Abstract
The protein α-synuclein is considered central to the pathogenesis of Parkinson disease (PD) on genetic and histopathological grounds. It is widely expressed in fetal life and continues to be highly expressed in adult neural tissues, red blood cells and platelets, while the remainder of adult tissues are reported to have little or no expression. Despite cellular and molecular evidence for a role in neuronal function including synaptic vesicle trafficking, neurotransmitter release, mitochondrial function, lipid metabolism, neurogenesis, neuroprotection, and neuromelanin biosynthesis, mice ablated for the gene encoding α-synuclein (Snca) have little or no neurological phenotype. Thus, nearly 20 years of intensive study have yet to reveal conclusively what the normal function of this highly abundant protein is in the nervous system. Interestingly, α-synuclein has also been shown to have enzymatic activity as a ferrireductase capable of reducing Fe+3 to Fe+2. Given its abundant expression in red blood cells, we set out to explore the role of α-synuclein in converting chemically-induced Fe+3 methemoglobin to normal Fe+2 hemoglobin. Initial in vivo experiments with the potent methemoglobin inducer, para-aminopropiophenone and its active metabolite, 4-hydroxy para-aminopropiophenone, demonstrated significantly greater and more prolonged methemoglobinemia in Snca−/− mice compared to Snca+/+ mice. In vitro experiments with red blood cells, however, and in vivo experiments in genetically engineered mouse strains that differ in their α-synuclein expression in various tissues, including the nervous system, red blood cells and liver, revealed that contrary to the initial hypothesis, a lack of expression of α-synuclein in red blood cells did not correlate with higher levels or more prolonged duration of methemoglobinemia. Instead, the greater sensitivity to chemically induced methemoglobinemia correlated with the absence of hepatic α-synuclein expression. We have uncovered a new and robust whole-animal phenotype in mice lacking α-synuclein that reflects its hitherto unrecognized role in xenobiotic detoxification.
Keywords: Alpha-synuclein, Methemoglobin, Parkinson disease, Para-aminopropiophenone, Hydroxylamine
1. Introduction
1.1. The α-synuclein protein is implicated Parkinson disease (PD)
The involvement of the α-synuclein protein in Parkinson disease (PD) rests on three primary strands of evidence: (1) missense and copy number mutations in the SNCA gene encoding α-synuclein cause autosomal dominant PD [30]; (2) polymorphic alleles near the SNCA gene, some of which have functional impact on α-synuclein expression, increase susceptibility to PD [30]; and (3) aggregated α-synuclein protein is a major constituent of Lewy bodies, the pathognomonic protein aggregates found in the nervous systems of sporadic PD patients [3], [28]. α-Synuclein has a wide tissue expression in fetal life [18] and continues to be very highly expressed in adult neural tissues, red blood cells (RBCs) and platelets [22], [25], [26], while the remainder of adult tissues of the rodent or human are reported to have little or no expression [31].
1.2. Functions of α-synuclein and results from knock-out mice
A bewildering array of cellular functions have been proposed for α-synuclein including synaptic vesicle trafficking, neurotransmitter release, mitochondrial function, lipid metabolism, neurogenesis, neuroprotection, and melanin biosynthesis [5], [6], [7], [11], [13]. Adding to the difficulty of determining its normal function is that mice ablated for the Snca gene appear to have little or no neurological phenotype [6], [7], [11], [13]. α-Synuclein has also been found to have enzymatic activity as an NADH-dependent ferrireductase capable of reducing Fe+3 to Fe+2 [5], [10]. As with the nervous system, no clear hematological phenotype has been reported in Snca−/− mice either. Thus, nearly 20 years of intensive study have yet to convincingly reveal α-synuclein's normal function in the nervous system or in hematopoietic tissues.
1.3. Hypothesis that α-synuclein is involved in recovery from chemically-induced methemoglobinemia
Genetic and biochemical data have clearly demonstrated that the soluble isoform of cytochrome b5 reductase-3 (E.C. 1.6.2.2), which utilizes electrons transferred from NADH via cytochrome b5, is the primary enzyme responsible for methemoglobin (methgb) reduction in the red blood cell [23]. Nonetheless, given α-synuclein's ferrireductase activity, combined with its high expression in red blood cells, we hypothesized that it might also function, perhaps in an ancillary role, in reducing Fe+3 in methgb to Fe+2 and regenerating normal hemoglobin. We therefore undertook a series of experiments exploring the role of α-synuclein in the recovery from chemically-induced methemoglobinemia. We used a series of genetically engineered mouse strains that differ in α-synuclein expression in various tissues, including the nervous system, RBCs and liver, to ask whether the presence or absence of α-synuclein in RBCs had an effect on recovery from methemoglobinemia. Initial in vivo experiments with the potent methgb inducer, 4-hydroxy para-aminopropiophenone (4-OHPAPP) and its pro-drug, para-aminopropiophenone (PAPP) [14], demonstrated a significant prolongation of methemoglobinemia in Snca−/− mice compared to Snca+/+ mice. In vitro experiments with RBCs, however, revealed that, contrary to the initial hypothesis, a lack of expression of α-synuclein in RBCs did not correlate with prolongation of methemoglobinemia. Instead, prolongation of chemically induced methemoglobinemia correlated with the absence of hepatic α-synuclein expression. We propose a new and robust whole-animal phenotype in Snca−/− mice, albeit one that is toxicological rather than neurological, that manifests as a defect in the detoxification of a xenobiotic in mice.
