera (Pentacam) in the patient from A. (C) Electron microscopy of an anterior lens capsule obtained at surgery showing the thinned capsule (arrow) and vertical tears (upper panel) compared with normal (arrow in lower panel). The abnormal lens was from a man with X-linked Alport syndrome, renal failure, lenticonus, and retinopathy.
Alport syndrome affects at least one in 10,000 individuals, and the diagnosis is important because of the risk of disease in other family members; also, early treatment with angiotensin-converting enzyme inhibitors delays the onset of end stage renal failure (8,9).
Inheritance of Alport syndrome is X-linked in nearly all families (85%), and mutations affect the COL4A5 gene, which codes for the collagen IV α5-chain (10,11). The diagnosis of X-linked Alport syndrome is often overlooked, especially in women, who are affected three times as often as men. Inheritance in the other 15% of Alport families is autosomal recessive with homozygous or compound heterozygous mutations in trans in the COL4A3 or COL4A4 gene, which corresponds to the collagen IV α3- or α4-chain (12,13). The occurrence of an autosomal dominant form of Alport syndrome is controversial. Thin basement membrane nephropathy represents the carrier state for autosomal recessive Alport syndrome, and affected individuals have a heterozygous COL4A3 or COL4A4 mutation (13,14) but no ocular or other extrarenal abnormalities (15).
The characteristic ocular features of Alport syndrome are corneal opacities, anterior lenticonus and cataract, central perimacular and peripheral coalescing fleck retinopathies, and temporal retinal thinning. Rarely, posterior polymorphous corneal dystrophy, a macular hole, or a maculopathy impairs vision. Lenticonus, corneal dystrophy, central and peripheral fleck retinopathies, temporal retinal thinning, and giant macular hole are all highly suspicious for the diagnosis of Alport syndrome.
Biochemistry of Collagen Type IV
Collagen IV is the most abundant protein found in basement membranes and is responsible for the membrane’s strength and integrity. It also contributes to many biologic functions through its interactions with other proteins and cells (16).
Collagen IV occurs as three heterotrimers (α1α1α2, α3α4α5, and α5α5α6) that form distinct networks (17). Individual chains have an intermediate collagenous sequence with glycine as every third amino acid, because it is the only residue small enough to fit inside the collagen helix. The collagen IV α1α1α2 network predominates in embryonic membranes and the adult vasculature. It is replaced by the α3α4α5 network in the adult glomerulus (glomerular basement membrane), cochlea (stria vascularis), cornea (Descemet’s and Bowman’s membranes) (18), lens capsule, and retina (inner limiting membrane and Bruch’s membrane) (19), and by the α5α5α6 network in the skin (17).
Collagen Mutations
In total, >1200 unique variants have been described in X-linked Alport syndrome (www.LOVD.nl). They are missense (40%), nonsense mutations and complex changes that result in a downstream nonsense change (40%), and splice site mutations (10%) (20). Missense mutations typically produce misfolded proteins that are retained within the endoplasmic reticulum and destroyed by the unfolded protein response (21). Nonsense mutations, such as for other inherited collagen diseases, probably result in nonsense-mediated decay, where most of the corresponding mRNA is degraded (21). Both missense and nonsense COL4 mutations have a positive-negative effect, causing the loss of not only the corresponding collagen chain but also, those with which it normally forms the α3α4α5 heterotrimer.
The loss of the collagen IV α3α4α5 network and the persistence of the immature α1α1α2 network produce abnormal membranes and the clinical features characteristic of Alport syndrome. The α1α1α2 network is less structurally sound with fewer intra- and interheterotrimer cross-links (22,23) and more proteolytic cleavage sites than the α3α4α5 network (16). It is also more susceptible to biomechanical strain (24), which is exaggerated by the overproduction of ectopic laminin chains (25), and induces matrix metalloproteinase activity (26–28).
Genotype-Phenotype Correlations
In X-linked Alport syndrome, the clinical phenotype depends on both the location of the mutation and the nature of the substituting residue (29–32) (Figure 1). Missense mutations near the carboxy terminus, large rearrangements, insertions and deletions, and nonsense mutations typically result in early-onset renal failure (before the age of 30 years), hearing loss, lenticonus, and retinopathy (29–31). Single-nucleotide substitutions that replace glycine with more highly charged or larger residues (arginine, aspartic, or glutamic acid) also produce a more severe phenotype (32). Missense mutations near the amino terminus usually result in mild disease. A genotype-phenotype correlation is less obvious in females with X-linked Alport syndrome because of the effects of Lyonization (33), but the same rules for phenotype severity appear to apply for autosomal recessive and X-linked inheritance (34).
