Abstract
The kidney filters vast quantities of Na at the glomerulus but excretes a very small fraction of this Na in the final urine. Although almost every nephron segment participates in the reabsorption of Na in the normal kidney, the proximal segments (from the glomerulus to the macula densa) and the distal segments (past the macula densa) play different roles. The proximal tubule and the thick ascending limb of the loop of Henle interact with the filtration apparatus to deliver Na to the distal nephron at a rather constant rate. This involves regulation of both filtration and reabsorption through the processes of glomerulotubular balance and tubuloglomerular feedback. The more distal segments, including the distal convoluted tubule (DCT), connecting tubule, and collecting duct, regulate Na reabsorption to match the excretion with dietary intake. The relative amounts of Na reabsorbed in the DCT, which mainly reabsorbs NaCl, and by more downstream segments that exchange Na for K are variable, allowing the simultaneous regulation of both Na and K excretion.
Keywords: renal physiology, Na transport, water-electrolyte balance
Introduction
Daily Na intake in the United States averages approximately 180 mmol (4.2 g) for men and 150 mmol (3.5 g) for women (1). Because Na is an immutable ion, mass balance requires that an equal amount must be removed from the body daily to prevent inappropriate gains or losses of Na and its accompanying anions, chloride and bicarbonate. Because these ions are the prime determinants of extracellular fluid volume, maintenance of extracellular fluid volume, arterial blood pressure, and organ perfusion depends on control of body Na content.
Under most conditions, the kidneys excrete >95% of the ingested Na at rates that match dietary Na intake. Under steady-state conditions, this process is remarkably precise in both healthy and diseased kidneys, and determinations of excretion are used to estimate Na intake (e.g., in determining adherence to a prescribed dietary regimen). Recent studies have shown the presence of a sizable subcutaneous Na pool that is not in solution equilibrium with the freely exchangeable extracellular Na. Long-term observations suggest that cyclical release of Na from this pool can lead to excretion rates that deviate from Na intake (2). The speed of achieving an intake/excretion match or a new steady state when Na intake varies is relatively slow; body Na content usually increases somewhat when Na intake increases and decreases somewhat when Na intake decreases (3). These changes in body Na content and blood volume are the likely cause for the relation between Na intake and BP. In normotensive individuals, the effect of variations in daily Na intake on BP is small, about 2 mmHg for a 50% reduction in Na intake (4). Nevertheless, such a reduction is associated with demonstrable health benefits, particularly in salt-sensitive persons, such as those with hypertension, patients with diabetes, African Americans, and individuals with chronic renal disease (4).
The precise adaptation of urinary Na excretion to dietary Na intake results from regulated processing of an ultrafiltrate of circulating plasma by the renal tubular epithelium. Perhaps the most striking aspect of this remarkable process is the gross inequality in the quantities of Na removed from plasma by ultrafiltration and those removed from the body by urinary excretion. The staggering mass of >500 g of Na is extracted daily from the plasma by ultrafiltration in order to excrete the ingested 3 g. The large filtered load results from the high extracellular Na concentration and the high rate of glomerular ultrafiltration. Whatever the evolutionary pressure behind this functional design may be, the necessity to retrieve almost all of the filtered Na before it reaches the urine represents a challenging regulatory and energetic demand that the tubular epithelium has to meet. Just as Na intake dictates the rate of Na excretion, Na filtration dictates the rate of Na reabsorption.
Na Reabsorption along the Nephron
Micropuncture and microperfusion studies have shown that all nephron segments contribute to the retrieval of filtered Na (with the exception of the thin descending limbs of the loop of Henle) (Figure 1). The reabsorption of Na is an energy-consuming process that is powered by a Na- and K-activated ATPase in the basolateral membranes of all Na-reabsorbing cells in the kidney. Oxygen consumption of the kidneys is similar to that of other major organs (approximately 6–8 ml/min per 100 g) and is extracted from a seemingly excessive blood supply. While the kidneys consume between 7% and 10% of total oxygen uptake, they receive about 20%–25% of cardiac output at rest. Two thirds or more of renal oxygen uptake are expended for the needs of the Na,K-ATPase, which is for active Na reabsorption.
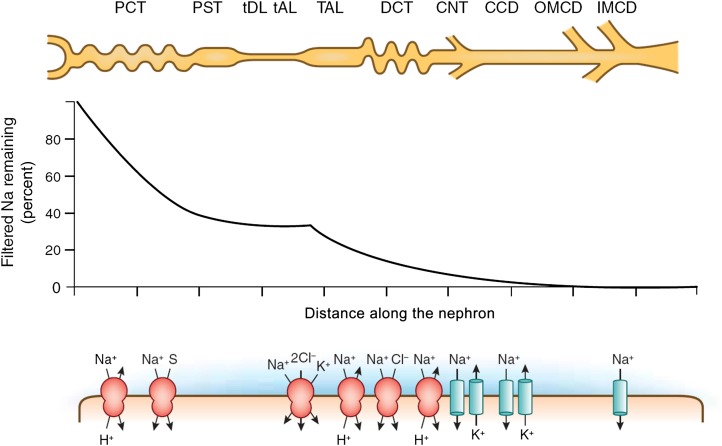
Figure 1.
Na transport along the nephron. The fraction of Na remaining in the ultrafiltrate is plotted as a function of distance along the nephron under conditions of normal (approximately 100 mmol/d) salt intake. The cartoon indicates the major nephron segments. The symbols below show the key Na transport mechanisms in each segment. CCD, cortical collecting duct; CNT, connecting tubule; DCT, distal convoluted tubule; IMCD, inner medullary collecting duct; OMCD, outer medullar collecting duct; PCT, proximal convoluted tubule; PST, proximal straight tubule; S, solute (various); tAL, thin ascending limb; TAL, thick ascending limb; tDL, thin descending limb.
As much as 60%–70% of total Na reabsorption takes place along the proximal convoluted tubule (PCT) and proximal straight tubule, and because reabsorption is near isotonic in this part of the nephron, this is also true for the reabsorption of water. While the thin descending limbs of the loop of Henle absorb water, but not Na, another 25%–30% of the filtered Na is reabsorbed by the ascending portion of this loop, mostly the thick limbs (TALH). Thus, by the time the filtered fluid reaches the macula densa cells at the transition to the distal convoluted tubule (DCT), about 90% of the filtered Na has been retrieved. In quantitative terms, Na reabsorption, therefore, is mostly a function of the proximal nephron (proximal tubule and loop of Henle) while the DCT and the cortical (CCD) and medullary (MCD) collecting ducts contribute no more than 5%–10% of total Na reabsorption. Nevertheless, because of the magnitude of the rate of ultrafiltration, 10% of the total daily filtered load is still an amount that is in the order of the rapidly exchangeable extracellular Na (about 60 g). In fact, it is Na reabsorption along the distal nephron that is highly regulated, and failure of the distal nephron to reabsorb Na is generally more deleterious to Na homeostasis than proximal nephron malabsorption.
Rates and Mechanisms of Na Transport along the Nephron
Proximal Tubule
The renal proximal tubule is a prototypical low-resistance epithelium characterized by low transepithelial voltage, high ion permeabilities, constitutively high water permeability, low transepithelial osmotic gradients, and near-isotonic fluid transport. Results from micropuncture studies indicate avid reabsorption of Na without measurable changes in Na concentration along the PCT. The magnitude of Na reabsorption per millimeter of nephron length in the rat is on the order of 300 pEq/min in PCT from superficial nephrons and even higher in PCTs from juxtamedullary nephrons. Transport rates along the proximal tubule decrease substantially with distance from the glomerulus, and this is accompanied by reductions in the number of mitochondria and the extent of surface membrane amplification both apically and basolaterally. For example, micropuncture studies have shown that fluid and, presumably, Na reabsorption in the rat fell by 75% over the initial 5 mm of proximal tubule length (5). As discussed in a previous article in this series, all Na absorption in the PCT is driven directly or indirectly by the action of the basolateral Na,K-ATPase (6). Active translocation of Na creates driving forces for Na-dependent cotransport and ion exchange, as well as diffusive gradients for paracellular ion movement. Quantitatively, apical Na/H exchange mediated by NHE3 is the most important reabsorptive mechanism. It mediates NaHCO3 uptake, generates an electrochemical gradient for paracellular salt absorption, and operates in tandem with Cl/base exchange in the later part of the proximal tubule. Active transport, electrodiffusion, and solvent drag each contribute approximately one third to total Na reabsorption in the proximal tubule (7).
