Abstract
Background:
Cerebral ischemia causes some disorders in behavioral patterns, including memory disorders and pain, which is due to the production of free radicals. Ferulago angulata, known in Iran as chavir, contains some bioactive compounds having antioxidant and free radical-scavenging properties.
Objectives:
This study aimed to evaluate the effect of two weeks oral administration of hydroalcoholic extract of F. angulate (100, 200 and 400 mg/kg) on pain as well as active and passive avoidance memories after permanent, bilateral common carotid artery occlusion or cerebral ischemia/hypoperfusion in male adult rats.
Materials and Methods:
In this study, 35 male rats were randomly allocated to test and control groups. To make animal model of permanent cerebral hypoperfusion/ischemia, carotid arteries were ligatured as upper and lower and cut bilaterally.
Results:
It was found that the administration of 400 mg/kg hydroalcoholic extract of F. angulate for two weeks after brain hypoperfusion ischemia increased the passive avoidance memory (P < 0.001) and latency time of painful tail reflex significantly (P < 0.05).
Conclusions:
Ferulago angulata extract, because of its antioxidant activities, is probably capable of removing free radicals and oxidant substances from brain and thus it can improve behavioral disorders in brain hypoperfusion ischemia model.
Keywords: Memory, Pain, Ischemia, Rat
1. Background
The most prevalent reason for ischemic stroke is a lack of sufficient blood flow to reach brain tissue (1). Interruption of forward blood flow at any point could lead to irreversible neural damage (1); stroke, depending on the location of a lesion, size of the region deprived from blood flow or extent of the induced bleeding, could cause a wide range of neurologic defects (2). Reduction of tissue oxygen in ischemic region of brain leads to disorder in performance of mitochondria and production of free radicals (3). Reduced activity and capacity of antioxidant enzymes and the increased number of free radicals cause serious damage to cell components such as lipid, protein and nucleic acid and ultimately induce cell death (4). Certain region of brain and some special types of neurons, including CA1 hippocampal pyramidal neurons, helios region and striatum are more sensitive to cerebral ischemia (5). Hippocampus and its related segments play a critical role in transmission of information from short-term to permanent memory (6). Occlusion of left and right common carotid artery for some minutes induces ischemia in this region (5) and damage to hippocampus causes amnesia, poor short-term and spatial memory and learning disorders. Also, existence of lesion causes development of different types of disorders in behavioral patterns (7, 8). Some studies have shown that survivors of stroke face pain resulted from stroke (9). Post-stroke pain could develop in slight, moderate or severe and constant or variable forms, in a definite part or the whole body (9). Ferulago angulata belongs to umbelliferae family. Its extract which contains phenolic compounds and has antioxidant and antidiabetic effects is considered one of the important herbs in Iran (10-12). According to studies by Sodeifian et al. (10) analysis of aerial parts of different species of Ferulago by ethanol has led to the identification of its several components, the most important of which are cis-ocimene, α-pinene, β-germacrene, σ-terpinene, trans-β-ocimene, germacrene D, limonene, bornyl acetate, myrcene, camphene, allo-neo-ocimene, β-pinene, bicyclogermacrene and sophrosyne, respectively (10). Phenolic compounds, due to having antioxidant properties, are capable of inhibiting free radicals; thus, they could be effective in preventing many diseases like cancer, cardiovascular and nervous diseases (13).
2. Objectives
In the current study, neuroprotective (neuronal protection) effects of F. angulata extract (FAE) were investigated in animal model of hypoperfusion-cerebral ischemia through studying the effect on pain (tail flick) and active and passive avoidance memories (shuttle box).