Cornea: Recurrent Corneal Ulcers, Corneal Clouding, and Posterior Polymorphous Corneal Dystrophy
Corneal disease is recognized infrequently in Alport syndrome. Erosions (3,35) result from an abnormal Bowman’s membrane in the corneal subepithelium (Figure 2A) and posterior polymorphous corneal dystrophy from an abnormal Descemet’s membrane in the subendothelium (Figure 2, B–D). The affected membranes lack the collagen IV α3α4α5 network, are weak, and adhere poorly to the epithelium, endothelium, and underlying stroma (36).
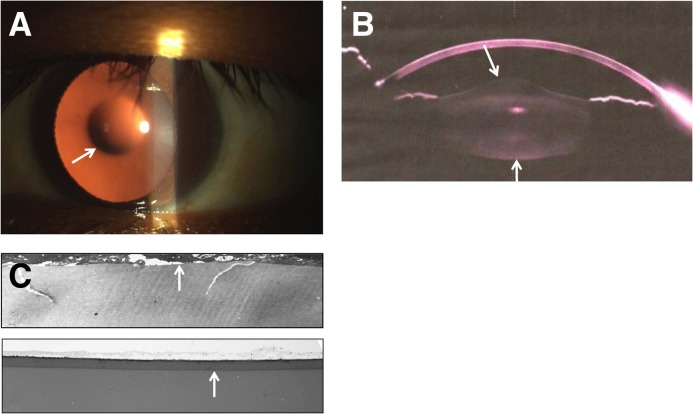
Figure 2.
Corneal abnormalities. (A) Mild scarring caused by recurrent corneal erosions shown on slit-lamp examination in a man with X-linked Alport syndrome (arrow), renal failure, and perimacular retinopathy. The patient’s mother is also affected with renal disease and similar corneal changes. (B) Posterior polymorphous corneal dystrophy (arrow) with diffuse and vesicular lesions posteriorly at the level of Descemet’s membrane on slit-lamp examination in a man with X-linked Alport syndrome, renal failure, lens replacement for lenticonus, and perimacular retinopathy. (C) A slit-lamp view of posterior polymorphous corneal dystrophy showing the characteristic doughnut-like vesicles posteriorly (arrow). (D) Specular microscopy of the corneal endothelium in the patient in C showing that the doughnut-like lesions are vesicles with thick dark borders around clusters of endothelial cells (arrow).
Superficial corneal erosions occur in <10% of patients (3,35) but are intermittent and hence, seem to be less common. Their onset may precede the diagnosis of Alport syndrome and is often in the late teenage years. They typically occur in individuals with early-onset renal failure and extrarenal features. Sometimes, corneal erosions are found in different members of the same family, but they do not appear to be associated with specific mutations.
Erosions are accompanied by episodes of unilateral or bilateral ocular pain occurring spontaneously at night or first thing in the morning, with watering, photophobia, and blurred vision (Figure 2A). Symptoms last 2–5 days and recur (35). Precipitants include working at a computer screen and irritation from the wind or contact lenses. On examination, the eye is red, and there are opacities and vesicles anteriorly at the epithelium on slit-lamp examination (37). Most episodes resolve with supportive measures, such as an eye pad, topical antibiotics, and pain relief, but sometimes, they result in corneal clouding and scars.
Posterior polymorphous corneal dystrophy is rare and more serious than the corneal erosions (Figure 2, B–D) (3,5,38). Again, patients may be asymptomatic, or complain of recurrent episodes of grittiness, watering, and photophobia. The diagnosis is made when multiple clusters of vesicles (“doughnuts”) or bands (“snail tracks”) are demonstrated at the posterior corneal surface on slit-lamp biomicroscopy or with specular microscopy, in vivo confocal microscopy, or high-resolution anterior segment optical coherence tomography (OCT; OCT is a little like ultrasound but uses the reflection of light rather than sound from surfaces in the eye to demarcate different layers) (39). The vesicles result from vacuolar degeneration of dying cells or multilayered epithelial cell protuberances from Descemet’s membrane (40–42). Treatment is usually symptomatic; sometimes, the dystrophy progresses, and corneal transplantation is required.
Corneal erosions are distinguished from posterior polymorphous corneal dystrophy by causing gritty eyes rather than visual loss, being more prevalent, and their location in the anterior cornea.