The discovery of claudin2 as a tight junction protein highly expressed along the PCT has greatly improved the understanding of the paracellular shunt properties in this part of the nephron. Expression studies in Madin-Darby canine kidney cells and the use of claudin2-deficient mice strongly indicate that claudin2 provides a cation-selective and water-permeable paracellular conductance in the PCT (8–11). Claudin2 appears to be a major molecular contributor to the low-resistance properties of the proximal epithelium.
The contributions of Na-dependent cotransporters linked to glucose, phosphate, amino acids, lactate, and other molecules to apical Na uptake are small. For example, with a glucose-to-Na stoichiometry of 1:1 and complete glucose reabsorption along the PCT, <5% of Na uptake along the PCT would be mediated by the Na/glucose cotransporter SGLT2.
Loop of Henle
The loop of Henle is heterogeneous, consisting of the thin descending limbs, the thin ascending limbs, and TALH. Thin descending limbs are inhomogeneous in both structural and functional aspects and also vary substantially between species. Generalizable characteristics include a very high water permeability due to the presence of AQP1, low permeabilities for Na and urea in the outer medulla with increasing values in the inner medulla, and very low levels of Na,K-ATPase activity throughout (12). Thus, this segment is unlikely to contribute measurably to renal Na retrieval. Its function is to be seen in the context of the renal concentrating mechanism. Thin ascending limbs reabsorb some Na, but because Na,K-ATPase levels are also very low, this absorption is presumably passive. Compared with the thin descending limbs of the loop of Henle, thin ascending limbs are more permeable to Na and urea and have 100-fold lower water permeability (12).
In contrast, TALH is a major Na-reabsorbing segment accounting for about 25%–30% of renal net Na retrieval. The Na/K/2Cl cotransporter NKCC2 and NHE3 are the predominant mechanisms of Na transport in the TALH (13). Na reabsorption in the absence of measurable water permeability is an essential prerequisite for the ability of the kidney to osmotically concentrate the urine above isotonicity (12).
Macula densa (MD) cells, a subset of 15–25 cells per nephron at the distal end of the TALH, share most transport properties with proper TALH cells even though they are distinct in other aspects. Na uptake is mostly through the A and B isoforms of NKCC2 with some contribution of NHE2, while Na extrusion is mediated by Na,K-ATPase. K recycling across apical renal outer medullary K channel and basolateral exit of Cl through a Cl channel also mimic TALH properties. In MD cells, enhanced uptake through NKCC2 is coupled to the generation of a vasoconstrictor signal to the afferent arterioles that mediates the tubuloglomerular feedback response (14). This signal entails the release of ATP into the juxtaglomerular interstitium and the subsequent generation of the nucleoside adenosine that constricts afferent arterioles through activation of A1 adenosine receptors. Furosemide and other NKCC2 inhibitors interrupt the MD signaling pathway and, therefore, uncouple the elevated NaCl concentrations from their constrictor effect on the preglomerular vasculature (15). The absence of a tubuloglomerular feedback–mediated suppression of GFR enhances the natriuretic effect of furosemide and the salt-losing phenotype of disease-causing mutations of NKCC2.
Distal Convoluted Tubule
Micropuncture measurements indicate that about 8%–10% of filtered Na enters the initial 20% of the distal convoluted tubule (16–18). At the most distal site that is accessible to micropuncture—just before the first coalescence of tubules to form the collecting duct—about 0.5%–2% of the filtered load remains in the urine. Thus, this part of the nephron reabsorbs 6%–10% of the filtered Na. However, the DCT as defined by these micropuncture-accessible points is heterogeneous, consisting of several different segments that can be distinguished anatomically as DCT1, DCT2, and the connecting tubule (CNT) (19).
The mechanisms of Na transport are different in the “early” or more proximal DCT (mainly DCT1) and in the “late DCT” (DCT2+CNT). In the early DCT, Na reabsorption is largely sensitive to thiazide diuretics, implicating the NaCl-cotransporter NCC. In addition, this segment can reabsorb HCO3− through an Na-H exchange process mediated by NHE2 (20), although this accounts for only about 10% of Na reabsorption in the DCT (21,22). In the late DCT, Na reabsorption is sensitive to amiloride (16), indicating transport through the epithelial Na channel (ENaC). A limited number of measurements of isolated perfused CNTs of rabbits (23,24), as well as patch-clamp measurements in rats and mice (25,26), are in accord with this picture. Immunocytochemistry has confirmed the physiologic and pharmacologic data in that NCC expression diminishes in the distal direction (from DCT1 to DCT2 to CNT) while that of ENaC increases (27).
Collecting Duct
The collecting duct, which is divided into the CCD, outer medullary collecting duct (OMCD), and inner medullary collecting duct (IMCD), handles only about 1% of filtered Na according to micropuncture analysis. However, transport of Na here is remarkable for two reasons. First, it is highly variable, helping to match Na excretion with Na intake. Second, the collecting duct can reduce urine Na concentrations to very low levels (<1 mM), establishing large transtubular Na gradients.
CCDs from rats fed normal chow transport very little Na in vitro (28,29). However, if the animals are pretreated with an Na-deficient diet or with mineralocorticoids, net fluxes of 35–100 pEq/mm per minute can be measured. Direct measurements of ENaC activity in principal cells suggest that Na channels form the apical entry step in these segments (25,30,31). Recent work has indicated that the Na-dependent Cl-HCO3 exchanger (SLC4A8), operating in parallel with the Cl-HCO3 exchanger pendrin, may provide an alternative pathway (32). Na channel densities are similar in the rat CCD and OMCD (33), indicating that transport capacities are similar in these two segments. In the rabbit, however, Na transport rates in the OMCD were lower (34).
Na transport in the IMCD is controversial. Although one study of isolated IMCD segments detected no net Na transport (35), others have found robust Na reabsorption (up to 140 pEq/mm per minute) (36,37). In the latter case, the transport mechanism differed from that of the rest of the collecting duct in that it was not associated with a negative lumen voltage. It was inhibited by the loop diuretic furosemide, consistent with a role of the triple cotransporter (NKCC2) as in the TALH. Finally, studies of the IMCD in situ using split-drop techniques (38) or microcatheterization (39) indicated even larger transport rates of up to 240 pEq/mm per minute, similar to those in the DCT. This reabsorption was partially inhibited by amiloride and sensitive to the mineralocorticoid state of the animal, similar to that in the CCD. However electrophysiologic measurements suggested a low ENaC activity in these segments (33). Thus, the rate and mechanism of Na reabsorption in the IMCD, as well as its role in Na homeostasis, are uncertain.
Urine Na Concentration
Rats fed a diet very low in Na can reduce the concentration of Na+ in urine to <1 mM. Humans are also capable of this degree of Na+ scavenging, as illustrated by the Yanomamo people of the Amazon basin whose diet is nearly devoid of Na and whose urinary Na excretion rates are <1 mmol/d (40). How and where this very dilute salt solution is produced is not entirely clear. The rabbit CCD is able to reduce luminal Na+ concentration to approximately 10–15 mM in vitro from an initial perfusate with 140 mM under conditions of very low flow rates (41). Further reductions of these levels could occur in the OMCD and IMCD if the paracellular resistance is sufficiently large to maintain Na gradients of this magnitude. In fact, measurements of IMCD segments in vitro indicate rather high rates of Na leak (approximately 100 pEq/min per millimeter) and low transepithelial resistance (about 50 Ω per cm2) (35,37,39,42,43). In contrast, epithelia known to produce or sustain very large Na concentration gradients, such as urinary bladder, have much higher junctional resistances (44–46). It is possible that the observed leak conductances in the IMCD result from tissue damage during the preparation or perfusion procedure. Alternatively, transport rates would need to be very high to maintain low Na concentrations in the relatively leaky tubules.
Flow Dependence of Na Transport
A basic form of communication between parts of the nephron is through changes in Na delivery that result from changes in GFR for the proximal tubule, and from changes in reabsorption in an upstream segment in all other parts of the nephron. In general, a change in delivery provokes a change in downstream absorption in the same direction, thereby blunting the effect of changes in Na input on the output to the downstream segment.