3. Materials and Methods
3.1. Animals and Experimental Procedure
Thirty-five adult male Wistar rats (220 ± 30 g) were obtained from Central Animal House of Jundishapur University of Medical Sciences, Ahvaz, Iran. They were housed individually in standard cages and maintained in a temperature-controlled room (21 ± 2°C) on a 12/12-h light/dark cycle, humidity of (50%- 55%) with food and water available ad libitum. The rats were randomly allocated to five equal numbered groups of 7 in each: 1) control; 2) ischemic group submitted to occlusion of both common carotid arteries (ischemia); 3) ischemic animals receiving two weeks administration of FAE (100 mg/kg, orally) (ischemia + FAE 100); 4) ischemic animals receiving two weeks administration of FAE (200 mg/kg, orally) (ischemia + FAE 200); 5) ischemic animals receiving two weeks administration of FAE (400 mg/kg, orally) (ischemia + FAE 400) (14).
3.2. FAE Preparation
FA plants were collected from the western parts of Iran (Khozestan). Miss Ghayour from Department of Botany, Islamic Azad University, Izeh Branch, Khozestan, Iran, identified the plant. The aerial parts of the plant were separated, air dried in shade for one week and milled to fine powder (electric mill, Panasonic Co. Japan). The powder was macerated in 75% ethanol for 72 hours at room temperature. The ethanol extract was evaporated to remove ethanol and FAE was obtained as a lyophilized powder (yield 35%) (14).
3.3. Brain Ischemia/Hypoperfusion Procedure
We used the Cechetti’s method (2010) with little modification. In summary, the rats were anesthetized by ketamine/xylazine (50.5 mg/kg, ip). A neck ventral midline incision was made and the common carotid arteries were then exposed and gently separated from the vagus nerve. Carotids were occluded at a 1-week interval between the interventions, the right common carotid being the first to be assessed and the left one being occluded 1 week later.
3.4. Passive Avoidance Memory
Using a shuttle box device, including two chambers of dark and light with the floor which was covered with steel metal wires with a 1-2 mm diameter in 1 cm distance and a device for generating an electrical current, a slight shock with 75 v and an alternating current of 0.3 mA was applied to sole of the animals for 3 sec only once (15). First, each animal was placed inside the shuttle box with an open guillotine door to get acquainted with the training device and freely move inside and outside the chamber. Then, the animal was put inside the light box and the latency time of animal's entrance into the dark box was recorded (learning). Upon the entrance of the animal into the dark chamber, a guillotine door was closed and an electrical shock was applied to its sole. After 24 hours (one day), latency time of the animal's entrance into the dark chamber (which had shock previously, but this time did not have any shock) was measured in terms of second as passive avoidance memory (step-through latency).
3.5. Pain Test
A tail-flick assay was performed using the tail-flick analgesia meter and radiant heat tail flick. A beam of light was focused on the dorsal surface of the tail approximately 8 cm from the tail’s tip. The intensity of the heat stimulus was adjusted so that the baseline latencies settled on 10 seconds. Each nociceptive test consisted of the mean value of three measurements in each rat (16).
3.6. Data Analysis
The data were expressed as mean ± SEM (Standard error mean). The significance level was determined by one-way ANOVA applying LSD’s post-hoc test (SPSS, 18). A value of P < 0.05 was considered significant.
4. Results
4.1. Passive Avoidance Memory
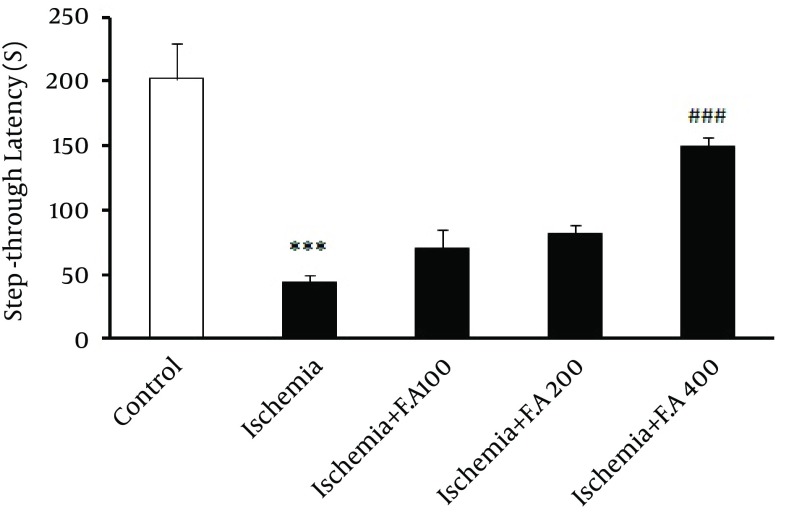
In this study, as seen in Figure 1, evaluating step-through latency time inside the shuttle box or latency of animals' entrance into the dark box (learning) showed a significant difference between the control and ischemic groups, which indicated decreased passive avoidance learning in the ischemic groups compared to the control. Moreover, the group receiving 400 mg/kg oral doses (gavage) of F. angulata for two weeks was significantly different from other ischemic groups, which indicated that this dose of F. angulata caused maximum increase in passive avoidance learning among other doses. Other doses of extract (100 and 200 mg/kg) did not show any significant difference compared to the other ischemia groups.