2. Methods
2.1. Mouse strains
The mice used in these experiments were all previously published [6], [16] and are summarized in Table 1. Experimental strains used were (1) mice transgenic for a P1-artificial chromosome (PAC) containing and expressing a wild-type human SNCA gene (PAC-Tg(SNCAWT)), (2) two independent lines of mice transgenic for a PAC containing and expressing an A53T mutant SNCA gene (PAC-Tg(SNCAA53T)), and two lines expressing an A30P mutant human SNCA gene (PAC-Tg(SNCAA30P)). The PAC-Tg(SNCAWT) line was bred onto the Snca−/− background and then intercrossed; mice homozygous for the transgene insertion and Snca knock-out allele (PAC-Tg(SNCAWT)+/+; Snca−/−) were selected for further study. The two PAC-Tg(SNCAA53T) lines were each bred onto the Snca−/− background to eliminate expression of the endogenous murine α-synuclein, intercrossed to be homozygous for the transgene insertion, and finally bred together to create a line of double transgenic animals that lacked murine Snca expression and were homozygous for both sites of the SNCAA53T PAC insertion (dbl-PAC-Tg(SNCAA53T)+/+;Snca−/−). The PAC-Tg(SNCAA30P) mice were similarly crossed to create double transgenic animals with both PAC-SNCAA30P insertion sites in an Snca−/− background (dbl-PAC-Tg(SNCAA30P)+/+;Snca−/−). However, because one of the two PAC-SNCAA30P lines carried its insertion on the X chromosome, only female dbl-PAC-Tg(SNCAA30P) were homozygous for both PAC insertions (four copies of the PAC insertions) whereas the males had three copies of the PAC insertions. Because all PAC transgenics were initially made in the FVB/N strain while the Snca knock-out was made in 129S6/SvEvTac, the PAC transgenic animals on a Snca−/− background were in a mixed FVB/N × 129S6/SvEvTac background. We therefore crossed wild-type FVB/N mice with wild-type or Snca knock-out (Snca−/−) 129S6//SvEvTac mice to create matched control cohorts of Snca+/+ or Snca−/− animals with a mixed background similar to the PAC transgenic animals.
Table 1.
Mouse strains used.
| Genotype | Abbreviated name |
|---|---|
| Snca+/+ | +/+ |
| Snca−/− | −/− (Snca knock-out) |
| PAC-Tg(SNCAWT)+/+; Snca−/− | wtPAC |
| PAC-dbTg(SNCAA53T)+/+; Snca−/− | dtgA53T |
| PAC-dblTg(SNCAA30P)+/+; Snca−/− | dtgA30P |
2.2. Reagents used
Para-aminopropiophenone (PAPP) was purchased from City Chemical LLC (West Haven, CT 06516) and 4-hydroxyl p-aminopropiophenone at >95% purity was provided by the Vanderbilt Institute of Chemical Biology, Chemical Synthesis Core (Vanderbilt University, Nashville, TN 37232-0412).
2.3. In vivo induction of methemoglobinemia
10 Snca+/+ and 11 Snca−/− mice received a single intraperitoneal injection of 33 nmoles/kg of PAPP dissolved at a concentration of 50 mg/ml in DMSO; 5 Snca+/+ and 6 Snca−/− mice received 40 nmoles/kg of 4-OHPAPP dissolved at a concentration of 50 mg/ml in DMSO. This dose is well below the LD50 of 2.3 mmole/kg for mice [27]. Control mice were injected with the same volume of DMSO without PAPP or 4-OHPAPP.
Blood samples were obtained by submandibular bleeding of mice using a 5 mm lancet (Goldenrod, Medipoint, Inc) into blood collection tubes prepared with K2EDTA·2H2O as an anticoagulant (Safe-T-Fill, Ram Scientific). Blood samples were taken at 1, 2 and 4 h after treatment for methgb measurements.
2.4. In vitro induction of methemoglobinemia
Blood was collected by submandibular bleeding into K2EDTA·2H2O collection tubes and blood from mice of the same genotype was pooled. RBCs were washed three times at room temperature in phosphate buffered saline, pH 7.4, and 328–330 Osm.
PAPP or PHAPP was dissolved in DMSO at 5 mg/ml and then diluted and added to washed erythrocytes from Snca+/+ and Snca−/− mice at 30% hematocrit at a final concentration of 3 μg/ml of drug and incubated at 37 °C. Blood was treated with DMSO vehicle alone as a control.
