Abstract
Background
Patients in the final stages of renal failure have accelerated inflammation conditions. Inflammation causes progressive kidney damage, faster progression of atherogenesis, chronic malnutrition and increased anemia, resulting in lower life expectancy of patients under dialysis. Statins have pleiotropic effects, because the drug has effects more than just decreasing lipids such as antioxidant effects, changes in endothelial dysfunction, stabilizing the plaque and immune system regulator.
Objectives
The aim of the study was to evaluate anti-inflammatory effect of simvastatin (one of the statins) in patients under hemodialysis.
Patients and Methods
In this clinical trial study, 40 patients under hemodialysis were studied for 12 weeks. Patients were divided into treatment (25 cases) and control groups (15 cases). The treatment group received a daily dosage of 20 mg of simvastatin, while the control group received no medication. The serum amounts of hs-CRP, IL6, Hb and WBC count were measured and compared at baseline and after 12 weeks. In addition, probable hepatic and muscular complications were studied in patients.
Results
At baseline, each of treatment and control groups had similar characteristics. During the study, the average level of CRP decreased in the treatment group (P = 0.04), while it was increased in the control group. The amount of serum IL-6 dropped in the treatment group (P = 0.01); however, it was increased in the control group. In both groups, the level of Hb increased significantly at the end of study in the treatment group (P = 0.007) and the control group (P = 0.016). The average WBC count decreased significantly in the treatment group and the control group (P = 0.003). There was no significant change in hepatic and muscular enzymes in the two groups.
Conclusions
End stage renal disease (ESRD) have accelerated inflammatory conditions. Simvastatin clearly lowers the serum levels of CRP and IL-6, and the white blood cell count in dialysis patients. Administering Simvastatin to dialysis patients is safe.
Keywords: Anti-inflammatory Agents, Simvastatin, Renal Dialysis
1. Background
It is common to see accelerated atherogenesis and resulting cardiovascular disease in patients with ESRD. Patients with chronic renal failure develop cardiovascular complications more likely than general population; these diseases are recognized as the most frequent causes of death in ESRD (end stage renal disease) (1). For these patients, in addition to common risk factors for accelerated atherosclerosis and cardiovascular disease, other recognized risk factors exist including: inflammation, proteinuria, lipoproteinemia, hyper homocysteinemia, anemia, hyperuricemia and metastatic calcification (2). Patients with chronic renal failure display higher inflammation levels and inflammatory factors in their blood compared to general population. Inflammatory factors increase vascular endothelial damage and thrombosis. Inflammation causes progression of kidney damage, as well as acceleration of decreased kidney function. In addition, higher levels of inflammation play a part in chronic rejection of kidney transplantation (3). Researches have shown that statin has an inhibitory effect on atherogenesis and lower instances of cardiovascular injuries (1, 4). Statin also inhibits the mevalonate path; this facilitates the effectiveness of both anti-inflammatory properties of the medication and what is called statin pleiotropic properties including antioxidation, anti-inflammation, regulation of the immune system and some others. Several indicators have shown that these drugs cause longer life expectancy in patients with chronic renal failure (5). In addition, it has been emphasized that statins are beneficial in other vascular, inflammatory and cancerous diseases (6). Statins have anti-inflammatory properties including increased function of the endothelium, reduced proliferation of mesangial cells and vascular smooth muscles and lowering endothelin, as well as protective properties for the kidney. Besides, they increase life expectancy of patients under dialysis (5, 7). In addition, by reducing inflammation and serum CRP level, they improve response to erythropoietin in these patients (8).
2. Objectives
In this research, while considering anti-inflammatory effects of statins as well as considering patients with kidney diseases, the level of reduction in inflammation was evaluated through measuring inflammatory factors.