Lens: Anterior Lenticonus and Cataracts
The demonstration of lenticonus is diagnostic for Alport syndrome (Figure 3). Anterior lenticonus is present in 50% of men, but not women, with X-linked disease, where it is associated with early-onset renal failure and perimacular retinopathy. In contrast, lenticonus is common in both men and women with autosomal recessive inheritance, and therefore, women with Alport syndrome and lenticonus are likely to have recessive disease (43,44).
Lenticonus results from the conical protrusion of the lens anteriorly through the thinnest and weakest part of the capsule (Figure 3B) (45). The absence of the α3α4α5 network from the capsule means that it develops partial splits that may rupture (Figure 3C). Cataracts develop from healing of small spontaneous ruptures (46). Lenticonus ceases to progress after cataract formation (47). Posterior lenticonus also occurs but is less common (48).
Lenticonus is often first seen in early middle age after the onset of renal failure. Patients have progressive difficulty in focusing because of their abnormal lens shape. The diagnosis is made when there is an oil droplet sign on direct ophthalmoscopy or slit-lamp examination (Figure 3A). Lenticonus worsens until visual symptoms require treatment, and most patients eventually require surgery. Treatment for both symptomatic lenticonus and cataract is lens removal and intraocular lens implantation (49). Lenticonus does not recur after lens replacement.
Retina: Central Fleck Retinopathy and Peripheral Coalescing Retinopathy
Common retinal abnormalities include central or perimacular fleck retinopathy and peripheral coalescing fleck retinopathy. They also include manifestations of temporal thinning (4), such as loss of the foveal reflex, a lozenge, disturbances of foveal pigmentation (50), including a bull’s eye or vitelliform maculopathy (7), and lamellar and giant macular hole (6,51) (Figure 4, Table 1).
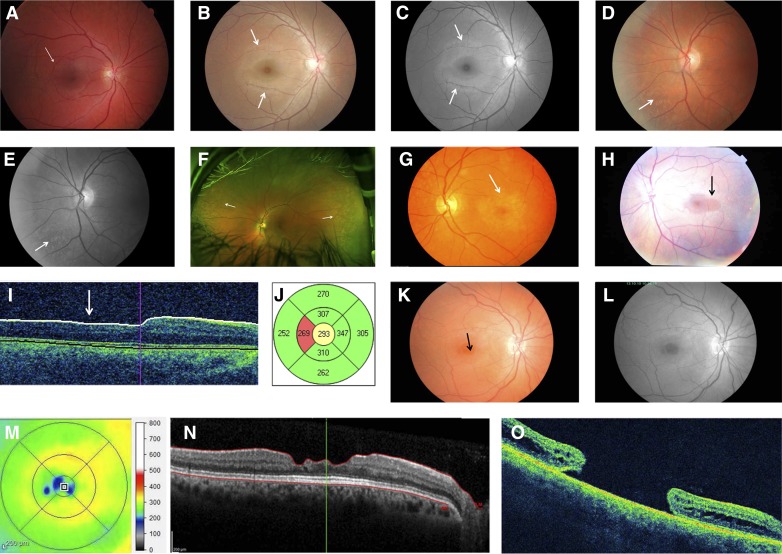
Figure 4.
Retinal abnormalities. (A) Central or perimacular fleck retinopathy sparing the foveola and located principally in the temporal retina (arrow) in a man with X-linked Alport syndrome, renal failure, and lenticonus. (B and C) Perimacular fleck retinopathy in a woman with X-linked Alport syndrome, renal impairment, and hearing loss (arrows). The flecks are pronounced in the temporal retina. They are more obvious and can be distinguished from the normal retinal sheen on the redfree image (arrows). (D and E) Peripheral coalescing fleck retinopathy in a woman with X-linked Alport syndrome, normal renal function, and hearing loss. The flecks are >2 disc diameters from the foveola and more obvious on the redfree image (arrows). (F) Peripheral retinopathy with widespread evenly distributed retinal flecks (arrows) in an ultrawide field scan (Optos camera) in a man with X-linked disease. The lozenge and central fleck retinopathy are not obvious in this view. (G) Pigment disturbance with bull’s eye maculopathy. There is a ring of hypopigmentation with central hyperpigmentation in a woman with X-linked Alport syndrome, renal impairment, and peripheral retinopathy (arrow). (H) Lozenge from temporal extension of the normal round foveolar reflex (arrow) caused by retinal thinning in a man with X-linked Alport syndrome, renal failure, hearing loss, lenticonus, and perimacular fleck retinopathy. (I) Temporal retinal thinning seen on a cross-section of the retina from a man with X-linked Alport syndrome, renal failure, and hearing loss. (J) Temporal thinning confirmed in the patient from I indicating that the temporal thickness is in the <5th percentile (red). (K–N) Retinal shadow suggesting (K) macular hole in a woman with autosomal recessive Alport syndrome, hearing loss, and lenticonus. (L) The holes are more obvious on a black and white image of the macula and temporal retina. (M) The lamellar holes are confirmed with three well demarcated areas of thinning seen on optimal coherence tomography (OCT) (<200-µm thick). (N) These correspond to three areas of lamellar thinning on OCT. (O) Full-thickness giant macular hole in a woman with Alport syndrome and renal failure.