2.5. Measurement of methemoglobinemia
The percent methgb in a blood sample was determined by a modification of a previously published method [24]. In brief, 80 μl of blood was added to 1.2 ml of distilled water and mixed by inversion to hemolyze the red blood cells and to ensure complete oxygenation of hemoglobin. The mixture was left at room temperature for 2–3 min, then 0.24 ml of 0.5 M phosphate buffer pH 6.5 (0.22 M Na2HP04, 0.28 M KH2PO4) was added and the buffered hemolysate was immediately cooled on wet ice, followed by centrifugation at 1600 × g for 5 min at 4 °C. The cleared, buffered hemolysate is designated solution S (sample). A second dilution, solution R (for reference), was made by adding 0.2 ml of solution S to 1.0 ml of 0.1 M phosphate buffer, pH 6.5. 1 mg of K3Fe(CN)6 was added by adding 5 μl of a 20% w/v (200 mg/ml) solution in 0.1 M phosphate buffer, and mixed by inversion and left to stand at least 5 min at room temperature. 1 ml of solution S was added to a cuvette (10-mm light path) and the absorbance at 630 nm was measured = S1. Immediately following measurement of S1, 0.5 mg of KCN was added by adding 5 μl of a 10% w/v solution (100 mg/ml) to the cuvette, and mixed by inversion. After 2–3 min to allow for air bubbles to rise from the solution, the absorbance at 630 nm was read = S2. Solution R was transferred to a cuvette as above and the absorbance read at 630 nm = R1. Absorbance was then re-read at A630 after adding 5 μl of a 10% (100 mg/ml) w/v solution of KCN, mixing, and allowing it to stand for 2–3 min = R2. Percent methgb was calculated as 100(S1 − S2)/6(R1 − R2). The blood was incubated at 37 °C and triplicate samples taken at various time points for methgb measurements.
2.6. Statistical methods
Statistical significance of elevations of methgb between various mouse strains in vivo was assessed by one-tailed Mann–Whitney test (Prism, GraphPad Software). Extrapolated Y-intercept and slopes for in vitro studies were calculated by linear regression (Prism, GraphPad Software).
2.7. Western blotting method and antibody for α-synuclein
Blood samples were prepared by mixing blood with phosphate buffered saline buffer containing 0.5% NP-40 containing protease and phosphatase inhibitor tablets (Roche) in 1:2 ratio, then centrifuged for 20 min at 4 °C at 5000 rpm. An equal volume of the blood/PBS supernatant and SDS buffer (30 mM Tris, pH 6.8, 2.5% SDS, 5% glycerol) were mixed and vortexed. Then an equal total volume of 2X Laemmli buffer containing 10% beta-mercaptoethanol was added and the sample immediately loaded onto the gel. Tissue samples were prepared as described previously from mice that been perfused with phosphate-buffered saline during euthanasia to flush blood out of the various tissues [16]. Proteins were subjected to SDS-PAGE (12% Mini-PROTEAN TGX™ Gels -BIO RAD) and analyzed by western blot. Mouse anti-Snca (BD Transduction Laboratories) and horseradish peroxidase goat anti-mouse IgG (GE Healthcare) and either Clarity (BioRad) or Prime ECL kits (for high sensitivity) were used. To further increase sensitivity, cross-linking of α-synuclein was carried out by exposing blots to 0.4% paraformaldehyde in PBS for 30 min at room temperature prior to blocking [17].
3. Results
3.1. Chemical induction of methemoglobinemia in vivo
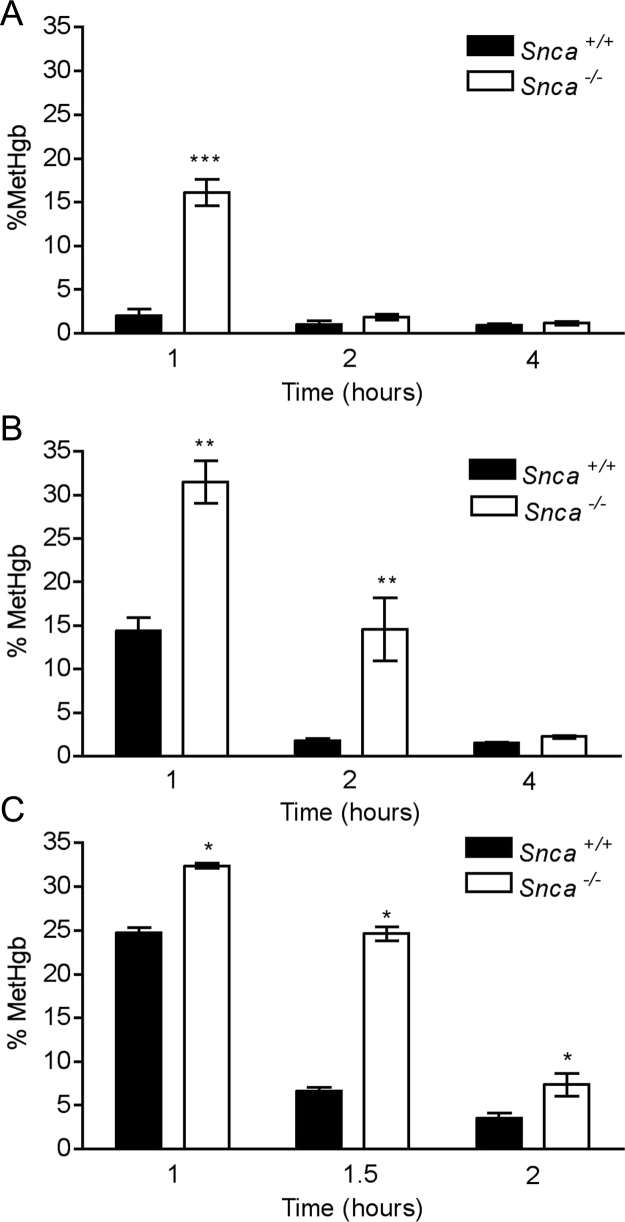
To test whether α-synuclein functions in vivo as a ferrireductase in RBCs, we asked whether Snca−/− mice would have elevated or more prolonged methemoglobinemia following exposure to a single intraperitoneal injection of PAPP (33 nmoles/kg in DMSO) or DMSO vehicle only. Blood was obtained 1, 2 and 4 h after injection for methgb measurement, expressed as a fraction of total hemoglobin (Fig. 1A). Wildtype (Snca+/+) mice had 2.4% methgb at 1 h following PAPP treatment, which then returned to baseline at 2 h. This time course is similar to what was previously observed in wildtype rats and mice treated with subcutaneous or intraperitoneal PAPP respectively [2], [27]. In contrast, mice lacking α-synuclein had 17% methgb 1 h after PAPP injection, a significantly greater level than in the wildtype mice (p = 0.00005, one-tailed Mann–Whitney test), with return nearly to baseline at 2 h and complete recovery by 4 h. Mice treated with DMSO vehicle only showed no methemoglobinemia. These experimental results were replicated 8 times with similar results (data not shown). The return of methgb levels to normal was not due to splenic sequestration or destruction of RBCs carrying elevated methgb since the hematocrits of both sets of animals were equal and normal, unchanged from baseline (data not shown). Thus, the methgb induction did not cause irreversible destruction of RBCs.