Proximal Tubule–Glomerulotubular Balance
Na reabsorption in the proximal tubule is highly and directly dependent on the rate of filtration, a phenomenon referred to as glomerulotubular balance (GTB). Adapting reabsorption to filtration has the dual function of minimizing Na and fluid losses when GFR increases, and of preventing cessation of tubular flow with decreasing GFR. The term GTB originally defined the relation between GFR and total urinary Na excretion (47). Numerous studies have shown that changes in GFR are accompanied by nearly proportional changes in tubular reabsorption so that Na excretion and fractional Na reabsorption, the reabsorption rate expressed as percentage of filtered rate, change very little with decreases or increases of GFR (48,49).
The existence of GTB in the PCT has been shown in numerous micropuncture and microperfusion studies in which variations of GFR were spontaneous or were induced by experimental interventions, such as reductions of renal perfusion pressure. The degree of GTB was often near-perfect, although some deviations from proportionality have been observed during low GFRs (48,50,51). Although GTB in the PCT is a robust and easily demonstrable phenomenon, its explanation has remained a challenge. Studies in intact animals support the notion that flow dependence of proximal fluid reabsorption is the result of changes in physical forces, such as hydrostatic and oncotic pressures in the peritubular capillary bed. Changes in proximal fluid reabsorption that correlate positively with changes in peritubular net absorption pressure have been observed during reduction of renal perfusion pressure, extracellular volume expansion, renal venous pressure elevation, and arterial hypertension (52–55).
An alternative explanation posits that reabsorbed substrates linked to Na reabsorption, such as glucose, amino acids, organic acids, and bicarbonate, are depleted faster along the proximal tubules at low, compared with high, flow rates, and that this would create flow dependence of Na uptake (56,57). Finally, studies in perfused tubules under a variety of conditions seem to indicate that tubular flow rate per se, independent of peritubular force variations or compositional changes, can directly affect Na and fluid reabsorption (58). In recent studies, flow-dependent reabsorption could be described by a model in which the bending moment or torque at the level of the microvilli acts as the signal for reabsorption (59). This is supported by the observation that increasing perfusion fluid viscosity, and, therefore, fluid shear stress, was associated with increased reabsorption over the entire flow range studied. In cultured proximal tubule cells, exposure to shear stress induces trafficking of NHE3 to the apical membrane, and this process can be prevented by disruption of cytoskeletal organization (60). Whether the central cilium, an epithelial cell organelle with the demonstrated ability to function as a flow sensor in a number of cells, could be involved in the regulation of proximal tubular reabsorption is an interesting but untested possibility.
Tubuloglomerular Feedback
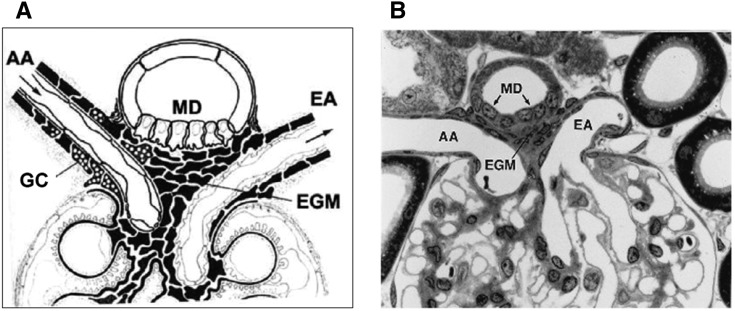
Reabsorption of the filtered Na load along the proximal tubule in proportion to GFR implies that rates of Na and fluid escaping absorption will also change in proportion to their rates of filtration. Thus, despite GTB, delivery to the tubular segments beyond the proximal tubule will increase whenever GFR increases. Furthermore, GTB would be unable to compensate for a primary impairment of proximal Na reabsorption due to transporter malfunction or dysregulation. Thus, Na homeostasis would be well served by a mechanism that senses an increase in NaCl delivery to the late nephron and would use this information to initiate a counter-regulatory response. Extensive research has established that the MD cells situated near the transition from the TALH to the DCT act as sensors of NaCl concentration, and that their output affects the tone of the afferent glomerular arterioles and, therefore, GFR (14). The assignment of the MD cells to this specific role is based on the cyto-architectural feature of a consistent and close anatomic contact between MD cells and glomerular arterioles in a region called the juxtaglomerular apparatus (Figure 2). As discussed below, NaCl concentration at the site of the MD cells is an indicator of GFR or of flow rate at the end of the proximal tubule because tubular flow rate is converted into a concentration signal by the function of the TALH (61). GTB and tubuloglomerular feedback are interdependent and arranged in a feedback circuit such that the fluid escaping absorption along the proximal tubule can exert negative control over GFR formation (62). In this circuit it is irrelevant whether the change in NaCl concentration at the sensor site is the result of a change in GFR or in proximal reabsorption (Figure 3).
Figure 2.
The juxtaglomerular apparatus. Schematic (A) and low-power electron micrograph (B) (original magnification, ×320) of the juxtaglomerular apparatus at the glomerular vascular pole. (Courtesy of B. Kaissling and W. Kriz). AA, afferent arteriole; EA, efferent arteriole; EGM, extraglomerular mesangium; GC granular cells; MD, macula densa.
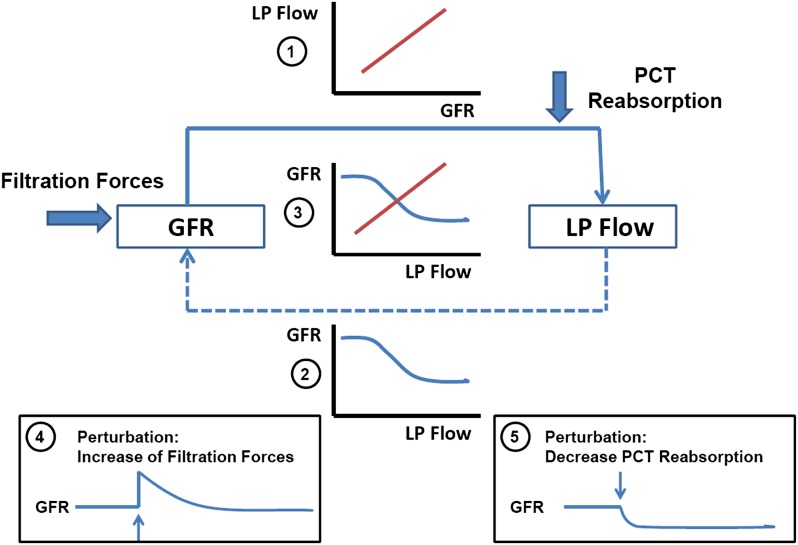
Figure 3.
Feedback relationship between GFR and proximal nephron Na reabsorption. While GFR directly determines Na reabsorption and thereby late proximal (LP) flow rate (solid arrow and inset 1), LP flow rate inversely affects GFR (broken arrow and inset 2). The intersection of the two relationships when plotted in the same graph (inset 3) defines the operating point of this feedback system. LP flow rate is an experimentally controllable surrogate of NaCl concentration at the macula densa sensor site. Bold arrows indicate two principal perturbations: a change in filtration forces and a change in Na reabsorption. Insets 4 and 5 indicate that GFR will tend to return to normal with changes in the former but deviate from normal with changes in the latter.
Tubuloglomerular feedback is tonically active and maintains MD NaCl—and normally also GFR—at levels below those observed without the MD input. The operating point of the system defined as the steady-state GFR at steady-state MD NaCl concentration is located within the steepest part of tubuloglomerular feedback function curve (63). Thus, both increases and decreases in MD NaCl will cause tubuloglomerular feedback–mediated adjustments of afferent arteriolar resistance. The power of tubuloglomerular feedback to compensate for a flow perturbation is maximal for small changes around the operating point of the system (64,65). An acute change in arterial blood pressure unveils the regulatory effect of tubuloglomerular feedback. Both increases and decreases in arterial pressure elicit parallel adjustments in renal arterial resistance that mainly reflect that of afferent arterioles. These autoregulatory adjustments are impaired in the absence of tubuloglomerular feedback, suggesting that MD input is required to maintain renal blood flow during changes in arterial pressure (66,67). In preventing arterial pressure from affecting blood flow and GFR, tubuloglomerular feedback acts in cooperation with at least one additional mechanism, probably the myogenic property of all vascular smooth muscle cells, to respond to increasing luminal pressure with increasing resistance (68).