Table 1.
Prevalence of ocular features in X-linked and autosomal recessive Alport syndrome
| Ocular Feature | X-Linked Alport Syndrome (%) | Autosomal Recessive Alport Syndrome (%) | |
|---|---|---|---|
| Men | Women | ||
| Recurrent corneal erosions | <10 | <10 | Not described |
| Posterior polymorphous corneal dystrophy | Rare | Rare | Not described |
| Lenticonus | 50 | <5 | 75 (52) |
| Central or perimacular fleck retinopathy | 70 | 20 | 75 (52) |
| Peripheral retinopathy | 80 | 50 | 75 (52) |
| Temporal retinal thinning | 55 | 30 | 90 |
| Lamellar macular hole | <5 | Not described | <5 |
| Other maculopathies | <5 | <5 | Not described |
Central fleck retinopathy is present in 60% of men and at least 15% of women with X-linked Alport syndrome and 50% of individuals with recessive disease (Figure 4, A–C) (52). It is more common with early-onset renal failure and lenticonus. Nearly all individuals with the central retinopathy also have a peripheral retinopathy, but the converse is not true (53). The central retinopathy varies from scattered whitish-yellow dots and flecks to a dense, almost confluent annulus around the region of temporal retinal thinning. The fleck retinopathy is associated with an abnormal inner limiting membrane (54).
The clear demarcation of the central retinopathy from the foveola is consistent with involvement of the inner limiting membrane/nerve fiber layer. This is not a true membrane but rather, results from fusion of the Muller cell end plates and incorporates the collagen IV α3α4α5 network. The central retinopathy probably represents an abnormality of these end plates. Thinning of the inner limiting membrane/nerve fiber layer may interfere with the nutrition of the overlying cells, removal of debris, and maintenance of the watertight barrier.
Visual acuity is essentially normal, although formal testing of retinal function shows minor abnormalities. The central retinopathy is best seen with color photographs and redfree images centered on the macula. Specialized tests of retinal function, such as electroretinogram and electrooculogram, are normal or nearly normal. The central retinopathy becomes more prominent with time. No treatment is required.
The peripheral fleck retinopathy is the most common retinal abnormality, occurring in most men and 25% of women with X-linked Alport syndrome and most individuals with recessive disease (Figure 4, D–F) (53). The peripheral retinopathy is characterized by asymmetric patches of confluent flecks (54). The fleck location in relation to the blood vessels and appearance on OCT suggest that they result mainly from an abnormality at the level of the retinal pigment epithelium/Bruch’s membrane. The finding of a peripheral retinopathy is a very helpful pointer to the diagnosis of Alport syndrome, especially in women with X-linked disease (53).
The peripheral retinopathy is associated with early-onset renal failure, lenticonus, and central retinopathy but also occurs in women with X-linked disease who have normal renal function (53). It is probably more common than the central retinopathy because of the periphery’s larger surface area.
Visual acuity is, again, normal. The peripheral retinopathy is best seen on ophthalmic examination or with retinal photographs that extend beyond the standard views into the periphery and with redfree retinal images. Again, tests of retinal function are normal (54). No treatment is required.
Temporal Retinal Thinning, Dull Macular Reflex, Lozenge, Foveopathy, and Macular Hole
Temporal retinal thinning is very common in men and women with X-linked Alport syndrome, and with recessive disease (Figure 4, G–J) (4,55). The lozenge (56), dull macular reflex (56,57), foveopathy, and lamellar and macular holes all affect the temporal retina (Figure 4, K–O) (6,51) and reflect retinal thinning of both the inner limiting membrane and Bruch’s membrane (4,19,44).
Temporal thinning is apparent on retinal color photography as the dull macular reflex or lozenge with a larger, more oval shape rather than the normal round foveal reflex. Thinning is confirmed with retinal thickness measurements in the <5th percentile on OCT (55). Although thinning is common in all forms of Alport syndrome and less sensitive diagnostically than a peripheral retinopathy, its demonstration is more objective. Thinning occurs with retinal ischemia but otherwise, not in non-Alport renal failure. Tests of retinal function are normal when there is thinning only. Vision is not affected.