Fig. 1.
(A) Time course of methemoglobinemia following induction by a single i.p. injection of PAPP (33 nmoles/kg BW) in 10 Snca+/+ and 11 Snca−/− mice. Methemoglobinemia was prolonged at 1 h in Snca−/− versus Snca+/+ mice (***p = 0.00005, one-sided Mann–Whitney test). Five mice of each genotype were available at 2 and 4 h. No significant difference between Snca+/+ and 11 Snca−/− mice at 2 and 4 h. (B) Time course of methemoglobinemia following induction by a single i.p. injection of 4-OHPAPP(44 nmoles/kg BW) in 5 Snca+/+ and 6 Snca−/− mice. Methemoglobinemia was prolonged at 1 h and 2 h in 6 Snca−/− versus 5 Snca+/+ mice (**p = 0.0022, one-sided Mann–Whitney test). (C) Time course of methemoglobinemia following induction by a single i.p. injection of a larger dose of 4-OHPAPP(60 nmoles/kg BW) in 4 Snca+/+ and 4 Snca−/− mice. Methemoglobinemia was prolonged at 1, 1.5 and 2 h in Snca−/− versus Snca+/+ mice (*p = 0.05, one-sided Mann–Whitney test).
Since PAPP is a pro-drug that must be converted in vivo to its active methgb-producing metabolite, 4-OHPAPP [14], we repeated the in vivo induction of methgb with a single intraperitoneal injection of 4-OHPAPP (40 nmoles/kg in DMSO). As before, DMSO served as a vehicle-only control. 4-OHPAPP induced methgb in both Snca+/+ and Snca−/− strains of mice (Fig. 1B). The Snca−/− mice, however, had significantly greater levels of methemoglobinemia at 1 h (32% compared to 14% in Snca+/+ mice, p = 0.0022, one-tailed Mann–Whitney test). Methemoglobinemia remained significantly elevated at 14% in Snca−/− 2 h after injection, as compared to the methgb levels in the Snca+/+ mice, which had returned to baseline by that time (p = 0.0022, one-tailed Mann–Whitney test). Methgb levels returned to baseline in both genotypes by 4 h after injection. Although there was some variability when an approximately 50% higher dose of 4-OHPAPP (60 nmoles/kg) was used, the more prolonged methemoglobinemia in Snca−/− mice versus wildtype mice was replicated (p = 0.05, one-tailed Mann–Whitney test) (Fig. 1C).
3.2. Chemical induction of methemoglobinemia in vitro
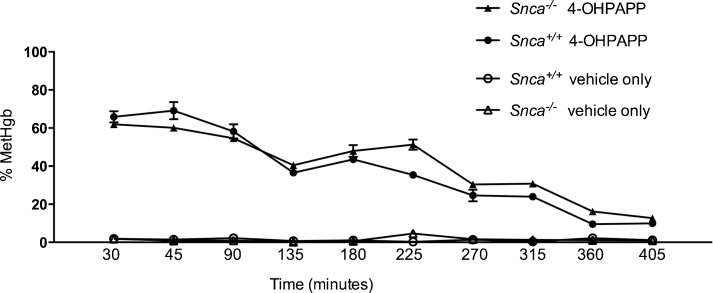
We then repeated the methgb induction experiments in vitro. We incubated RBCs from Snca+/+ and Snca−/− mice with 3 μg/ml PAPP, 3 μg/ml 4-OHPAPP, or DMSO vehicle alone. As expected, in vitro treatment of RBCs with the pro-drug PAPP did not induce methgb (data not shown). RBCs treated in vitro with 4-OHPAPP showed rapid and robust methgb production. What was surprising, however, was that the percent methgb at a given concentration of 4-OHPAPP was similar in both mouse strains and then slowly declined over a period of more than 6 h with a similar time courses (Fig. 2). Linear regression analysis comparing the slopes and extrapolated Y-intercepts were not statistically significantly different. There was also no significant hemolysis observed during the 6 h ours of incubation. There was, therefore, little evidence that α-synuclein in RBCs had an appreciable effect on the induction of, or recovery from, methgb induced by 4-OHPAPP in intact RBCs.