Figure 1. Mean ± SD of Step-Through Latency Time Inside the Shuttle Box Between Control Group, Ischemia and Ischemic Group Orally Receiving 100, 200 and 400 mg/kg FAE for two weeks.
*, This indicates a significant difference compared to the control; #, This indicates a significant difference compared to the ischemia (*** P < 0.001, ### P < 0.001).
4.2. Evaluating Tail Flick Pain Threshold
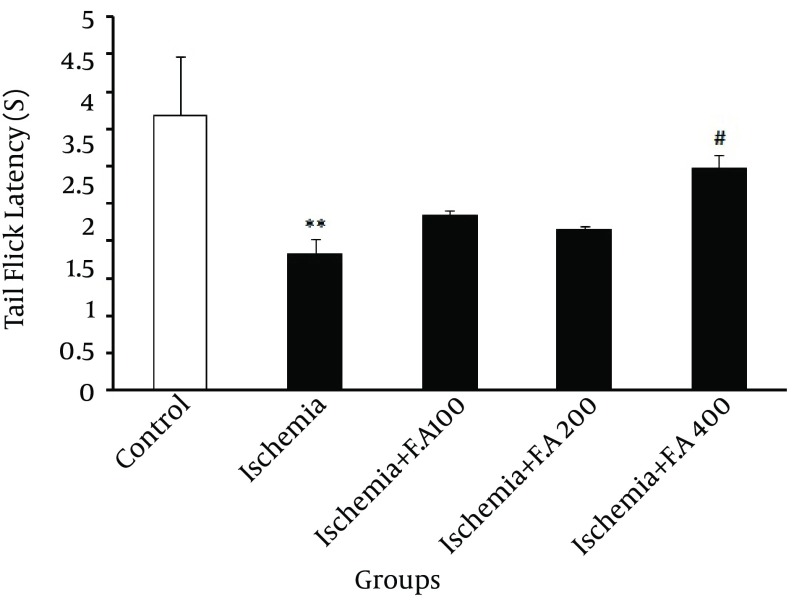
In Figure 2, evaluating the pain threshold was significantly different from time period of emergence of painful tail reflex or getting tail from heat focus in the control and ischemic groups, which indicated decreased latency time of emergence of painful tail reflex in the ischemic group. Also, among the groups receiving F. angulata extract for two weeks, only the group receiving 400 mg/kg dose was significantly different from the other ischemia groups, demonstrating that this dose of F. angulata caused a maximum increase in latency time of emergence of painful tail reflex among other doses.
Figure 2. Mean ± SD of Tail Flick Latency Between Control Group, Ischemia and Ischemic Group Orally Receiving 100, 200 and 400 mg/kg FAE for Two Weeks.
*, This indicates a significant difference compared to the control; #, this indicates a significant difference compared to the ischemia (# P < 0.05, ** P < 0.01).