Foveopathy
Hypopigmentation occurs in Alport syndrome but is typically overlooked (58). It is often present together with perimacular flecks or other ocular features. Vision and visual fields are not usually affected. Occasionally, severe forms, such as a bull’s eye or vitelliform retinopathy, are found (7).
Lamellar and Giant Macular Holes
Lamellar or partial-thickness macular holes are uncommon in men with X-linked Alport syndrome and men and women with recessive disease (55). Full-thickness holes are even less common. Macular holes occur at a younger age and are larger (giant holes) than the spontaneous holes in patients who do not have Alport syndrome. Holes may be bilateral, asymmetric, or unilateral. They start with multiple small defects (59) hollowed out from the surface of the inner limiting membrane (6) due to accelerated passage of fluid through the defective Bruch’s membrane, and followed by fusion of the microcysts, when the abnormal membrane breaks down (51). Thus, full-thickness macular holes arise from collagen IV abnormalities in Bruch’s membrane and the internal limiting membrane together with anomalous vitreoretinal traction, retinal detachment (60), and anterior lens capsule rupture (61).
Patients with macular holes have difficulty with central vision and metamorphopsia (where straight lines are distorted). Holes sometimes only become evident when there is no improvement in vision after surgery for lenticonus. Lamellar holes are not necessarily seen on retinal photographs, and OCT is required for their demonstration. They may be confused with a retinal lozenge. Holes in Alport syndrome respond less well to surgical closure and often result in a permanent loss of vision.
Clinical Usefulness of Ophthalmic Features
The ocular features of Alport syndrome are explained by distribution of the collagen IV α3α4α5 network in basement membranes of the eye, mutations that result in the loss of this network, basement membrane thinning, lamellation and rarefaction, and the intraocular mechanical stresses.
Most of the ocular features in Alport syndrome do not affect vision but are useful diagnostically, and in some cases, they suggest the likelihood of early-onset renal failure and the mode of inheritance.
Some ocular features (lenticonus and central and peripheral retinopathy) are common in Alport syndrome, and their presence confirms the diagnosis (Table 2). Retinal temporal thinning is very common and suggests Alport syndrome in an individual with hematuria or renal failure. Posterior polymorphous corneal dystrophy and macular hole are rare but also suggest this diagnosis. Ocular features are less sensitive but more specific than hearing loss in Alport syndrome, because hearing loss occurs with other inherited renal diseases and in dialysis patients.
Table 2.
Usefulness of ocular features in diagnosis, predicting early-onset renal failure, and identifying mode of inheritance
| Ocular Feature | Diagnostic | Severe Mutations | Early-Onset Renal Failure | Distinguish Autosomal Recessive Alport Syndrome from X-Linked Alport Syndrome in Women |
|---|---|---|---|---|
| Posterior polymorphous corneal dystrophy | Yes (38,40) | Yes | Yes | ? |
| Lenticonus | Yes (50) | Yes | Yes | Yes (52) |
| Central retinopathy | Yes | Yes (31) | Yes (31) | Yes (52) |
| Retinal thinning | Yes (55) | No | No | No |
| Giant macular hole | Yes (51) | Yes | Yes | Yes |
| Peripheral retinopathy | Yes (54) | No (31) | No | No (52) |
In addition, ocular features may help distinguish between X-linked and autosomal recessive inheritance. Thus, a peripheral retinopathy in the mother of a boy with hematuria indicates not only the diagnosis of Alport syndrome but also, that inheritance is X-linked. Lenticonus, central retinopathy, and macular hole are rare in women with X-linked disease. Thus, a woman referred for investigation of hematuria who has any of these features is likely to have not only Alport syndrome but also, autosomal recessive inheritance.
Some ocular features are associated with early-onset renal failure in X-linked Alport syndrome. Thus, lenticonus and central retinopathy usually indicate renal failure onset before the age of 30 years. Early-onset renal failure occurs more often with COL4A5 mutations such as large rearrangements, nonsense mutations, etc. Lenticonus and central retinopathy also seem to be more common in autosomal recessive inheritance caused by nonsense mutations (34).
Which Ocular Investigations Should Be Performed?
Why are ocular abnormalities in Alport syndrome often overlooked? The fleck retinopathy does not affect vision and can be subtle or even confused with a youthful retinal sheen. Ophthalmologists see many minor retinal changes in their routine practice, and, unless vision is abnormal, may not report these.