Tubuloglomerular feedback regulation of preglomerular resistance also creates a functional link between primary changes in PCT reabsorption and filtered load. Micropuncture studies in NHE3- or AQP1-deficient mice have documented unchanged delivery of fluid into the DCT despite the expected major reductions in PCT fluid reabsorption (69,70). Normalization of distal delivery in these cases was due entirely to a reduction of GFR. The cause for the GFR reduction was tubuloglomerular feedback activation because it was not seen when tubuloglomerular feedback was interrupted. Similar correlations between inhibition of PCT fluid transport and GFR have been observed in a range of settings, including administration of carbonic anhydrase inhibitors, claudin2-deficient mice, mice with selective deletion of angiotensin II type 1 receptors for angiotensin II in the proximal tubule, and a mouse model of methyl malonic aciduria (11,71–73). The causes for the GFR reduction in some of these cases appear multifactorial. In addition to tubuloglomerular feedback, they may include effects of elevated tubular pressure and of the constrictor consequences of extracellular volume depletion. Nevertheless, the decrease in GFR is the major reason why catastrophic salt losses are usually not encountered with major failures of proximal reabsorption. The correlation between GFR and proximal reabsorption also holds during primary increments of PCT reabsorption. For example, there is evidence to support the notion that the rise of GFR in rats fed a high-protein diet and in streptozotocin-induced diabetes mellitus is the consequence of tubuloglomerular feedback due to enhanced proximal nephron Na reabsorption (74,75).
Thick Ascending Limbs of the Loop of Henle
Much of the evidence for flow-dependent NaCl reabsorption along the loop of Henle is based on in vivo microperfusion studies of the entire loop, including the pars recta. Nevertheless, the furosemide sensitivity of NaCl reabsorption leaves little doubt that flow dependence of loop Na transport is mediated mainly by the TALH (15,76). A 4-fold increase in flow rate caused a doubling of NaCl reabsorption, indicating that, in contrast to the proximal tubule, fractional reabsorption of NaCl fell with increasing flow rates (15). Flow dependence of TALH NaCl transport results from dependence of NKCC2 activity on luminal NaCl concentration (77). At low flows, luminal NaCl decreases along the length of the TALH from an isotonic or hypertonic starting value to a minimum of about 30 mM at the exit of the cortical TALH. If flow increases (as a result of GFR increases or proximal transport decreases), the fall in NaCl concentration is slowed, and the higher luminal concentrations enhance NaCl transport. Under these conditions, minimum concentrations are not attained, resulting in flow-dependent increases in NaCl concentration at the TALH exit (15,76,77,61).
TALH cells release several inhibitory mediators in response to increased flow that may partially counteract these effects. For example, an increase in shear stress stimulates the release of ATP, probably mediated by the central cilium (78). Luminal ATP increases cytoplasmic Ca2+ concentration ([Ca]i) through activation of apical P2Y receptors (78,79). [Ca]i then activates NOS3, leading to flow-dependent release of nitric oxide (80). Increased [Ca]i also activates phospholipase A2 and causes the formation of 20-HETE, another inhibitor of TALH NaCl transport (81). The release of these agents may explain why relative changes of TALH NaCl transport are less than those in delivery.
Distal Convoluted Tubule
Although the physiologic significance is less well understood, Na transport in distal tubular segments also depends on rates of Na and/or fluid delivery. In vivo microperfusion measurements (82) indicated a 4-fold increase in net Na transport by the rat DCT when perfused with solutions containing 148 mM Na (similar to plasma) compared with 75 mM Na (similar to distal tubular fluid in vivo). The effect presumably involves NCC because it was largest in the early part of the DCT. The mechanism behind this effect is not clear. It does not seem to involve a simple substrate activation of transport as the measured apparent Km values of both Na and Cl for transport by NCC are <10 mM (83). It is also not the result of maintaining luminal Na along the nephron as there were only small differences between perfused and collected fluid. It therefore appears to be a regulatory process involving luminal or intracellular Na or Cl concentrations as the perfusion rates were constant and shear stress did not change.
Cortical Collecting Duct
In vitro perfusion of isolated rabbit CCD showed that Na transport, mediated by ENaC, increases with perfusion rate over the physiologic range (84,85). In this case, the Na concentration remained constant as the volume flow increased. Again, a simple substrate effect can be ruled out, indicating regulation of the system. Here the most likely signal is mechanical. In a heterologous expression system, the channels were activated by shear stress on the membrane (86). It is also possible that increased flow dilutes an inhibitory factor such as ATP that might be secreted into the tubular lumen (87,88).
Inner Medullary Collecting Duct
As assessed by in vivo microcatheterization of the IMCD, rates of Na+ reabsorption were proportional to rates of Na delivery to the segment and modified by infusion of KCl (43). This phenomenon was not observed in isolated perfused tubules (37), but in that preparation high flow rates abolish the effect of atrial natriuretic peptide (ANP) to inhibit absorption.
Chronic Effects
In addition to the acute effects described above, chronic changes in Na delivery from upstream segments can further modulate downstream transport systems. Treatment of animals with furosemide inhibits Na reabsorption in the TALH and increases delivery to the distal nephron. This increases the reabsorptive capacity of the DCT (17,89) and is accompanied by ultrastructural changes in DCT cells (17,90), including increased cell volume (hypertrophy), increased luminal and basolateral surface area, and mitochondrial density. The remodeling of the cells continued in the CNT and CCD (91), implying that these segments were also affected by chronic increases in delivery rates. These changes attenuate the effects of the diuretic.
The signaling pathways involved in these events are unknown, but it is possible that increased intracellular Na+ (or Cl−) contributes to the phenomena. DCT cells also undergo hypertrophy in animal models of familial hyperkalemia with hypertension (19) that involve primary upregulation of NCC (92,93), thus increasing NaCl entry into the epithelial cells. Conversely, in NCC-knockout mice, the DCT cells undergo atrophy (94). In the rat CCD, mineralocorticoid infusion also produces hypertrophy (95) associated with increased Na/K-ATPase activity (96). Activation of the pump was prevented by blocking Na channels with amiloride, suggesting that increased Na entry into the cells was required for this effect.
Effects of Dietary Na and Extracellular Volume
While the responses to flow and Na delivery tend to keep Na outflow constant, matching Na excretion to Na intake requires that the kidneys produce urine with highly variable Na content. Na intake as determined by 24-hour urinary Na excretion varies in different populations from <1 mmol/d to 250 mmol/d (97). Changes in intake and body Na content are sensed through alterations in effective arterial blood volume and its effect on pressure-sensitive receptors in the vascular wall, the renal afferent arteriole, and the heart. The activation state of these receptors leads to changes in renal effector systems, such as the renin–angiotensin II–aldosterone axis, the renal sympathetic nervous system, and the release of vasopressin and ANP.
The neural and hormonal effector systems regulated by extracellular fluid volume cause changes of Na excretion mainly by changes in tubular Na reabsorption, especially in the distal nephron (Figure 4). Even with extreme variations in dietary intake, changes in fractional reabsorption are not very large because of the excess amounts of Na filtered under all circumstances. Renal fractional Na reabsorption with plausible 10-fold changes in Na intake vary only between approximately 97% and 99.7%. Thus, even with extreme intake variations, Na reabsorption fluctuates by only about 3% of filtered Na. Because of the gross imbalance between filtered and excreted Na it is not easy to exclude a role of a small change in GFR in the altered rate of Na excretion.
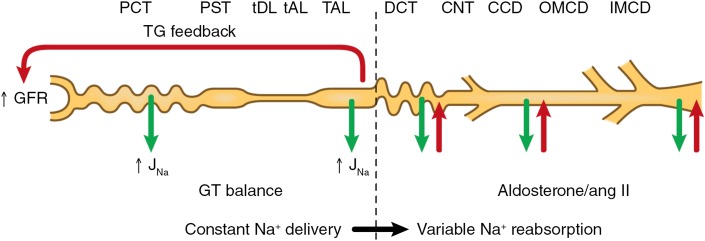
Figure 4.