5. Discussion
Results of the current study showed that ischemia caused disorders in behavioral patterns in a way that passive avoidance memory (latency time of entrance into dark area after 24 hours in shuttle box) and pain (latency time of emergence of painful reflex in tail) decreased in ischemic group and also it was observed that a two-week injection of F. angulata improved the disorders resulting from ischemia so that, among the injected doses of this study (100, 200 and 400 mg/kg), 400 mg/kg dose had the highest effect on increasing passive avoidance memory. Furthermore, the maximum increase of pain threshold and 100 and 200 mg/kg doses did not have such effects, which indicated effectiveness of this dose of the extract on learning disorders and ischemia-caused pain. Decreased amount of cerebral metabolites caused by decreased blood flow leads to decreased oxygen storage and consequently death of brain tissue and stroke (17). Oxidative stress as a main factor in inducing ischemic brain damage (18) causes disorders in oxidant-antioxidant balance and leads to probable damage (18). Also, it has a central role in pathogenesis of neurodegenerative and neurologic diseases like Alzheimer's and Parkinson, trauma and stroke (4, 19). Several studies have shown that production of free radicals increases after ischemia and Siesjo was a pioneer in proposing existence of several paths through which free radicals like peroxynitrite were produced during ischemia (20). Research evidence has shown that free radicals have an undeniable role in formation and expansion of edema and secondary lesions after stroke (21, 22). These lesions include several sensory and motion lesions like cognitive damage (passive avoidance learning) and pain disorders (23). Oxidative damage in peripheral neural cells often causes increased activity of glial cells and neural fibers and leads to release of pre-inflammatory factors, cytokine and glutamate. Also, it is considered a reinforcing factor of sensitivity to painful stimuli and peripheral neuropathic pain (24). Kirk et al. demonstrated that combination antioxidant treatment decreased pain and led to improvement of chronic pancreatitis (25). Moreover, in the present study, FAE increased pain threshold in ischemic rats, which confirmed previous studies. In several studies including those by it has been shown that one of the first parts that are destroyed after ischemia is hippocampus, which is a key region in memory and learning process (26-29). Recent studies have focused on the effect of using antioxidants on treatment of nervous system disorders and probably ischemia (30). This is on the assumption that, Oxidative stress occurs when there is an imbalance between the production of free radicals and antioxidants available. Accordingly, the administration of antioxidant supplementation can be used to scavenge free radicals and prevention of disease progression (30). The conducted studies in this regard have shown that many herbal substances and extracts like olive and saffron have antioxidant properties and thus have preventive effects on memory disorders resulting from ischemia and also reinforcing effects on memory and learning and the protective effect on neurons (31, 32). Moreover, antioxidant effects of some herbs and their main components improve weakness in learning resulting from ethanol in small rats (32). On the other hand, studies conducted in 2011 represented phenolic and antioxidant properties of FAE (4, 5). Also, these studies have stated that phenolic compounds, due to having antioxidant activity, can be be effective in preventing many diseases like ischemia through inhibiting free radicals and protecting cells against oxidative reactions (10, 13, 32). Ferulago angulata extract is considered a medicinal herb in western Asia (31) and is used in drug and food industries due to its polyphenolic compounds and great antioxidant properties (10, 11). This herb contains essential oils, which are used as food containers (33). Moreover, some species of this herb are used as flavoring and nutritious substances and sedatives and have digestive, antifungal and antimicrobial properties (10, 34, 35). Its extracted essence is also useful in cosmetics and perfume industry (10). According to studies conducted by Taran et al. (12) a subspecies of this herb called F. angulata carduchorum subsp. which belongs to this species (F.a) has antibacterial, antifungal (through inhibiting microorganisms) and antimicrobial properties against different infectious microbes; some of its compound with antimicrobial and antifungal effects include β–pinene, caryophyllene oxide, α–terpineol and 4-terpineol (12). F. angulata species has stronger antimicrobial properties than other species of this family (umbelliferae) (12).
it could be stated that post-ischemic functional disorders are mainly resulted from damage to cerebral cells due to produced oxidant substances (36), which attenuates passive avoidance memory and decreases pain threshold in rats. F. angulata extract, due to containing phenolic and antioxidant compounds, being able to remove oxidant substances from specific and important brain regions and consequently decreasing oxidative stress, could improve learning disorders and pain in hypoperfusion ischemic model.