Fig. 2.
Time course of percent methemoglobin in washed RBCs from Snca+/+ and Snca−/− mice exposed to 3 μg/ml 4-OHPAPP. Each point represents the average of three measurements at a given time point for each genotype and treatment (4-OHPAPP versus vehicle only).
3.3. Sensitivity of transgenic mouse lines with differing expression in red blood cells and other tissues to chemically-induced methemoglobinemia
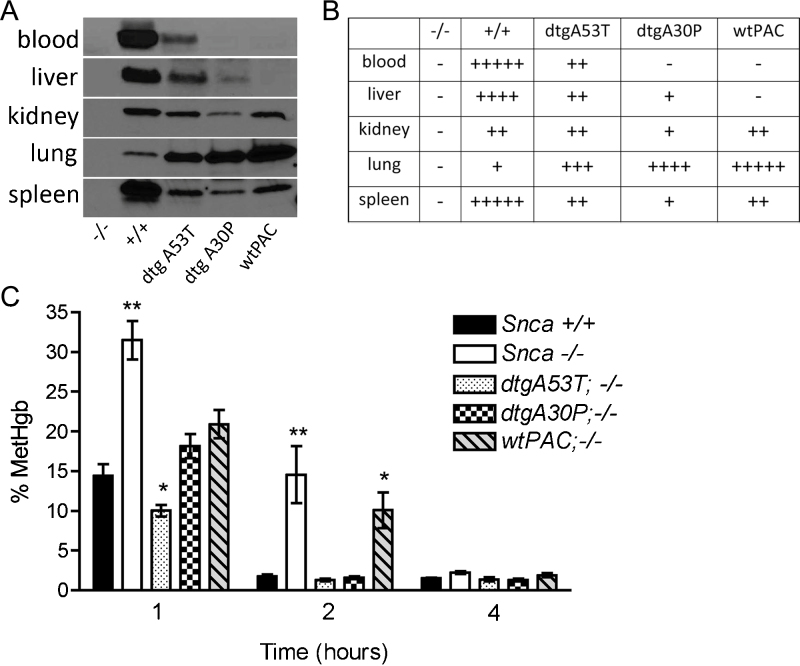
These results indicate that while expression of α-synuclein affects the response of mice to 4-OHPAPP and PAPP treatments, the different response is not due to α-synuclein's expression in RBCs. To investigate an alternative possibility, that α-synuclein is involved in detoxification of 4-OHPAPP, we used three transgenic mice in which the SNCA gene is expressed differently in various non-neuronal tissues. We have previously generated and published a series of transgenic mice made with human P1-artificial chromosomes (PACs) containing the wildtype, A53T mutant and A30P mutant versions of the human SNCA gene, and crossed them all onto a mouse background ablated for endogenous murine Snca (Table 1) [16]. In these mice, therefore, the only α-synuclein being made is human, encoded by the transgenic PACs. The transgenic mice show robust expression in the central and enteric nervous systems [16]. They do not, however, match wildtype mice in their expression of α-synuclein in non-neural tissues such as blood, liver, kidney, lung and spleen (Fig. 3A and B). In particular, while some of the PAC transgenic mice do express α-synuclein in RBCs and liver, others do not. These mice, therefore, offered an opportunity to dissect the role played by α-synuclein expression in different tissues in the persistent methgb we observed in Snca−/− mice following PAPP or 4-OHPAPP treatment. The fact that some of these mice expressed A53T or A30P mutant rather than wildtype α-synuclein is not an impediment since Davies et al. [10] found no difference in ferrireductase activity between wildtype α-synuclein and proteins with either the A53T or A30 P mutations.
Fig. 3.
(A) Western blot of α-synuclein in red blood cells, liver, kidney, lung and spleen. Each lane is labeled with the name of the mouse strain with defined various genotypes as described in Table 1. +/+ refers to Snca+/+, −/− refers to Snca−/−, dtgA53T refers to PAC-dbTg(SNCAA53T)+/+; Snca−/−, dtgA30P refers to PAC-dblTg(SNCAA30P)+/+; Snca−/−. wtPAC refers to PAC-Tg(SNCAWT)+/+; Snca−/−. (B) Table giving approximate expression levels of α-synuclein derived from western blot in Fig. 3A. The greater the number of “+” signs, the stronger the expression. A “−” sign means no signal. (C) Time course of methemoglobinemia following induction by a single i.p. injection of 4-OHPAPP(44 nmoles/kg BW) in mouse strains with defined genotypes as described in Table 1. Each measurement is the average of 5 mice. At 1 h, methemoglobinemia was elevated more than two-fold in Snca−/− versus Snca+/+ (**p = 0.0011, one-tailed Mann–Whitney test) and showed a barely significant decrease in A53T versus Snca+/+ (*p = 0.024, one-tailed Mann–Whitney test). At 2 h, methemoglobinemia was prolonged 5–7 fold in Snca−/− and wtPAC lines versus Snca+/+ mice (**p = 0.0022, *p = 0.016, respectively, one-tailed Mann–Whitney test). All lines recovered equally by 4 h.
