Abstract
Aims
Although current American Heart Association guidelines address C-reactive protein concentration and cardiovascular disease risk, it remains unclear whether this paradigm is consistent across populations with differing disease burdens. Individuals with Type 2 diabetes mellitus represent one group at increased risk of cardiovascular disease and subsequent mortality. This study aimed to examine the relationship between C-reactive protein concentrations and risk for all-cause mortality in European Americans with Type 2 diabetes from the Diabetes Heart Study.
Methods
A total of 846 European Americans with Type 2 diabetes and baseline measures of C-reactive protein were evaluated. Vital status was determined after a follow-up period of 7.3 ± 2.1 years (mean ± SD). C-reactive protein concentrations were compared between living and deceased subgroups along with other known risk factors for cardiovascular disease, including blood lipids. Logistic regression was performed to determine risk for mortality associated with increasing C-reactive protein concentrations.
Results
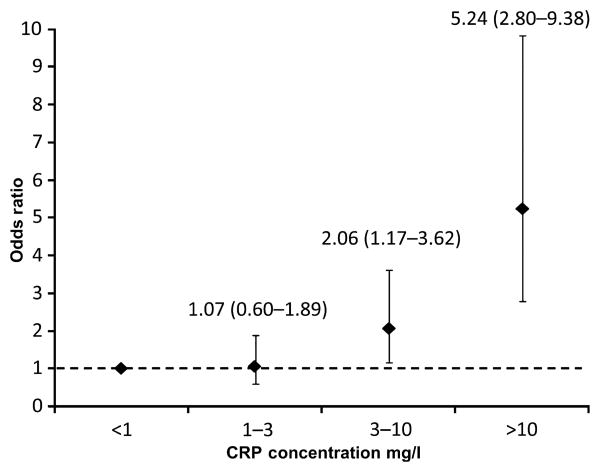
At follow-up 160 individuals (18.7%) were deceased. No significant differences in baseline serum glucose or lipid measures were observed between living and deceased subgroups. Baseline C-reactive protein concentrations were significantly higher in the deceased subgroup (9.37 ± 15.94) compared with the living subgroup (5.36 ± 7.91 mg/l; P < 0.0001). Participants with C-reactive protein concentrations of 3–10 mg/l were approximately two times more likely to be deceased at follow-up (OR 2.06; 95% CI 1.17–3.62); those with C-reactive protein >10 mg/l were more than five times more likely to be deceased (OR 5.24; CI 2.80–9.38).
Conclusions
This study documents the utility of C-reactive protein in predicting risk for all-cause mortality in European Americans with Type 2 diabetes and supports its use as a screening tool in risk prediction models.
Keywords: inflammation, mortality, risk-prediction, Type 2 diabetes
Introduction
The volume of literature examining the utility of C-reactive protein (CRP) in prediction of cardiovascular disease events is vast. Several large meta-analyses, including from 24 to 83 investigations, consistently report positive associations between increasing CRP concentration and risk for cardiovascular disease events after adjustment for other conventional cardiovascular disease risk factors [1–4]. Based on this literature, the American Heart Association has established CRP > 3 mg/l as a threshold beyond which risk for cardiovascular disease events is increased above the population average (http://www.americanheart.org/presenter.jhtml?identifier=4648). Further, definition of cardiovascular disease risk groups based on CRP concentrations has also been proposed with CRP < 1 mg/l considered to reflect low risk, CRP 1–3 mg/l reflecting medium risk, CRP 3–10 mg/l reflecting high risk and CRP > 10 mg/l considered as very high risk [5,6].
In addition to contributing to risk for cardiovascular disease events, CRP has been investigated as a predictor of mortality; however, it remains uncertain as to whether this relationship holds consistently across populations with differing disease burdens. One prospective study examining cardiovascular disease trends in a predominately non-diabetic sample (approximately 7% diabetes affected) reported CRP concentrations >3 mg/l to be associated with 1.88 times greater risk of all-cause mortality [7]. Another study in a sample with established cardiovascular disease risk (about 15% diabetes affected) reported CRP concentrations > 3 mg/l to be associated with a 1.38 times greater risk for all-cause mortality, but upon stratification by cardiovascular disease severity found that CRP was only predictive of mortality when detectable atherosclerosis was present [8]. It is unknown whether CRP is of similar value in predicting mortality in individuals with Type 2 diabetes mellitus, a group at markedly increased risk of cardiovascular disease and associated mortality. Of particular interest, CRP concentrations are elevated in patients with Type 2 diabetes [9–11], with mean values above the American Heart Association risk threshold of 3 mg/l, supporting higher inherent risk in this group. The aim of this study was to examine the relationship between CRP concentrations and risk for mortality in European Americans with Type 2 diabetes from the Diabetes Heart Study.