Acknowledgments
The authors would like to thank the Research Deputy of Islamic Azad University, Izeh Branch for its support to carry out this study.
Footnotes
Funding/Support:This article was extracted from Mrs. Sahar Mirzapour Msc thesis. The research protocol number 13330519902001 was adopted by Islamic Azad University, Shahrekord Branch based on research ethics charter laws. This project was financially supported by the student herself.
References
- 1.Sodifilan GH, Ansaria K, Bamoniri K, Mirjalili B. Ischemic Stroke: Pathophysiology and Principles of Localization. Nephrol. 2011:1–16. [Google Scholar]
- 2.Black J, Hawks J. Medical-surgical nursing clinical management for positive outcomes. Nephrol. 2010:183–232. [Google Scholar]
- 3.Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 1997;75(10-11):1149–63. doi: 10.1139/y97-146. [DOI] [PubMed] [Google Scholar]
- 4.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Kesner RP, Adelstein TB, Crutcher KA. Equivalent spatial location memory deficits in rats with medial septum or hippocampal formation lesions and patients with dementia of the Alzheimer's type. Brain Cogn. 1989;9(2):289–300. doi: 10.1016/0278-2626(89)90038-9. [DOI] [PubMed] [Google Scholar]
- 6.Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL, Jr. Role of hippocampal CA3 mu-opioid receptors in spatial learning and memory. J Neurosci. 2004;24(12):2953–62. doi: 10.1523/JNEUROSCI.5569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmanzadeh F, Fathollahi Y, Semnanian S, Shafizadeh M. Dependence on morphine impairs the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Brain Res. 2003;965(1-2):108–13. doi: 10.1016/s0006-8993(02)04144-6. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadiasl N, Alaei H, Hanninen O. Effect of exercise on learning, memory and levels of epinephrine in rats' hippocampus. J Sports Sci Med. 2003;2(3):106–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain. 1995;61(2):187–93. doi: 10.1016/0304-3959(94)00144-4. [DOI] [PubMed] [Google Scholar]
- 10.Sodeifian GH, Ansari K, Bamoniri A, Mirjalili B. Study of chemichal composition of the essential oil of ferulago angulata (Schelchet) boiss. from iran using super critical fluid extraction and nano scale injection. Dig J Nanomater Biostruct. 2011:161–8. [Google Scholar]
- 11.Demetzos C, Perdetzoglou D, Gazouli M, Tan K, Economakis C. Chemical analysis and antimicrobial studies on three species of Ferulago from Greece. Planta Med. 2000;66(6):560–3. doi: 10.1055/s-2000-8652. [DOI] [PubMed] [Google Scholar]
- 12.Taran M, Ghasempour HR, Shirinpour E. Antimicrobial activity of essential oils of Ferulago angulata subsp. carduchorum. J J Microb. 2011;3(1):10–4. [Google Scholar]
- 13.Hosseini N, Akbari M, Ghafarzadegan R, Changizi Ashtiyani S, Shahmohammadi R. Total Phenol, Antioxidant and Antibacterial Activity of the Essential Oil and Extracts of Ferulago angulata ssp. J Med Plant. 2012;11(43):80–9. [Google Scholar]
- 14.Rafieirad M, Zangenehnezhad Z, Eydipour Z. Effects of oral ferulago angulate hydroalcoholic extract on liped peroxidation induced by ischemia /hypoperfusion in the hippocampus rat. Int J Biol Pharm Allied Sci. 2014;3:157–64. [Google Scholar]
- 15.Tamburella A, Micale V, Mazzola C, Salomone S, Drago F. The selective norepinephrine reuptake inhibitor atomoxetine counteracts behavioral impairments in trimethyltin-intoxicated rats. Eur J Pharmacol. 2012;683(1-3):148–54. doi: 10.1016/j.ejphar.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 16.Rafiei Rad M, Chardachirik SV. Gallic acid improves memory and pain in diabetic rats. Lorestan Univ Medl Sci. 2013:33–41. [Google Scholar]