Effects of glomerulotubular (GT) balance and tubuloglomerular (TG) feedback on delivery of Na to the distal nephron. These feedback loops minimize changes in GFR and in the amount of Na escaping the TALH. Changes in Na reabsorption in the distal nephron, including the DCT, CNT, and collecting duct, effect variations in Na excretion. ang II, angiotensin II; CCD, cortical collecting duct; CNT, connecting tubule; DCT, distal convoluted tubule; J(Na-subscript), sodium flux; OMCD, outer medullary collecting duct; PCT, proximal convoluted tubule; PST, proximal straight tubule; tAL, thin ascending limb; TAL, thick ascending limb of Henle's loop; tDL, thin descending limb.
Proximal Nephron
Most evidence suggests that the role of the proximal tubule in the response to changes in Na intake is small. Typically, variations in NaCl intake are not associated with consistent and reproducible changes in GFR or proximal fluid reabsorption (98,99). Accordingly, Na delivery to the early DCT as assessed by micropuncture does not change dramatically with Na intake (17,18) (Figure 4).
The limited role of the PCT in the regulation of the excretion of Na following changes in dietary intake is puzzling because some of the effector systems responding to effective circulating volume (ECV) have clear effects on NaCl reabsorption in the PCT. Activation of the renal sympathetic nervous system stimulates Na transport through α1 receptors, while renal denervation can reduce rates of Na reabsorption by 40% (100,101). During anesthesia, these changes were not accompanied by measurable alterations in renal hemodynamics, but this is less clear in conscious animals (101). In addition, angiotensin II, in a physiologic dose range, appears to stimulate NaHCO3 reabsorption through activation of NHE3 and to enhance fluid reabsorption through its effect on filtration fraction and peritubular oncotic pressure (102–104). Thus, a reduction of ECV with stimulation of the renal nervous system and renin–angiotensin system should lead to stimulation of Na transport in the PCT. Furthermore, dopamine produced in the PCT is known to inhibit both Na,K-ATPase and NHE3 (105,106). Because dopamine synthesis is enhanced in ECV expansion, this would be another mediator that should contribute to the regulation of PCT NaCl transport with changes in dietary salt content (106). The reasons for the apparent neutral net effects of these potent mediators on the regulation of PT absorption by dietary salt intake are complex and will be difficult to untangle. Intra- and intersegmental compensation is likely to occur with blunting of early proximal events by downstream segments. Furthermore, possible interactions between these different agents and between them and other potential transport modulators (such as endothelin, nitric oxide, 20-HETE, cardiotonic steroids, and nucleotides/nucleosides) could make the final outcome, with respect to net effects on Na transport in the complete system, highly unpredictable. Finally, results from in vitro experimental models could lead to conclusions that do not apply to in vivo conditions.
More dramatic changes in extracellular fluid volume, such as those experimentally induced by the infusion of large fluid volumes, increase GFR and do indeed often reduce fractional fluid reabsorption (107). These factors combine to cause marked elevations in fluid delivery to the loops of Henle. Furthermore, long-term activation or inhibition of angiotensin II–mediated transport in the proximal tubule can affect BP, presumably through progressive changes of body Na content (71,108).
Distal Convoluted Tubule
In vivo microperfusion measurements in rat kidney showed that Na reabsorption in the early DCT doubled under chronic conditions when dietary Na intake was reduced for >1 week (17). This was accompanied by a similar increase in Cl reabsorption and was blocked by thiazides, indicating an effect on NCC-mediated transport (Figure 5). Biochemical measurements on rat kidney indicated an increase in the surface expression of this transporter in Na-depleted animals (109). The signals mediating this response have not been established. The renin–angiotensin–aldosterone (RAA) axis is stimulated under these conditions, and long-term infusion of aldosterone increased NCC expression in some (110,111), but not all (109,112), studies. However, mineralocorticoids may not be the major regulators of NCC because a high-K diet increases aldosterone secretion but decreases cotransporter activity. Angiotensin II is another candidate for upregulating NCC. In microperfusion experiments, application of angiotensin II increased DCT Na transport by both early and late DCT segments, suggesting upregulation of Na/H exchange and Na channels, respectively (21). Acute changes in the hormone level in vivo led to redistribution of NCC between the cell surface and intracellular vesicles of DCT cells (113). Long-term infusion of this peptide increases NCC expression in rat kidney (114).
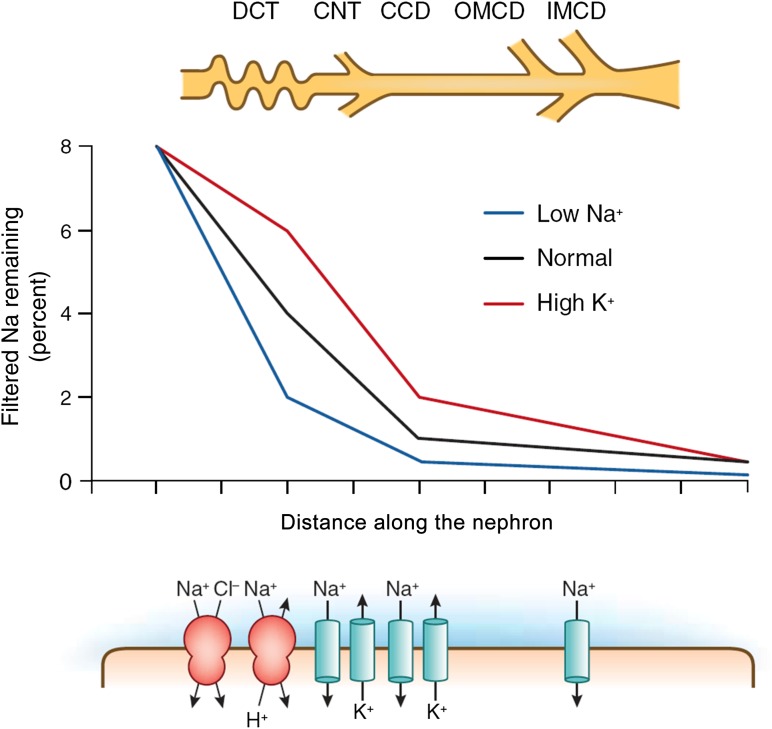
Figure 5.
Variations in the profile of Na transport during dietary Na restriction (blue line) and dietary K loading (red line). With Na restriction, transport by all distal segments is stimulated. With K loading, Na channel–mediated transport in the CNT and collecting duct, which effectively exchanges Na for K, is augmented, while NaCl cotransport in the DCT is diminished. CCD, cortical collecting duct; CNT, connecting tubule; DCT, distal convoluted tubule; IMCD, inner medullary collecting duct; OMCD, outer medullary collecting duct.
CNT/CCD
Dietary Na depletion strongly increases Na transport by the CCD measured in vitro (28,29,115) (Figure 5). Na channel activity rises in parallel (30), consistent with upregulation of ENaC as the major event in this process. Electrophysiologic measurements indicated similar responses in the CNT (25) and in the late DCT (DCT2) (26). In the latter case, the dependence of the channels on dietary salt was shifted with activity appearing at higher levels of Na intake.
The increased Na channel activity observed in the CCD when animals are Na-depleted can be quantitatively mimicked by treatment with aldosterone (30), suggesting that increases in this hormone account for the chronic response of this segment. Indeed, these segments are collectively referred to as the aldosterone-sensitive distal nephron (ASDN). However angiotensin II acutely increased ENaC activity (116). Furthermore, chronic angiotensin II elevation can also increase ENaC activity independent of aldosterone (117), indicating separate effects of these two arms of the RAA axis on channel-mediated Na reabsorption.
Dietary Na depletion also upregulates the bicarbonate-dependent NaCl transporter in the CCD (32). This system also appears to be inhibited by ANP (118), a hormone released from the heart in response to expansion of plasma volume (119).
Inner Medullary Collecting Duct
Split-drop experiments in the IMCD indicated that this segment could also participate in the response to dietary Na depletion (38). Transport was stimulated modestly by aldosterone and inhibited by amiloride, consistent with an upregulation of ENaC as in the CCD. This idea is supported by biochemical measurements showing increased abundance of α- and βENaC and increased proteolytic cleavage of α- and γENaC in the inner medulla of the rat kidney (33). ANP decreased amiloride-sensitive O2 consumption in isolated IMCD cells (120) and net Na reabsorption in IMCD measured in vivo (43) or in vitro (37).