Patients and methods
Participant ascertainment and Diabetes Heart Study design have described previously [12–14]. All protocols were approved by the Institutional Review Board at Wake Forest School of Medicine and participants provided written informed consent before participation.
At recruitment participants underwent examination including interview for medical history and health behaviours, anthropometric measures, resting blood pressure, electrocardiography, fasting blood sampling for laboratory analyses, urine collection and an extensive assessment of subclinical cardiovascular disease, including coronary artery calcified plaque. Participants were required to be free of all infectious illness symptoms at the time of the study visit. Coronary artery calcified plaque was measured using fast-gated helical computed tomography scanning, with calcification quantified as previously described and recorded as an Agatston score [15]. Standard laboratory analyses included a serum lipid profile, glucose, HbA1C and CRP. The CRP concentrations were classified as: low (<1 mg/l), medium (1–3 mg/l), high (3–10 mg/l) and very high (> 10 mg/l) risk categories.
Participants were followed over an approximate 7-year period and vital status recorded based on family report and/or confirmation against the Social Security Death Index maintained by the United States Social Security Administration. The length of follow-up for each participant was determined from the date of the initial study visit to the beginning of 2011, or to the confirmed date of death.
Descriptive statistics are expressed as mean ± standard deviation. Variables were transformed, as appropriate, before analysis to approximate conditional normality. C-reactive protein concentrations were log transformed and compared between living and deceased subgroups using a t-test for unpaired samples and logistic regression completed to determine the risk for mortality associated with a 1 SD increase in log CRP concentration adjusted for potential confounders. A proportional hazards model was also completed to confirm these findings using length of follow-up to reflect survival time. Odds ratios and 95% confidence intervals were determined. Analyses were completed using SAS Enterprise Guide v4.2 (SAS Institute Inc, Cary, NC, USA). Statistical significance was accepted at P < 0.05.
Results
A total of 846 European Americans with Type 2 diabetes from 403 families were evaluated. Overall, 160 individuals (18.7%) were deceased over an average follow-up period of 7.3 ± 2.1 years. The deceased group was significantly older, more likely to be male, had longer Type 2 diabetes duration, lower BMI and higher coronary artery calcified plaque scores (Table 1). There were no significant differences in baseline serum glucose or lipid measures between living and deceased groups.
Table 1. Demographic characteristics and baseline laboratory measures, including C-reactive protein (CRP) concentrations, in living and deceased sub-groups (based on vital status at about 7 years).
| Living (n = 686) | Deceased (n = 160) | P | |
|---|---|---|---|
| Age | 61.5 ± 8.6 | 66.3 ± 9.1 | < 0.0001 |
| Gender (%male) | 47.1% | 56.3% | 0.0432 |
| Diabetes duration (years) | 10.1 ± 6.9 | 13.3 ± 8.2 | < 0.0001 |
| BMI (kg/m2) | 32.5 ± 6.4 | 30.9 ± 6.4 | 0.0060 |
| Hypertension (%) | 87.9% | 92.5% | 0.1250 |
| Self-reported previous cardiovascular disease (%) | 36.4% | 58.8% | < 0.0001 |
| Smoking – past or current (%) | 57.9% | 63.1% | 0.2464 |
| Current medication use | |||
| Insulin | 23.6% | 40.0% | < 0.0001 |
| Oral hypoglycaemics | 80.5% | 75.0% | 0.1290 |
| Lipid lowering medications | 47.6% | 45.8% | 0.7788 |
| Anti-hypertensives | 77.8% | 86.3% | 0.0221 |
| Serum glucose (mmol/l) | 8.12 ± 2.94 | 8.44 ± 3.82 | 0.8229 |
| HbA1C (%, mmol/mol) | 7.4 ± 1.4 (50 ± 20) | 7.7 ± 1.9 (52 ± 26) | 0.0797 |
| Total cholesterol (mmol/L) | 4.68 ± 1.30 | 4.73 ± 1.15 | 0.9726 |
| HDL cholesterol (mmol/l) | 1.12 ± 0.31 | 1.07 ± 0.33 | 0.1088 |
| LDL cholesterol (mmol/l) | 2.62 ± 0.83 | 2.68 ± 0.84 | 0.4585 |
| Triglycerides (mmol/L) | 2.32 ± 1.47 | 2.44 ± 1.74 | 0.7970 |
| Coronary artery calcified plaque (Agatston Score) | 1555 ± 3353 | 3360 ± 3467 | < 0.0001 |
| CRP (mg/L) | 5.36 ± 7.91 | 9.37 ± 15.94 | < 0.0001 |
| CRP risk groups | |||
| Low (< 1 mg/l) | 19.4% | 14.4% | 0.0016 |
| Medium (1–3 mg/l) | 34.1% | 24.4% | |
| High (3–10 mg/l) | 30.9% | 32.5% | |
| Very high (> 10 mg/l) | 15.6% | 28.8% | |
| Follow-up duration (years) | 7.9 ± 1.5 | 4.8 ± 2.5 | < 0.0001 |
All P-values < 0.05 were in bold typeface.