3. Patients and Methods
In a clinical trial research, 50 patients undergoing hemodialysis at Imam Khomeini Hospital of Ahvaz were recruited. Exclusion criteria were current infection, malignancy, inflammation, major illness or recent surgery, recent cardiovascular illness, major internal illnesses, genetic hyperlipidemia and hypercholesterolemia needing treatment. In addition, patients with conditions such as myopathy and hepatic malfunction that could potentially increase side effects of drugs were excluded. Furthermore, those taking drugs like nicotinic acid, gemfibrozil and cyclosporine were also excluded. In total, 10 patients were excluded. Two cases were excluded due to tuberculosis, 1 due to cellulitis, 2 for positivity of HCV Ab, 2 due to lack of cooperation and 3 due to hypercholesterolemia that needed treatment. Finally 40 patients, 27 males and 13 female, were included. They were randomly divided into two groups; 25 patients received drugs, while 15 received none. The average age in both groups was 47. The two groups were similar regarding age, gender, history of cardiovascular disease, diabetes, smoking, dyslipidemia, duration of dialysis, consumption of antioxidants and ACEI (angiotensin converting enzyme inhibitor) at baseline. The study condition was thoroughly explained to patients and their written consent was obtained. Participants could leave the project at any time. The study was approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences. All patients had been under hemodialysis for longer than six months.
At baseline, inflammatory condition of patients was ascertained by measuring the levels of CRP (c reactive protein), IL6 (interleukin 6), WBC (white blood cell) count as well as Hb (hemoglobin) level; measurements were made for lipid profiles and levels of hepatic and muscular enzymes. Patients in the treatment group were given one 20 mg tablet of simvastatin, manufactured by Tehran Darou Company, daily. Other previously prescribed drugs were continued. During the study, patient was observed and monthly examined and laboratory tests for CPK, LDH, AST and ALT were performed to assess hepatic and muscular complications. Subsequently, after 12 weeks, the above–mentioned markers were measured and compared with baseline measurements. Eliza kit, made in Italy, was used to measure CRP and Human Eliza kit (manufactured by Bender Med system, Germany) was used to determine IL-6 serum level. Normal level of CRP was considered as < 3 mg/L, normal level of SGOT: 9-40 IU/L, normal level of LDH: 113-134 IU/L and normal level of CPK as 24-195 IU/L. Data was analyzed by SPSS version 17 software (IBM Statistical Package for the Social Science) and P < 0.05 was considered as significant.