In assessing patients with possible Alport syndrome, it is important to work closely with an interested ophthalmologist who performs a formal ophthalmic examination with slit-lamp examination as well as retinal photography and OCT. These tests are widely available, inexpensive, noninvasive and acceptable to patients. Corneal abnormalities are best seen on slit-lamp examination. Lenticonus appears as a bubble in the red reflex on ophthalmoscopy, although an ophthalmologist will use a retinoscope. The central retinopathy is usually evident on retinal photographs but more obvious with redfree images. The peripheral retinopathy may be obvious on standard retinal views, but peripheral examination may be necessary. OCT demonstrates temporal retinal thinning, especially in men with X-linked disease and individuals with recessive inheritance.
When a boy presents for investigation of Alport syndrome, ocular features are less likely to be present, but his mother should be examined, especially for the peripheral retinopathy.
The recent “Expert Guidelines for the Management of Alport Syndrome and Thin Basement Membrane Nephropathy” recommend that Alport syndrome be diagnosed with genetic testing (62). The finding of any of the ocular features of Alport syndrome, although they rarely interfere with vision, provides additional evidence supporting this request and also, in rare instances, for explaining vision impairment.
Disclosures
None.
Acknowledgments
We thank the many patients and their clinicians who assisted with these studies and in particular, members of the Department of Ophthalmology at the Nair Hospital (Mumbai, India) and Dr. Peter Crowley and Paul Martinello of the Austin Health Department of Anatomical Pathology (Heidelberg, Australia) for the lens electron micrographs.
Some of this work was presented in poster format at the Annual Meeting at Ahmedabad India, February 3–6, 2011.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Colville DJ, Savige J: Alport syndrome. A review of the ocular manifestations. Ophthalmic Genet 18: 161–173, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Savige J, Colville D: Opinion: Ocular features aid the diagnosis of Alport syndrome. Nat Rev Nephrol 5: 356–360, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Rhys C, Snyers B, Pirson Y: Recurrent corneal erosion associated with Alport’s syndrome. Rapid communication. Kidney Int 52: 208–211, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Usui T, Ichibe M, Hasegawa S, Miki A, Baba E, Tanimoto N, Abe H: Symmetrical reduced retinal thickness in a patient with Alport syndrome. Retina 24: 977–979, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bower KS, Edwards JD, Wagner ME, Ward TP, Hidayat A: Novel corneal phenotype in a patient with Alport syndrome. Cornea 28: 599–606, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Rahman W, Banerjee S: Giant macular hole in Alport syndrome. Can J Ophthalmol 42: 314–315, 2007 [PubMed] [Google Scholar]
- 7.Fawzi AA, Lee NG, Eliott D, Song J, Stewart JM: Retinal findings in patients with Alport Syndrome: Expanding the clinical spectrum. Br J Ophthalmol 93: 1606–1611, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Temme J, Kramer A, Jager KJ, Lange K, Peters F, Müller GA, Kramar R, Heaf JG, Finne P, Palsson R, Reisæter AV, Hoitsma AJ, Metcalfe W, Postorino M, Zurriaga O, Santos JP, Ravani P, Jarraya F, Verrina E, Dekker FW, Gross O: Outcomes of male patients with Alport syndrome undergoing renal replacement therapy. Clin J Am Soc Nephrol 7: 1969–1976, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, Höcker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dötsch J, Müller-Wiefel DE, Hoyer P, Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Müller GA, Weber M, Study Group Members of the Gesellschaft für Pädiatrische Nephrologie : Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 81: 494–501, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Feingold J, Bois E, Chompret A, Broyer M, Gubler MC, Grünfeld JP: Genetic heterogeneity of Alport syndrome. Kidney Int 27: 672–677, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K: Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248: 1224–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schröder CH, Smeets HJ, Reeders ST: Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet 8: 77–81, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Buzza M, Wang YY, Dagher H, Babon JJ, Cotton RG, Powell H, Dowling J, Savige J: COL4A4 mutation in thin basement membrane disease previously described in Alport syndrome. Kidney Int 60: 480–483, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Buzza M, Wilson D, Savige J: Segregation of hematuria in thin basement membrane disease with haplotypes at the loci for Alport syndrome. Kidney Int 59: 1670–1676, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Savige J, Rana K, Tonna S, Buzza M, Dagher H, Wang YY: Thin basement membrane nephropathy. Kidney Int 64: 