- 17.Johansson BB. Current trends in stroke rehabilitation. A review with focus on brain plasticity. Acta Neurol Scand. 2011;123(3):147–59. doi: 10.1111/j.1600-0404.2010.01417.x. [DOI] [PubMed] [Google Scholar]
- 18.Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207(Pt 18):3221–31. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 19.Tarawneh R, Galvin JE. Potential future neuroprotective therapies for neurodegenerative disorders and stroke. Clin Geriatr Med. 2010;26(1):125–47. doi: 10.1016/j.cger.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siesjo BK. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1(2):155–85. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14(8):1505–17. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39(1):51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging. 2000;21(2):321–30. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 24.Serpell M. Anatomy, physiology andpharmacology of pain. Anaesth Intensive Care Med. 2005;24(10):7–10. doi: 10.1383/anes.6.1.7.57133. [DOI] [Google Scholar]
- 25.Kirk GR, White JS, McKie L, Stevenson M, Young I, Clements WD, et al. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. J Gastrointest Surg. 2006;10(4):499–503. doi: 10.1016/j.gassur.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Butler TL, Kassed CA, Sanberg PR, Willing AE, Pennypacker KR. Neurodegeneration in the rat hippocampus and striatum after middle cerebral artery occlusion. Brain Res. 2002;929(2):252–60. doi: 10.1016/s0006-8993(01)03371-6. [DOI] [PubMed] [Google Scholar]
- 27.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–20. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt W, Reymann KG. Proliferating cells differentiate into neurons in the hippocampal CA1 region of gerbils after global cerebral ischemia. Neurosci Lett. 2002;334(3):153–6. doi: 10.1016/s0304-3940(02)01072-8. [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Liu YM, Fratkins JD, LeBlanc MH. Grape seed extract suppresses lipid peroxidation and reduces hypoxic ischemic brain injury in neonatal rats. Brain Res Bull. 2005;66(2):120–7. doi: 10.1016/j.brainresbull.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Biessels GJ, Kerssen A, de Haan EH, Kappelle LJ. Cognitive dysfunction and diabetes: implications for primary care. Prim Care Diabetes. 2007;1(4):187–93. doi: 10.1016/j.pcd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, et al. Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled mediterranean herbs and aromatic plants. J Agric Food Chem. 2002;50(23):6882–90. doi: 10.1021/jf020540a. [DOI] [PubMed] [Google Scholar]
- 32.Saleem S, Ahmad M, Ahmad AS, Yousuf S, Ansari MA, Khan MB, et al. Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J Med Food. 2006;9(2):246–53. doi: 10.1089/jmf.2006.9.246. [DOI] [PubMed] [Google Scholar]
- 33.Ghasempour HR, Shirinpour E, Haidari H. Iran J Sci Tech. The constituents of essential oils os Ferulago angulata (schlecht) boiss at two different habitats, nevakoh and shahoo, zagroos mountain, western iran. 2007:309–12. [Google Scholar]
- 34.Baser KH, Demirci B, Demirci F, Hashimoto T, Asakawa Y, Noma Y. Ferulagone: a new monoterpene ester from Ferulago thirkeana essential oil. Planta Med. 2002;68(6):564–7. doi: 10.1055/s-2002-32557. [DOI] [PubMed] [Google Scholar]
- 35.Oztürk B, Gür S, Coskun M, Kosan M, Erdurak C, Hafez G, et al. 237 Relaxant effect of ferulago syriaca root extract on human corpus cavernosum. Eur Urol Suppl. 2004;3(2):62. doi: 10.1016/S1569-9056(04)90238-2. [DOI] [Google Scholar]
- 36.Rafie rad M, Sarkaki A, Hosaini E. The effect of grape seed extract on lipid peroxidation induced by ischemia / hypoperfusion in rat Stratum tissue. J Anim Biol. 2011:37–44. [Google Scholar]