Following in vivo exposure of the different PAC transgenic mice to 4-OHPAPP, we observed persistence of methgb, similar to what was seen with Snca−/− mice, in the wtPAC line, while others, including the dtgA53T and dtgA30P, demonstrated a rapid reversal of the methgb levels, similar to what has been seen in Snca+/+ mice (Fig. 3C). By comparing the pattern of expression (Fig. 3A and B) with the recovery of the different lines from 4-OHPAPP induced methemoglobinemia (Fig. 3C), it is clear that only α-synuclein expression in liver, which is absent in the Snca−/− and wtPAC lines, correlated with prolonged methemoglobinemia. Furthermore, the lack of α-synuclein expression in red cells in the dtgA30P line, which showed rapid reversal of methemoglobinemia, and the robust α-synuclein expression in kidney, lung and spleen in the wtPAC line, which showed prolonged methemoglobinemia, indicate there is no correlation between expression in those tissues and prolonged methemoglobinemia. We conclude, therefore, that the persistence of methgb in Snca−/− mice did not correlate with expression of α-synuclein in RBCs, kidney, lung or spleen but, instead, correlated with liver expression.
4. Conclusions
4.1. Para-aminopropiophenone and 4-hydroxy para-aminopropiophenone
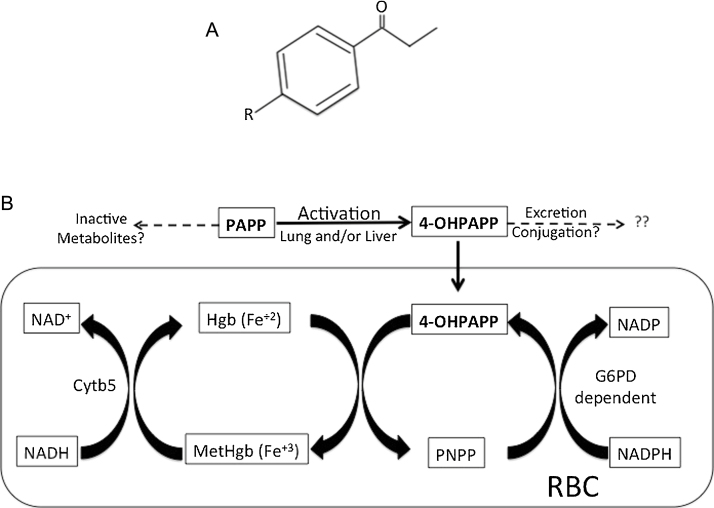
The high levels of α-synuclein in red blood cells, combined with its ferrireductase activity, led us to perform a series of experiments exploring the role of α-synuclein in reversing chemically-induced methemoglobinemia, despite there being extensive evidence that soluble cytochrome b5 reductase-3 is the primary enzyme responsible for methgb reduction in the red blood cell [23]. For these experiments, we used the compound PAPP and its active metabolite 4-OHPAPP (Fig. 4A). As with other derivatives of aniline, PAPP is converted into its hydroxylamine derivative 4-OHPAPP, which then directly generates methgb [1], [15]. Whether the activation of PAPP to 4-OHPAPP occurs in the liver [9] and/or in the lung [29] is not known. Serum levels of 4-OHPAPP following PAPP administration are quite low but the compound is still able to generate far more methgb than would be predicted by simple stoichiometry because, once inside the RBC, it binds tightly to hemoglobin, oxidizes hemoglobin to generate methgb and is converted to p-nitrosopropiophenone (PNPP). PNPP is then converted back to 4-OHPAPP by NADPH generated by the pentose monophosphate pathway, a cycle known as the Kreisprozess (Fig. 4B) [1]. Little is known about the metabolic fate of 4-OHPAPP outside the Kreisprozess [1], [19].
Fig. 4.
(A) Structure of compounds referred to in text. R NH2 in p-aminopropiophenone (PAPP), R NHOH in 4-hydroxyl p-aminopropiophenone; R NO2 in p-nitrosopropiophenone (PNPP). (B) Metabolism of PAPP and pathways involved in generating methgb (adapted from Baskin and Fricke [1], Jagow et al. [32], Bright and Marrs [4], Marrs and Bright [20], and Marrs et al. [21]).
4.2. Rejecting the hypothesis that α-synuclein participates directly in correcting methemoglobinemia
A direct role for α-synuclein in recovery from methgb induction was initially supported by in vivo experiments in which either the prodrug PAPP or its active metabolite 4-OHPAPP delivered i.p. showed markedly prolonged methgb levels in Snca−/− mice as compared to Snca+/+ mice. This hypothesis, however, had to be discarded when in vitro experiments with blood samples from Snca−/− and Snca+/+ mice showed no difference in the persistence of methgb induced by 4-OHPAPP. We decided to take advantage of a series of transgenic mice that either expressed or did not express wildtype or mutant α-synuclein in RBCs and other non-neuronal tissues such as liver, kidney, lung and spleen to examine the relationship between tissue expression of α-synuclein and recovery from 4-OHPAPP treatment in vivo. Detecting even the low levels of expression of α-synuclein in some of these tissues is made possible by crosslinking α-synuclein on the western blot membrane to increase antibody reactivity and using highly sensitive Western blotting reagents. Using this technique, wildtype Snca+/+ mice showed expression in the liver, as did some of the transgenic animals, although below the level present in the wildtype mice. We found prolongation of methemoglobinemia following 4-OHPAPP treatment correlated with a lack of expression of α-synuclein in liver, and not in RBC, kidney, lung or spleen. Although this was a surprising result in view of older published literature asserting α-synuclein was poorly expressed, if at all, in adult liver [18], [31], our observation of liver expression is supported by the PaxDb database of protein abundance, which indicates there is expression of the protein in liver, albeit at baseline levels that are ∼1/30th to 1/40th that of brain [33]. Baseline expression levels, however, may be an underestimate as it has been shown that α-synuclein expression can be strongly transcriptionally upregulated by liver X receptor ligands [8].