1169–1178, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Parkin JD, San Antonio JD, Pedchenko V, Hudson B, Jensen ST, Savige J: Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum Mutat 32: 127–143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV: Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci 48: 4989–4999, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savige J, Liu J, DeBuc DC, Handa JT, Hageman GS, Wang YY, Parkin JD, Vote B, Fassett R, Sarks S, Colville D: Retinal basement membrane abnormalities and the retinopathy of Alport syndrome. Invest Ophthalmol Vis Sci 51: 1621–1627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertz JM, Thomassen M, Storey H, Flinter F: Clinical utility gene card for: Alport syndrome. Eur J Hum Genet 20: 20, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bateman JF, Boot-Handford RP, Lamandé SR: Genetic diseases of connective tissues: Cellular and extracellular effects of ECM mutations. Nat Rev Genet 10: 173–183, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Reeders ST: The alpha chains of type IV collagen. Contrib Nephrol 117: 80–104, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG: Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of alpha3, alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J Biol Chem 273: 8767–8775, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Kalluri R, Shield CF, Todd P, Hudson BG, Neilson EG: Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest 99: 2470–2478, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrahamson DR, Isom K, Roach E, Stroganova L, Zelenchuk A, Miner JH, St John PL: Laminin compensation in collagen alpha3(IV) knockout (Alport) glomeruli contributes to permeability defects. J Am Soc Nephrol 18: 2465–2472, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF, 3rd, Werb Z, Kalluri R: Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med 3: e100, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao VH, Meehan DT, Delimont D, Nakajima M, Wada T, Gratton MA, Cosgrove D: Role for macrophage metalloelastase in glomerular basement membrane damage associated with Alport syndrome. Am J Pathol 169: 32–46, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meehan DT, Delimont D, Cheung L, Zallocchi M, Sansom SC, Holzclaw JD, Rao V, Cosgrove D: Biomechanical strain causes maladaptive gene regulation, contributing to Alport glomerular disease. Kidney Int 76: 968–976, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Verellen C, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Krejcova S, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC: X-linked Alport syndrome: Natural history in 195 families and genotype- phenotype correlations in males. J Am Soc Nephrol 11: 649–657, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Gross O, Netzer KO, Lambrecht R, Seibold S, Weber M: Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: Impact on clinical counselling. Nephrol Dial Transplant 17: 1218–1227, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Tan R, Colville D, Wang YY, Rigby L, Savige J: Alport retinopathy results from “severe” COL4A5 mutations and predicts early renal failure. Clin J Am Soc Nephrol 5: 34–38, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Persikov AV, Pillitteri RJ, Amin P, Schwarze U, Byers PH, Brodsky B: Stability related bias in residues replacing glycines within the collagen triple helix (Gly-Xaa-Yaa) in inherited connective tissue disorders. Hum Mutat 24: 330–337, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Dahan K, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC: X-linked Alport syndrome: Natural history and genotype-phenotype correlations in girls and women belonging to 195 families: A “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol 14: 2603–2610, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Storey H, Savige J, Sivakumar V, Abbs S, Flinter FA: COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J Am Soc Nephrol 24: 1945–1954, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke JP, Clearkin LG, Talbot JF: Recurrent corneal epithelial erosions in Alport’s syndrome. Acta Ophthalmol (Copenh) 69: 555–557, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Ramamurthi S, Rahman MQ, Dutton GN, Ramaesh K: Pathogenesis, clinical features and management of recurrent corneal erosions. Eye (Lond) 20: 635–644, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Herwig MC, Eter N, Holz FG, Loeffler KU: Corneal clouding in Alport syndrome. Cornea 30: 367–370, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Teekhasaenee C, Nimmanit S, Wutthiphan S, Vareesangthip K, Laohapand T, Malasitr P, Ritch R: Posterior polymorphous dystrophy and Alport syndrome. Ophthalmology 98: 1207–1215, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Grupcheva CN, Chew GS, Edwards M, Craig JP, McGhee CN: Imaging posterior polymorphous corneal dystrophy by in vivo confocal microscopy. Clin Experiment Ophthalmol 29: 256–259, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Sabates R, Krachmer JH, Weingeist TA: Ocular findings in Alport’s syndrome. Ophthalmologica 186: 204–210, 1983 [DOI] [PubMed] [Google Scholar]