4. Results
In this clinical trial study, 40 patients under dialysis were assessed in 12 weeks. They were divided into two groups; 25 cases received simvastatin and 15 cases were control. The mean age of treatment group was 47.3 ± 6.8 and in control group was 45.4 ± 6.4 years. Baseline characteristics of study group are shown in Table 1.
Table 1. Baseline Characteristics of Study Groups a.
| Treatment (n = 25) | Control (n = 15) | |
|---|---|---|
| Age, y | 47.3 ± 6.8 | 45.4 ± 6.4 |
| Gender | ||
| Male | 17 | 10 |
| Female | 8 | 5 |
| Smokers | 26 | 24 |
| Cardiovascular disease, % | 40 | 45 |
| Diabetes | 52 | 52 |
| Vitamin E consumption, % | 25 | 30 |
a Data are presented as Mean ± SD.
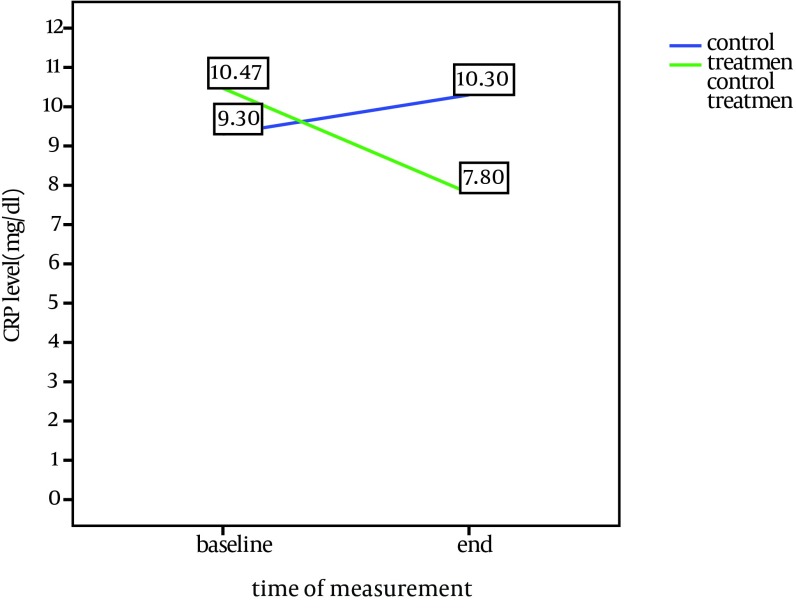
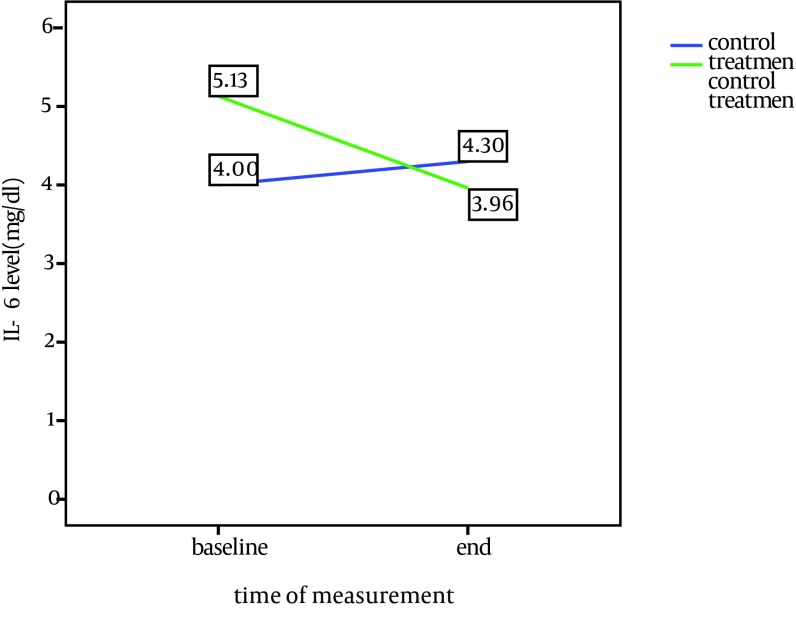
The average CRP level was higher than normal in all patients at baseline. During the study, the average level of CRP decreased in the treatment group (P = 0.04), while increased in the control group. The amount of serum IL-6 dropped in the treatment group (P = 0.01); however, it was increased in the control group. In both groups, the level of Hb increased significantly at the end of study in the treatment group (P = 0.007) and the control group (P = 0.016). The average WBC count decreased significantly in the treatment group and the control group (P = 0.003). There was no significant change in hepatic and muscular enzymes in the two groups.
Comparison of baseline and end of study characteristics in treatment and control groups is shown in Table 2. CRP and IL-6 changes during the study in both groups are shown in Figures 1 and 2.
Table 2. Comparison of Baseline and End of Study Characteristics in Treatment and Control Groups a.
| Variable | Control Group | Treatment Group | ||||
|---|---|---|---|---|---|---|
| Baseline | End of Study | P Value | Baseline | End of Study | P Value | |
| IL-6, pg/mL | 4 | 4.3 | NS | 5.13 | 3.96 | 0.01 |
| Hb, mg/dL | 9.9 | 10.65 | 0.016 | 10.1 | 10.9 | 0.007 |
| WBC, /µL | 7.18 × 103 | 7.15 × 103 | 0.3 | 8.6 × 103 | 6.7 × 103 | 0.003 |
| CRP, mg/L | 9.3 | 10.3 | NS | 10.47 | 7.8 | 0.04 |
| SGOT, IU/L | 22.6 | 19.7 | NS | 18.6 | 20.4 | NS |
| LDH, IU/L | 304 | 254 | NS | 186 | 205 | NS |
| CPK, IU/L | 61.3 | 80 | NS | 56.8 | 70 | NS |
a Abbreviations: NS, non-significant; WBC, white blood cell, LDH, Lactate dehyrogenase; CPK, reatine phosphokinase; SGOT, Serum glutamic oxaloacetic transaminase; CRP, C-reactive protein.
Figure 1. CRP Changes During the Study in the Both Groups.
Figure 2. IL-6 Changes During the Study in the Both Groups.