4.3. Implications of these findings for Parkinson disease research
In summary, we have shown that prolongation of methemoglobinemia following PAPP or 4-OHPAPP treatment is a novel phenotype for Snca−/− mice. The phenotype does not reflect a direct effect of α-synuclein on reduction of methemoglobin since there was no difference in vitro between RBCs with and without α-synuclein in their response to chemically induced methemoglobinemia. Instead, the persistence of methemoglobinemia following PAPP or 4-OHPAPP treatment in vivo correlated with the absence of even the normally low levels of α-synuclein in the liver. The increased sensitivity of Snca−/− mice to PAPP-induced methemoglobinemia cannot simply be the result of increased conversion of the prodrug PAPP to 4-OHPAPP since 4-OHPAPP itself results in an even more striking difference in the level and duration of methemoglobinemia in Snca−/− mice than does PAPP. We believe the methemoglobinemia induced by 4-OHPAPP simply serves as a biomarker for α-synuclein's role in the metabolism of 4-OHPAPP and, by extension, potentially other xenobiotic toxicants as well. Such a role adds a new dimension to the longstanding debate over the pathogenesis of PD, i.e. whether the disease is caused by environmental exposure to toxicants [12] or by a genetic susceptibility conferred by, among other genetic factors, increased expression or mutation of α-synuclein [30]. Instead, we propose that a direct interaction between α-synuclein and the environmental toxicants it participates in detoxifying could cause damage to this and other proteins, leading to aggregation and the initiation of a process that ultimately leads to PD.
Transparency document
Acknowledgements
Supported by grant NIH/NIEHS grant R01 ES017793.
We thank Dr. Ianai Fishbein for discussions and careful reading of the manuscript.
References
- 1.Baskin S.I., Fricke R.F. The pharmacology of p-aminopropiophenone in the detoxification of cyanide. Cardiovasc. Drug Rev. 1992;10:358–375. [Google Scholar]
- 2.Beutler E., Mikus B.J. The effect of sodium nitrite and para-aminopropriophenone administration on blood methemoglobin levels and red blood cell survival. Blood. 1961;18:455–467. [PubMed] [Google Scholar]
- 3.Braak H., Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv. Anat. Embryol. Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 4.Bright J.E., Marrs T.C. Kinetics of methaemoglobin production (2). Kinetics of the cyanide antidote p-aminopropiophenone during oral administration. Hum. Toxicol. 1986;5:303–307. doi: 10.1177/096032718600500502. [DOI] [PubMed] [Google Scholar]
- 5.Brown D.R. Alpha-synuclein as a ferrireductase. Biochem. Soc. Trans. 2013;41:1513–1517. doi: 10.1042/BST20130130. [DOI] [PubMed] [Google Scholar]
- 6.Cabin D.E., Shimazu K., Murphy D., Cole N.B., Gottschalk W., McIlwain K.L., Orrison B., Chen A., Ellis C.E., Paylor R., Lu B., Nussbaum R.L. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J. Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandra S., Gallardo G., Fernandez-Chacon R., Schluter O.M., Sudhof T.C. Alpha-synuclein cooperates with CSP alpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Cheng D., Kim W.S., Garner B. Regulation of alpha-synuclein expression by liver X receptor ligands in vitro. Neuroreport. 2008;19:1685–1689. doi: 10.1097/WNR.0b013e32831578b2. [DOI] [PubMed] [Google Scholar]
- 9.Coleman M.D., Kuhns M.J. Bioactivation of the cyanide antidote 4-aminopropiophenone (4-PAPP) by human and rat hepatic microsomal enzymes: effect of inhibitors. Environ. Toxicol. Pharmacol. 1999;7:75–80. doi: 10.1016/s1382-6689(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 10.Davies P., Moualla D., Brown D.R. Alpha-synuclein is a cellular ferrireductase. PLoS ONE. 2011;6:e15814. doi: 10.1371/journal.pone.0015814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis C.E., Murphy E.J., Mitchell D.C., Golovko M.Y., Scaglia F., Barcelo-Coblijn G.C., Nussbaum R.L. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol. Cell. Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman S.M. Environmental toxins and Parkinson's disease. Annu. Rev. Pharmacol. Toxicol. 2014;54:141–164. doi: 10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- 13.Golovko M.Y., Faergeman N.J., Cole N.B., Castagnet P.I., Nussbaum R.L., Murphy E.J. Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry. 2005;44:8251–8259. doi: 10.1021/bi0502137. [DOI] [PubMed] [Google Scholar]
- 14.Graffe W., Kiese M., Rauscher E. The formation in vivo of p-hydroxylaminopropiophenone from p-aminopropiophenone and its action in vivo and in vitro. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1964;249:168–175. doi: 10.1007/BF00247008. [DOI] [PubMed] [Google Scholar]