Effects of Dietary K
Changes in dietary K intake also affect Na transport along the nephron. If this occurs with constant Na intake, achieving Na balance demands that K excretion must vary without altering net Na excretion. In this case, the distribution of Na reabsorption along the nephron shifts between more proximal segments (where the K is reabsorbed with Na) and the CNT/CCD (where K is secreted by principal cells in exchange for Na [121]) (Figure 5).
Acute increases in plasma K are natriuretic (122,123). This effect may reflect reduced Na+ reabsorption in the proximal tubule (124), the TALH (125), or the DCT (126). As argued earlier, perturbations in the more proximal segments tend to be largely compensated by GTB and tubuloglomerular feedback. However, inhibition of reabsorption in the DCT will directly increase delivery of Na+ to the ASDN, stimulating both Na+ reabsorption and K+ secretion. Acute K loading leads to a decrease in the phosphorylation of the thiazide-sensitive cotransporter NCC (126), consistent with reduced activity (19).
Under more chronic conditions, the total amount of NCC as well as phospho-NCC in the cell (127) decreases as K intake increases. The surface expression of NCC also changes reciprocally with dietary K (128). At the same time, the activity of ENaC in the CCD (129) and CNT (25,130) rises with the K load, together with parallel upregulation of apical K+ channels (129,131) and the Na,K-ATPase (129). These responses will shift Na+ transport from the DCT, where Na+ is reabsorbed together with Cl−, to the ASDN, where K+ secretion balances, at least in part, the transport of Na+. In this way both Na and K can remain in balance. Achievement of Na balance in the presence of elevated aldosterone levels has been called “aldosterone escape.”
The opposite shift will occur when dietary K intake is restricted: NCC surface expression and presumably activity increase, limiting Na delivery to downstream K-secreting segments. This response is exaggerated with simultaneous Na and K depletion (132). Here, ENaC activity is suppressed, despite low salt intake. NCC abundance increases strongly, indicating that much of the burden of Na retention falls on the DCT under these conditions.
The RAA axis plays an important role in shifting Na reabsorption from one segment to the other. K loading increases aldosterone production independent of angiotensin II, contributing to the upregulation of ENaC, although an aldosterone-independent pathway may also be important (129). The identities of signals regulating NCC are uncertain. Angiotensin II can stimulate NCC expression independent of aldosterone (114), and it is possible that this hormone may mediate, at least in part, the effects of changes in dietary K. A low-K diet increases plasma renin activity (133), and angiotensin II levels are likely to change reciprocally with dietary K intake.
Disclosures
None.
Acknowledgments
L.G.P. acknowledges the support of Grant R01-DK27847 from the National Institutes of Health. J.S. was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. U.S. Department of Agriculture, Agricultural Research Service. Nutrient intakes from food: Mean amounts consumed per individual, by gender and age. In: What We Eat In America, NHANES 2009-2010, 2012. Available at: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0708/Table_1_NIN_GEN_07.pdf. Accessed June 25, 2014.
- 2.Titze J: Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens 23: 101–105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinhardt HW, Behrenbeck DW: [Studies in awake dogs on the regulation of the sodium balance. I. The significance of the extracellular space for the regulation of the daily sodium balance]. Pflugers Arch Gesamte Physiol Menschen Tiere 295: 266–279, 1967 [PubMed] [Google Scholar]
- 4.He FJ, Li J, Macgregor GA: Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 4: CD004937, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Liu FY, Cogan MG: Axial heterogeneity of bicarbonate, chloride, and water transport in the rat proximal convoluted tubule. Effects of change in luminal flow rate and of alkalemia. J Clin Invest 78: 1547–1557, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curthoys NP, Moe OW: Proximal tubule function and response to acidosis [published online ahead of print May 1, 2014]. Clin J Am Soc Nephrol 10.2215/CJN.10391012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frömter E, Rumrich G, Ullrich KJ: Phenomenologic description of Na+, Cl- and HCO-3 absorption from proximal tubules of rat kidney. Pflugers Arch 343: 189–220, 1973 [DOI] [PubMed] [Google Scholar]
- 8.Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M: Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M: Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A 107: 8011–8016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Günzel D, Fromm M: Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 123: 1913–1921, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Schnermann J, Huang Y, Mizel D: Fluid reabsorption in proximal convoluted tubules of mice with gene deletions of claudin-2 and/or aquaporin1. Am J Physiol Renal Physiol 305: F1352–F1364, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sands JM, Layton HE: The urine concentrating mechanism and urea transporters. In: The Kidney Physiology and Pathophysiology, edited by Alpern RJ, Caplan MJ, Moe OW, 5th Ed., London, Waltham, San Diego, Elsevier Academic Press, 2013, pp 1463–1510 [Google Scholar]
- 13.Mount DB: Renal physiology: thick ascending limb. Clin J Am Soc Nephrol 2014, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnermann J, Castrop H: Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: The Kidney. Physiology and Pathophysiology, edited by Alpern RJ, Caplan MJ, Moe OW, 5th Ed., London, Waltham, San Diego, Elsevier Academic Press, 2013, pp 757–801 [Google Scholar]
- 15.Wright FS, Schnermann J: Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest 53: 1695–1708, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costanzo LS: Comparison of calcium and sodium transport in early and late rat distal tubules: effect of amiloride. Am J Physiol 246: F937–F945, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Ellison DH, Velázquez H, Wright FS: Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest 83: 113–126, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malnic G, Klose RM, Giebisch G: Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol 211: 529–547, 1966 [DOI] [PubMed] [Google Scholar]
- 19.Subramanya AR, Ellison DH: Distal convoluted tubule [Published online ahead of print May 22, 2014]. Clin J Am Soc Nephrol 10.2215/CJN.0592061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, Hropot M, Aronson PS, Giebisch G: Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Physiol Renal Physiol 281: F1117–F1122, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Giebisch G: Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol 271: F143–F149, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Malnic G, Giebisch G, Chan YL: Renal bicarbonate reabsorption in the rat. IV. Bicarbonate transport mechanisms in the early and late distal tubule. J Clin Invest 91: 2776–2784, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida AJ, Burg MB: Sodium transport in the rabbit connecting tubule. Am J Physiol 243: F330–F334, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Shareghi GR, Stoner LC: Calcium transport across segments of the rabbit distal nephron in vitro. Am J Physiol 235: F367–F375, 1978 [DOI] [PubMed] [Google Scholar]
- 25.Frindt G, Palmer LG: Na channels in the rat connecting tubule. Am J Physiol Renal Physiol 286: F669–F674, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Nesterov V, Dahlmann A, Krueger B, Bertog M, Loffing J, Korbmacher C: Aldosterone-dependent and -independent regulation of the epithelial sodium channel (ENaC) in mouse distal nephron. Am J Physiol Renal Physiol 303: F1289–F1299, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B: Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279: F252–F258, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Reif MC, Troutman SL, Schafer JA: Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J Clin Invest 77: 1291–1298, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomita K, Pisano JJ, Knepper MA: Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest 76: 132–136, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pácha J, Frindt G, Antonian L, Silver RB, Palmer LG: Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 25–42, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer LG, Frindt G: Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci U S A 83: 2767–2770, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frindt G, Ergonul Z, Palmer LG: Na channel expression and activity in the medullary collecting duct of rat kidney. Am J Physiol Renal Physiol 292: F1190–F1196, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Stokes JB, Ingram MJ, Williams AD, Ingram D: Heterogeneity of the rabbit collecting tubule: Localization of mineralocorticoid hormone action to the cortical portion. Kidney Int 20: 340–347, 1981 [DOI] [PubMed] [Google Scholar]