- 41.Sekundo W, Lee WR, Kirkness CM, Aitken DA, Fleck B: An ultrastructural investigation of an early manifestation of the posterior polymorphous dystrophy of the cornea. Ophthalmology 101: 1422–1431, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Laganowski HC, Sherrard ES, Muir MG: The posterior corneal surface in posterior polymorphous dystrophy: A specular microscopical study. Cornea 10: 224–232, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Cheong HI, Kashtan CE, Kim Y, Kleppel MM, Michael AF: Immunohistologic studies of type IV collagen in anterior lens capsules of patients with Alport syndrome. Lab Invest 70: 553–557, 1994 [PubMed] [Google Scholar]
- 44.Streeten BW, Robinson MR, Wallace R, Jones DB: Lens capsule abnormalities in Alport’s syndrome. Arch Ophthalmol 105: 1693–1697, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Ohkubo S, Takeda H, Higashide T, Ito M, Sakurai M, Shirao Y, Yanagida T, Oda Y, Sado Y: Immunohistochemical and molecular genetic evidence for type IV collagen alpha5 chain abnormality in the anterior lenticonus associated with Alport syndrome. Arch Ophthalmol 121: 846–850, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Sonarkhan S,Ramappa M, Chaurasia S, Mulay K: Bilateral anterior lenticonus in a case of Alport syndrome: A clinical and histopathological correlation after successful clear lens extraction [published online ahead of print June 26, 2014]. BMJ Case Rep 2014 10.1136/bcr-2013-202036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi J, Na K, Bae S, Roh G: Anterior lens capsule abnormalities in Alport syndrome. Korean J Ophthalmol 19: 84–89, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Vedantham V, Rajagopal J, Ratnagiri PK: Bilateral simultaneous anterior and posterior lenticonus in Alport’s syndrome. Indian J Ophthalmol 53: 212–213, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Liu YB, Tan SJ, Sun ZY, Li X, Huang BY, Hu QM: Clear lens phacoemulsification with continuous curvilinear capsulorhexis and foldable intraocular lens implantation for the treatment of a patient with bilateral anterior lenticonus due to Alport syndrome. J Int Med Res 36: 1440–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Nielsen CE: Lenticonus anterior and Alport’s syndrome. Acta Ophthalmol (Copenh) 56: 518–530, 1978 [DOI] [PubMed] [Google Scholar]
- 51.Mete UO, Karaaslan C, Ozbilgin MK, Polat S, Tap O, Kaya M: Alport’s syndrome with bilateral macular hole. Acta Ophthalmol Scand 74: 77–80, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Sivakumar V, Mohammad M, Colville D, Storey H, Flinter F, Dagher H, Savige J: Clinical and genetic features in autosomal recessive and X-linked Alport syndrome. Pediatr Nephrol 29: 391–396, 2014 [DOI] [PubMed] [Google Scholar]
- 53.Shaw EA, Colville D, Wang YY, Zhang KW, Dagher H, Fassett R, Guymer R, Savige J: Characterization of the peripheral retinopathy in X-linked and autosomal recessive Alport syndrome. Nephrol Dial Transplant 22: 104–108, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Govan JA: Ocular manifestations of Alport’s syndrome: A hereditary disorder of basement membranes? Br J Ophthalmol 67: 493–503, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmed F, Kamae KK, Jones DJ, Deangelis MM, Hageman GS, Gregory MC, Bernstein PS: Temporal macular thinning associated with X-linked Alport syndrome. JAMA Ophthalmol 131: 777–782, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colville D, Wang YY, Tan R, Savige J: The retinal “lozenge” or “dull macular reflex” in Alport syndrome may be associated with a severe retinopathy and early-onset renal failure. Br J Ophthalmol 93: 383–386, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Cervantes-Coste G, Fuentes-Paez G, Yeshurun I, Jimenez-Sierra JM: Tapetal-like sheen associated with fleck retinopathy in Alport syndrome. Retina 23: 245–247, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Setälä K, Ruusuvaara P: Alport syndrome with hereditary macular degeneration. Acta Ophthalmol (Copenh) 67: 409–414, 1989 [DOI] [PubMed] [Google Scholar]
- 59.Saika S, Hayashi Y, Miyamoto T, Yoshitomi T, Ohnishi Y: Multiple retinal holes in the macular region: A case report. Graefes Arch Clin Exp Ophthalmol 240: 578–579, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Shaikh S, Garretson B, Williams GA: Vitreoretinal degeneration complicated by retinal detachment in alport syndrome. Retina 23: 119–120, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Pelit A, Oto S, Yilmaz G, Akova YA: Spontaneous rupture of the anterior lens capsule combined with macular hole in a child with Alport’s syndrome. J Pediatr Ophthalmol Strabismus 41: 59–61, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F: Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol 24: 364–375, 2013 [DOI] [PubMed] [Google Scholar]