Baseline CRP concentrations were significantly higher in the deceased subgroup (living 5.36 ± 7.91 mg/l; deceased 9.37 ± 15.94 mg/l; P < 0.0001). Classification of CRP concentrations into low-, medium-, high- and very high-risk categories revealed a significantly different distribution of concentrations between the living and deceased subgroups (P = 0.0016), with a greater proportion of the deceased sub-group in the very high-risk category (Table 1).
Logistic regression revealed CRP as a predictor of mortality in a model including age, sex and BMI as covariates. A one SD increase in logCRP (equivalent to approximately 3.6 mg/l) was associated with about a twofold increased risk for mortality (OR 1.89; 95% CI 1.52–2.35; P < 0.0001). Adjustment for diabetes duration, smoking status (past/current) and history of previous cardiovascular disease did not influence the ability of CRP to predict morality (OR 1.87; 95% CI 1.50–2.32; P < 0.0001), and neither did adjustment for coronary artery calcification as a measure of subclinical cardiovascular disease burden (OR 1.78; 95% CI 1.42–2.23; P < 0.0001). Adjustment for medication use (insulin, oral hypoglycaemics, lipid-lowering medications and anti-hypertensives) also had minimal impact on the ability of CRP to predict mortality (OR: 1.71; 1.35–2.15; P <0.0001). Finally, stratification of CRP concentrations into risk categories confirmed that increasing CRP concentrations (relative to ≤ 1 mg/l) were associated with increasing risk for mortality (OR: 1.07–5.24) after adjustment for age, sex and BMI (Fig. 1). This stepwise increase in risk was maintained following further adjustment for diabetes duration and coronary artery calcified plaque (Supporting Information, Figure S1). A proportional hazard model confirmed the each one unit increase in log CRP (equivalent to around 2.7 mg/l) was associated with a 1.5-fold increase in risk for mortality (HR 1.536; 95% CI 1.334–1.769; P < 0.0001).
Figure 1.
Odds ratios for all-cause mortality for CRP risk categories (model corrected for age, gender and BMI).
Discussion
This study examined the association between CRP and all-cause mortality in a sample of European Americans with confirmed Type 2 diabetes. The findings demonstrated that CRP concentrations predict risk for all-cause mortality and that this prediction was independent of known confounders such as age, BMI, smoking, diabetes duration, history of prior cardiovascular disease, medication use and burden of subclinical cardiovascular disease (i.e. coronary artery calcified plaque). The potential utility of CRP as an independent predictor of mortality has also recently been described by Mohlenkamp et al. [16], in an otherwise healthy, predominately non-diabetic cohort. Notably, the risk for mortality among individuals with CRP concentrations above the American Heart Association risk threshold was higher in this sample of Type 2 diabetics than has been reported previously in the literature for predominately non-diabetic samples (HR: 1.38–1.88) [7,8]. These results support the utility of CRP in risk assessment of high-risk populations, where levels of risk may be further amplified.
Although a number of studies have previously examined the utility of CRP in prediction of cardiovascular disease and, to a lesser extent, mortality, the current study was limited to European Americans with Type 2 diabetes and high frequencies of other known cardiovascular disease risk factors, including dyslipidaemia, hypertension and subclinical cardiovascular disease [13]. Maintenance of the CRP–mortality paradigm in this high-risk sample provides additional evidence for the utility of CRP risk prediction across populations with differing disease burdens. Also of note was the fact that CRP concentrations were, on average, greater than twofold higher in this group with Type 2 diabetes compared to other investigations, including a large meta-analysis of CRP in primarily non-diabetic individuals without a history of cardiovascular disease [4] and another independent study examining relationships between vascular calcification, inflammation and mortality in an otherwise healthy cohort [16]. The elevated CRP concentrations observed here are consistent with a chronic inflammatory state in Type 2 diabetes which may contribute, in part, to the subsequent development of diabetic macrovascular and microvascular complications, and ultimately death.