- 35.Sands JM, Nonoguchi H, Knepper MA: Hormone effects on NaCl permeability of rat inner medullary collecting duct. Am J Physiol 255: F421–F428, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Kudo LH, van Baak AA, Rocha AS: Effect of vasopressin on sodium transport across inner medullary collecting duct. Am J Physiol 258: F1438–F1447, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Rocha AS, Kudo LH: Atrial peptide and cGMP effects on NaCl transport in inner medullary collecting duct. Am J Physiol 259: F258–F268, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Ullrich KJ, Papavassiliou F: Sodium reabsorption in the papillary collecting duct of rats. Effect of adrenalectomy, low Na+ diet, acetazolamide, HCO-3-free solutions and of amiloride. Pflugers Arch 379: 49–52, 1979 [DOI] [PubMed] [Google Scholar]
- 39.Sonnenberg H, Honrath U, Wilson DR: Effects of amiloride in the medullary collecting duct of rat kidney. Kidney Int 31: 1121–1125, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Mancilha-Carvalho JJ, de Oliveira R, Esposito RJ: Blood pressure and electrolyte excretion in the Yanomamo Indians, an isolated population. J Hum Hypertens 3: 309–314, 1989 [PubMed] [Google Scholar]
- 41.Grantham JJ, Kurg MB, Obloff J: The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J Clin Invest 49: 1815–1826, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai M, Yoshitomi K: Electrophysiological study of inner medullary collecting duct of hamsters. Pflugers Arch 416: 180–188, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Sonnenberg H, Honrath U, Chong CK, Wilson DR: Atrial natriuretic factor inhibits sodium transport in medullary collecting duct. Am J Physiol 250: F963–F966, 1986 [DOI] [PubMed] [Google Scholar]
- 44.Erlij D: Basic electrical properties of tight epithelia determined with a simple method. Pflugers Arch 364: 91–93, 1976 [DOI] [PubMed] [Google Scholar]
- 45.Higgins JT, Jr, Gebler B, Frömter E: Electrical properties of amphibian urinary bladder epithelia. II. The cell potential profile in necturus maculosus. Pflugers Arch 371: 87–97, 1977 [DOI] [PubMed] [Google Scholar]
- 46.Lewis SA, Eaton DC, Diamond JM: The mechanism of Na+ transport by rabbit urinary bladder. J Membr Biol 28: 41–70, 1976 [DOI] [PubMed] [Google Scholar]
- 47.Smith HW: The Kidney. Structure and Function in Health and Disease, New York, Oxford University Press, 1951 [Google Scholar]
- 48.Landwehr DM, Schnermann J, Klose RM, Giebisch G: Effect of reduction in filtration rate on renal tubular sodium and water reabsorption. Am J Physiol 215: 687–695, 1968 [DOI] [PubMed] [Google Scholar]
- 49.Lindheimer MD, Lalone RC, Levinsky NG: Evidence that an acute increase in glomerular filtration has little effect on sodium excretion in the dog unless extracellular volume is expanded. J Clin Invest 46: 256–265, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glabman S, Aynedjian HS, Bank N: Micropuncture study of the effect of acute reductions in glomerular filtration rate on sodium and water reabsorption by the proximal tubules of the rat. J Clin Invest 44: 1410–1416, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnermann J, Wahl M, Liebau G, Fischbach H: Balance between tubular flow rate and net fluid reabsorption in the proximal convolution of the rat kidney. I. Dependency of reabsorptive net fluid flux upon proximal tubular surface area at spontaneous variations of filtration rate. Pflugers Arch 304: 90–103, 1968 [DOI] [PubMed] [Google Scholar]
- 52.Brenner BM, Troy JL: Postglomerular vascular protein concentration: Evidence for a causal role in governing fluid reabsorption and glomerulotublar balance by the renal proximal tubule. J Clin Invest 50: 336–349, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brenner BM, Troy JL, Daugharty TM: On the mechanism of inhibition in fluid reabsorption by the renal proximal tubule of the volume-expanded rat. J Clin Invest 50: 1596–1602, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falchuk KH, Brenner BM, Tadokoro M, Berliner RW: Oncotic and hydrostatic pressures in peritubular capillaries and fluid reabsorption by proximal tubule. Am J Physiol 220: 1427–1433, 1971 [DOI] [PubMed] [Google Scholar]
- 55.Lewy JE, Windhager EE: Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol 214: 943–954, 1968 [DOI] [PubMed] [Google Scholar]
- 56.Barfuss DW, Schafer JA: Flow dependence of nonelectrolyte absorption in the nephron. Am J Physiol 236: F163–F174, 1979 [DOI] [PubMed] [Google Scholar]
- 57.Burg M, Patlak C, Green N, Villey D: Organic solutes in fluid absorption by renal proximal convoluted tubules. Am J Physiol 231: 627–637, 1976 [DOI] [PubMed] [Google Scholar]
- 58.Bartoli E, Conger JD, Earley LE: Effect of intraluminal flow on proximal tubular reabsorption. J Clin Invest 52: 843–849, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T: Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci U S A 101: 13068–13073, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T: Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol 290: F289–F296, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Wright FS: Flow-dependent transport processes: Filtration, absorption, secretion. Am J Physiol 243: F1–F11, 1982 [DOI] [PubMed] [Google Scholar]
- 62.Briggs J: A simple steady-state model for feedback control of glomerular filtration rate. Kidney Int Suppl 12[Suppl. 12]: S143–S150, 1982 [PubMed] [Google Scholar]
- 63.Briggs JP, Schubert G, Schnermann J: Quantitative characterization of the tubuloglomerular feedback response: effect of growth. Am J Physiol 247: F808–F815, 1984 [DOI] [PubMed] [Google Scholar]
- 64.Moore LC, Mason J: Perturbation analysis of tubuloglomerular feedback in hydropenic and hemorrhaged rats. Am J Physiol 245: F554–F563, 1983 [DOI] [PubMed] [Google Scholar]
- 65.Thomson SC, Blantz RC: Homeostatic efficiency of tubuloglomerular feedback in hydropenia, euvolemia, and acute volume expansion. Am J Physiol 264: F930–F936, 1993 [DOI] [PubMed] [Google Scholar]
- 66.Moore LC, Schnermann J, Yarimizu S: Feedback mediation of SNGFR autoregulation in hydropenic and DOCA- and salt-loaded rats. Am J Physiol 237: F63–F74, 1979 [DOI] [PubMed] [Google Scholar]
- 67.Navar LG, Burke TJ, Robinson RR, Clapp JR: Distal tubular feedback in the autoregulation of single nephron glomerular filtration rate. J Clin Invest 53: 516–525, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holstein-Rathlou NH, Wagner AJ, Marsh DJ: Tubuloglomerular feedback dynamics and renal blood flow autoregulation in rats. Am J Physiol 260: F53–F68, 1991 [DOI] [PubMed] [Google Scholar]
- 69.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J: Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol 277: F447–F453, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Schnermann J, Chou C-L, Ma T, Traynor T, Knepper MA, Verkman AS: Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci U S A 95: 9660–9664, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM: AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manoli I, Sysol JR, Li L, Houillier P, Garone C, Wang C, Zerfas PM, Cusmano-Ozog K, Young S, Trivedi NS, Cheng J, Sloan JL, Chandler RJ, Abu-Asab M, Tsokos M, Elkahloun AG, Rosen S, Enns GM, Berry GT, Hoffmann V, DiMauro S, Schnermann J, Venditti CP: Targeting proximal tubule mitochondrial dysfunction attenuates the renal disease of methylmalonic acidemia. Proc Natl Acad Sci U S A 110: 13552–13557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tucker BJ, Steiner RW, Gushwa LC, Blantz RC: Studies on the tubulo-glomerular feedback system in the rat. The mechanism of reduction in filtration rate with benzolamide. J Clin Invest 62: 993–1004, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seney FD, Jr, Persson EG, Wright FS: Modification of tubuloglomerular feedback signal by dietary protein. Am J Physiol 252: F83–F90, 1987 [DOI] [PubMed] [Google Scholar]
- 75.Thomson SC, Deng A, Komine N, Hammes JS, Blantz RC, Gabbai FB: Early diabetes as a model for testing the regulation of juxtaglomerular NOS I. Am J Physiol Renal Physiol 287: F732–F738, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Morgan T, Berliner RW: A study by continuous microperfusion of water and electrolyte movements in the loop of Henle and distal tubule of the rat. Nephron 6: 388–405, 1969 [DOI] [PubMed] [Google Scholar]
- 77.Greger R: Ion transport mechanisms in thick ascending limb of Henle’s loop of mammalian nephron. Physiol Rev 65: 760–797, 1985 [DOI] [PubMed] [Google Scholar]
- 78.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J: Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Praetorius HA, Leipziger J: Primary cilium-dependent sensing of urinary flow and paracrine purinergic signaling. Semin Cell Dev Biol 24: 3–10, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Cabral PD, Hong NJ, Garvin JL: ATP mediates flow-induced NO production in thick ascending limbs. Am J Physiol Renal Physiol 303: F194–F200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang W, Lu M, Balazy M, Hebert SC: Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. Am J Physiol 273: F421–F429, 1997 [DOI] [PubMed] [Google Scholar]