- 15.Harrison J.H., Jr., Jollow D.J. Contribution of aniline metabolites to aniline-induced methemoglobinemia. Mol. Pharmacol. 1987;32:423–431. [PubMed] [Google Scholar]
- 16.Kuo Y.M., Li Z., Jiao Y., Gaborit N., Pani A.K., Orrison B.M., Bruneau B.G., Giasson B.I., Smeyne R.J., Gershon M.D., Nussbaum R.L. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum. Mol. Genet. 2010;19:1633–1650. doi: 10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee B.R., Kamitani T. Improved immunodetection of endogenous alpha-synuclein. PLoS ONE. 2011;6:e23939. doi: 10.1371/journal.pone.0023939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ltic S., Perovic M., Mladenovic A., Raicevic N., Ruzdijic S., Rakic L., Kanazir S. Alpha-synuclein is expressed in different tissues during human fetal development. J. Mol. Neurosci.: MN. 2004;22:199–204. doi: 10.1385/jmn:22:3:199. [DOI] [PubMed] [Google Scholar]
- 19.Marino M.T., Urquhart M.R., Sperry M.L., Bredow J.V., Brown L.D., Lin E., Brewer T.G. Pharmacokinetics and kinetic-dynamic modelling of aminophenones as methaemoglobin formers. J. Pharm. Pharmacol. 1997;49:282–287. doi: 10.1111/j.2042-7158.1997.tb06796.x. [DOI] [PubMed] [Google Scholar]
- 20.Marrs T.C., Bright J.E. Kinetics of methaemoglobin production. (1). Kinetics of methaemoglobinaemia induced by the cyanide antidotes, p-aminopropiophenone, p-hydroxyaminopropiophenone or p-dimethylaminophenol after intravenous administration. Hum. Toxicol. 1986;5:295–301. doi: 10.1177/096032718600500501. [DOI] [PubMed] [Google Scholar]
- 21.Marrs T.C., Inns R.H., Bright J.E., Wood S.G. The formation of methaemoglobin by 4-aminopropiophenone (PAPP) and 4-(N-hydroxy) aminopropiophenone. Hum. Exp. Toxicol. 1991;10:183–188. doi: 10.1177/096032719101000306. [DOI] [PubMed] [Google Scholar]
- 22.Nakai M., Fujita M., Waragai M., Sugama S., Wei J., Akatsu H., Ohtaka-Maruyama C., Okado H., Hashimoto M. Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson's disease, in erythropoietic lineage. Biochem. Biophys. Res. Commun. 2007;358:104–110. doi: 10.1016/j.bbrc.2007.04.108. [DOI] [PubMed] [Google Scholar]
- 23.Percy M.J., Lappin T.R. Recessive congenital methaemoglobinaemia: cytochrome b(5) reductase deficiency. Br. J. Haematol. 2008;141:298–308. doi: 10.1111/j.1365-2141.2008.07017.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodkey F.L., O’Neal J.D. Effects of carboxyhemoglobin on the determination of methemoglobin in blood. Biochem. Med. 1974;9:261–270. doi: 10.1016/0006-2944(74)90061-1. [DOI] [PubMed] [Google Scholar]
- 25.Scherzer C.R., Grass J.A., Liao Z., Pepivani I., Zheng B., Eklund A.C., Ney P.A., Ng J., McGoldrick M., Mollenhauer B., Bresnick E.H., Schlossmacher M.G. GATA transcription factors directly regulate the Parkinson's disease-linked gene alpha-synuclein. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10907–10912. doi: 10.1073/pnas.0802437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin E.C., Cho S.E., Lee D.K., Hur M.W., Paik S.R., Park J.H., Kim J. Expression patterns of alpha-synuclein in human hematopoietic cells and in Drosophila at different developmental stages. Mol. Cells. 2000;10:65–70. doi: 10.1007/s10059-000-0065-x. [DOI] [PubMed] [Google Scholar]
- 27.Smith R.P., Alkaitis A.A., Shafer P.R. Chemically induced methemoglobinemias in the mouse. Biochem. Pharmacol. 1967;16:317–322. doi: 10.1016/0006-2952(67)90033-0. [DOI] [PubMed] [Google Scholar]
- 28.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. α-Synuclein in lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 29.Tepperman J., Bodansky O. The role of hepatic detoxification in p-aminopropiophenone induced methemoglobinemia. J. Pharmacol. Exp. Ther. 1946;88:287–299. [PubMed] [Google Scholar]
- 30.Trinh J., Farrer M. Advances in the genetics of Parkinson disease. Nat. Rev. Neurol. 2013;9:445–454. doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- 31.Ueda K., Fukushima H., Masliah E., Xia Y., Iwai A., Yoshimoto M., Otero D.A., Kondo J., Ihara Y., Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Jagow R., Kiese M., Renner G. Urinary excretion of n-hydroxy derivatives of some aromatic amines by rabbits, guinea pigs, and dogs. Biochem. Pharmacol. 1966;15:1899–1910. doi: 10.1016/0006-2952(66)90219-x. [DOI] [PubMed] [Google Scholar]
- 33.Wang M., Weiss M., Simonovic M., Haertinger G., Schrimpf S.P., Hengartner M.O., von Mering C. PaxDb, a database of protein abundance averages across all three domains of life. Mol. Cell. Proteomics. 2012;11:492–500. doi: 10.1074/mcp.O111.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.