It is noteworthy that the average CRP concentration in the Diabetes Heart Study sample is above the American Heart Association risk threshold of CRP > 3 mg/l, yet in this high-risk group, CRP concentrations are predictive of risk for all-cause mortality over a follow-up period of around 7 years. The length of this intervening period suggests the possible utility of CRP as a screening tool for the implementation of aggressive intervention and management approaches in high-risk individuals. Further, the apparent continuous relationship between CRP and risk for mortality beyond the American Heart Association risk threshold in high-risk subjects with Type 2 diabetes suggests that interventions that elicit even modest reductions in CRP concentrations should be explored as a possible benefit in the context of risk for mortality in already high-risk groups.
In conclusion, findings from the current study demonstrate that CRP concentrations predict risk for mortality in European Americans with Type 2 diabetes. It remains unclear whether CRP has a causal role in Type 2 diabetes-associated mortality by promoting inflammation or merely reflects underlying disease pathogenesis.
Supplementary Material
Figure S1. Odds ratios for all-cause mortality for CRP risk categories for corrected models.
Acknowledgments
This study was supported in part by R01 HL67348, R01 HL09230, and R01 NS058700 to D.W.B. The authors thank the other investigators, the staff, and the participants of the DHS study for their valuable contributions.
Abbreviation
- CRP
C-reactive protein
Footnotes
Competing interests: Nothing to declare.
Supporting Information: Additional Supporting Information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the US Preventive Services Task Force. Ann Intern Med. 2009;151:483–495. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 2.Hemingway H, Philipson P, Chen R, Fitzpatrick NK, Damant J, Shipley M, et al. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7:e1000286. doi: 10.1371/journal.pmed.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, McCormack V, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol. 2009;38:217–231. doi: 10.1093/ije/dyn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 6.Hamer M, Chida Y, Stamatakis E. Association of very highly elevated C-reactive protein concentration with cardiovascular events and all-cause mortality. Clin Chem. 2010;56:132–135. doi: 10.1373/clinchem.2009.130740. [DOI] [PubMed] [Google Scholar]
- 7.Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984–1998. Clin Chem. 2008;54:335–342. doi: 10.1373/clinchem.2007.100271. [DOI] [PubMed] [Google Scholar]
- 8.Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, et al. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. 2007;116:32–38. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 9.Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabak AG, Kivimaki M, Brunner EJ, Lowe GD, Jokela M, Akbaraly TN, et al. Changes in C-reactive protein levels before type 2 diabetes and cardiovascular death: the Whitehall II study. Eur J Endocrinol. 2010;163:89–95. doi: 10.1530/EJE-10-0277. [DOI] [PubMed] [Google Scholar]
- 11.Bowden DW, Lohman K, Hsu FC, Langefeld CD, Carr JJ, Lenchik L, et al. Hormone replacement therapy is associated with increased C-reactive protein in women with Type 2 diabetes in the Diabetes Heart Study. Diabet Med. 2006;23:763–767. doi: 10.1111/j.1464-5491.2006.01912.x. [DOI] [PubMed] [Google Scholar]
- 12.Bowden DW, Rudock M, Ziegler J, Lehtinen AB, Xu J, Wagenknecht LE, et al. Coincident linkage of type 2 diabetes, metabolic syndrome, and measures of cardiovascular disease in a genome scan of the diabetes heart study. Diabetes. 2006;55:1985–1994. doi: 10.2337/db06-0003. [DOI] [PubMed] [Google Scholar]
- 13.Bowden DW, Cox AJ, Freedman BI, Hugenschimdt CE, Wagenknecht LE, Herrington D, et al. Review of the Diabetes Heart Study (DHS) Family of studies: a comprehensively examined sample for genetic and epidemiological studies of type 2 diabetes and its complications. Rev Diabet Stud. 2010;7:188–201. doi: 10.1900/RDS.2010.7.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowden DW, Lehtinen AB, Ziegler JT, Rudock ME, Xu J, Wagenknecht LE, et al. Genetic epidemiology of subclinical cardiovascular disease in the diabetes heart study. Ann Hum Genet. 2008;72:598–610. doi: 10.1111/j.1469-1809.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal S, Morgan T, Herrington DM, Xu J, Cox AJ, Freedman BI, et al. Coronary calcium score and prediction of all-cause mortality in diabetes: the diabetes heart study. Diabetes Care. 2011;34:1219–1224. doi: 10.2337/dc11-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohlenkamp S, Lehmann N, Moebus S, Schmermund A, Dragano N, Stang A, et al. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol. 2011;57:1455–1464. doi: 10.1016/j.jacc.2010.10.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Odds ratios for all-cause mortality for CRP risk categories for corrected models.