- 82.Ellison DH, Velázquez H, Wright FS: Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol 253: F546–F554, 1987 [DOI] [PubMed] [Google Scholar]
- 83.Monroy A, Plata C, Hebert SC, Gamba G: Characterization of the thiazide-sensitive Na(+)-Cl(-) cotransporter: A new model for ions and diuretics interaction. Am J Physiol Renal Physiol 279: F161–F169, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Engbretson BG, Stoner LC: Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am J Physiol 253: F896–F903, 1987 [DOI] [PubMed] [Google Scholar]
- 85.Woda CB, Bragin A, Kleyman TR, Satlin LM: Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 86.Carattino MD, Sheng S, Kleyman TR: Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem 279: 4120–4126, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Ma HP, Li L, Zhou ZH, Eaton DC, Warnock DG: ATP masks stretch activation of epithelial sodium channels in A6 distal nephron cells. Am J Physiol Renal Physiol 282: F501–F505, 2002 [DOI] [PubMed] [Google Scholar]
- 88.Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD: Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol 294: F38–F46, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Stanton BA, Kaissling B: Regulation of renal ion transport and cell growth by sodium. Am J Physiol 257: F1–F10, 1989 [DOI] [PubMed] [Google Scholar]
- 90.Kaissling B, Stanton BA: Adaptation of distal tubule and collecting duct to increased sodium delivery. I. Ultrastructure. Am J Physiol 255: F1256–F1268, 1988 [DOI] [PubMed] [Google Scholar]
- 91.Kaissling B, Le Hir M: Functional morphology of kidney tubules and cells in situ. Methods Enzymol 191: 265–289, 1990 [DOI] [PubMed] [Google Scholar]
- 92.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP: Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S: Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE: Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J Biol Chem 273: 29150–29155, 1998 [DOI] [PubMed] [Google Scholar]
- 95.Wade JB, Stanton BA, Field MJ, Kashgarian M, Giebisch G: Morphological and physiological responses to aldosterone: Time course and sodium dependence. Am J Physiol 259: F88–F94, 1990 [DOI] [PubMed] [Google Scholar]
- 96.Palmer LG, Antonian L, Frindt G: Regulation of the Na-K pump of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 43–57, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Intersalt Cooperative Research Group: Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297: 319–328, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schor N, Ichikawa I, Brenner BM: Glomerular adaptations to chronic dietary salt restriction or excess. Am J Physiol 238: F428–F436, 1980 [DOI] [PubMed] [Google Scholar]
- 99.Vallon V, Huang DY, Deng A, Richter K, Blantz RC, Thomson S: Salt-sensitivity of proximal reabsorption alters macula densa salt and explains the paradoxical effect of dietary salt on glomerular filtration rate in diabetes mellitus. J Am Soc Nephrol 13: 1865–1871, 2002 [DOI] [PubMed] [Google Scholar]
- 100.Bello-Reuss E, Colindres RE, Pastoriza-Muñoz E, Mueller RA, Gottschalk CW: Effects of acute unilateral renal denervation in the rat. J Clin Invest 56: 208–217, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johns EJ, Kopp UC: Neural control of renal function. In: The Kidney Physiology and Pathophysiology, edited by Alpern RJ, Caplan MJ, Moe OW, 5th Ed., London, Waltham, San Diego, Elsevier Academic Press, 2013, pp 451–486 [Google Scholar]
- 102.Cogan MG: Angiotensin II: A powerful controller of sodium transport in the early proximal tubule. Hypertension 15: 451–458, 1990 [DOI] [PubMed] [Google Scholar]
- 103.Geibel J, Giebisch G, Boron WF: Angiotensin II stimulates both Na(+)-H+ exchange and Na+/HCO3- cotransport in the rabbit proximal tubule. Proc Natl Acad Sci U S A 87: 7917–7920, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schuster VL, Kokko JP, Jacobson HR: Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J Clin Invest 73: 507–515, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aperia A, Bertorello A, Seri I: Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol 252: F39–F45, 1987 [DOI] [PubMed] [Google Scholar]
- 106.Jose PA, Felder RA, Eisner GM: Paracrine regulation of renal function by dopamine. In: The Kidney Physiology and Pathophysiology, edited by Alpern RJ, Caplan MJ, Moe OW, London, Waltham, San Diego, Elsevier Academic Press, 2013, pp 539–591 [Google Scholar]
- 107.Osgood RW, Reineck HJ, Stein JH: Further studies on segmental sodium transport in the rat kidney during expansion of the extracellular fluid volume. J Clin Invest 62: 311–320, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li H, Weatherford ET, Davis DR, Keen HL, Grobe JL, Daugherty A, Cassis LA, Allen AM, Sigmund CD: Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol 301: R1067–R1077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frindt G, Palmer LG: Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol 297: F1249–F1255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA: The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van der Lubbe N, Lim CH, Meima ME, van Veghel R, Rosenbaek LL, Mutig K, Danser AH, Fenton RA, Zietse R, Hoorn EJ: Aldosterone does not require angiotensin II to activate NCC through a WNK4-SPAK-dependent pathway. Pflugers Arch 463: 853–863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang XY, Masilamani S, Nielsen J, Kwon TH, Brooks HL, Nielsen S, Knepper MA: The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest 108: 215–222, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB: Angiotensin II provokes acute trafficking of distal tubule NaCl cotransporter (NCC) to apical membrane. Am J Physiol Renal Physiol 293: F662–669, 2007 [DOI] [PubMed] [Google Scholar]
- 114.van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ: Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79: 66–76, 2011 [DOI] [PubMed] [Google Scholar]
- 115.Schwartz GJ, Burg MB: Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol 235: F576–F585, 1978 [DOI] [PubMed] [Google Scholar]
- 116.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O: Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O: Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension 62: 1111–1122, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nonoguchi H, Sands JM, Knepper MA: ANF inhibits NaCl and fluid absorption in cortical collecting duct of rat kidney. Am J Physiol 256: F179–F186, 1989 [DOI] [PubMed] [Google Scholar]
- 119.Maack T: Role of atrial natriuretic factor in volume control. Kidney Int 49: 1732–1737, 1996 [DOI] [PubMed] [Google Scholar]
- 120.Zeidel ML, Seifter JL, Lear S, Brenner BM, Silva P: Atrial peptides inhibit oxygen consumption in kidney medullary collecting duct cells. Am J Physiol 251: F379–F383, 1986 [DOI] [PubMed] [Google Scholar]
- 121.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE: Collecting duct principal cell transport processes and their regulation [published online ahead of print May 29, 2014]. Clin J Am Soc Nephrol 10.2215/CJN.05760513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Buren M, Rabelink TJ, van Rijn HJ, Koomans HA: Effects of acute NaCl, KCl and KHCO3 loads on renal electrolyte excretion in humans. Clin Sci (Lond) 83: 567–574, 1992 [DOI] [PubMed] [Google Scholar]
- 123.Wright FS, Strieder N, Fowler NB, Giebisch G: Potassium secretion by distal tubule after potassium adaptation. Am J Physiol 221: 437–448, 1971 [DOI] [PubMed] [Google Scholar]
- 124.Brandis M, Keyes J, Windhager EE: Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol 222: 421–427, 1972 [DOI] [PubMed] [Google Scholar]
- 125.Stokes JB: Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle’s loop. J Clin Invest 70: 219–229, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 127.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S: Expression and phosphorylation of the Na+-Cl- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Frindt G, Palmer LG: Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Palmer LG, Antonian L, Frindt G: Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frindt G, Shah A, Edvinsson J, Palmer LG: Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol 296: F347–F354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang WH, Schwab A, Giebisch G: Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol 259: F494–F502, 1990 [DOI] [PubMed] [Google Scholar]
- 132.Frindt G, Houde V, Palmer LG: Conservation of Na+ vs. K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol 301: F14–F20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kotchen TA, Guthrie GP, Jr, Galla JH, Luke RG, Welch WJ: Effects of NaCl on renin and aldosterone responses to potassium depletion. Am J Physiol 244: E164–E169, 1983 [DOI] [PubMed] [Google Scholar]