5. Discussion
In this research, the average level of CRP in all patients was higher than normal showing the necessity of treatments for reduction of inflammatory conditions because of their harmful effects. In the treatment group, the level of CRP decreased significantly in the end of study (P = 0.04). In comparison, in the control group, there was a slight increase. This shows the effectiveness of treatment with statins, which is in accordance with previous studies. In the Navarro study in Spain, CRP was reduced from 5.4 mg/L to 2.3 mg/L in patients under dialysis and those with diabetes or hyperlipidemia (2). In the Vernaglione study, CRP decreased from 9 mg /L to 5 mg/L in patients under dialysis, and in Chang et al. study from 0.23 to 0.12 mg/dL (4, 9). In the Panichi et al. study of patients with chronic renal failure (CRF), CRP decreased from 2.6 to 2 mg/L (10). Tsirpanlis et al. studied the effects of fluvastatin on patients under dialysis; there was no change in CRP in this study, but IL-6 decreased (11). In a study by Judith on patients under dialysis and those with hyperlipidemia , there was a drop in LDL, but there was no significant change in the serum CRP level, which might be due to using a different technique in dialysis and low number of patients under study (12).
In this study after treatment, the level of serum IL-6 decreased from 5.13 pg/mL to 3.69 pg/mL in the treatment group (P = 0.01). The average amount of IL-6 in a healthy population was 1.3 ± 3 pg/mL. In contrast, there was an increase of 0.3 pg/mL in the control group. Tonelli showed a decrease of IL-6 following treatment with fluvastatin in patients under dialysis (3). Similarly, in the Panichi et al. study on patients with CRF, IL-6 and CRP decreased with nearly 45 mL/min GFR from 5.1 pg/mL to 3.5 pg/mL (10). However, in Chang study, in contrast to CRP, there was no obvious change in IL-6, which was contributed to its diurnal changes even in healthy population and probably due to independence of production path of CRP from IL-6 (4).
Therefore, in this study both CRP and IL-6 showed significant decrease after treatment with simvastatin. Another point is that the higher the mentioned factors were, the more noticeable the change after treatment was. It seems that WBC level in patients had a significant decrease after treatment with simvastatin, and these changes were more notable in patients with higher level as well; although the changes cannot be completely attributed to simvastatin.
Hemoglobin changes were significant in both treatment and control groups. However, we expected that decrease in inflammatory conditions would increase erythropoiesis. This could be due to our inconsideration of other factors such as patients receiving erythropoietin and food supplements. Furthermore, we expected to observe an increase in Hb due to controlling uremic syndrome following dialysis. Finally, there were only minute changes, all within normal limits, in the amounts of hepatic and muscular enzymes after treatment; this indicates safety of using simvastatin in this group of patients.
Based on this study, considering its effect on inflammatory indicators such as IL-6 and CRP, simvastatin has the capability to control inflammatory conditions in patients with dialysis.
No particular complications were observed with these drugs in patients under hemodialysis.
Acknowledgments
This paper was issued from the thesis of Dr. Afagh Atrian (No: 2337). Special thanks to Golestan Hospital Clinical Development Research Unit for their help. Authors are thankful to Seyed Mahmoud Latifi for his cooperation in editing.
Footnotes
Funding/Support:Financial support was provided by Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
References
- 1.van den Akker JM, Bredie SJ, Diepenveen SH, van Tits LJ, Stalenhoef AF, van Leusen R. Atorvastatin and simvastatin in patients on hemodialysis: effects on lipoproteins, C-reactive protein and in vivo oxidized LDL. J Nephrol. 2003;16(2):238–44. [PubMed] [Google Scholar]
- 2.Navarro JF, Mora C, Muros M, Garcia-Idoate G. Effects of atorvastatin on lipid profile and non-traditional cardiovascular risk factors in diabetic patients on hemodialysis. Nephron Clin Pract. 2003;95(4):c128–35. doi: 10.1159/000074838. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G, Cholesterol. et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68(1):237–45. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang JW, Yang WS, Min WK, Lee SK, Park JS, Kim SB. Effects of simvastatin on high-sensitivity C-reactive protein and serum albumin in hemodialysis patients. Am J Kidney Dis. 2002;39(6):1213–7. doi: 10.1053/ajkd.2002.33393. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg B, Brannstrom M, Bucht B, Crougneau V, Dimeny E, Ekspong A, et al. Safety and efficacy of atorvastatin in patients with severe renal dysfunction. Scand J Urol Nephrol. 2005;39(6):503–10. doi: 10.1080/00365590510031255. [DOI] [PubMed] [Google Scholar]
- 6.Endres M. Statins: potential new indications in inflammatory conditions. Atheroscler Suppl. 2006;7(1):31–5. doi: 10.1016/j.atherosclerosissup.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.D'Amico G. Statins and renal diseases: from primary prevention to renal replacement therapy. J Am Soc Nephrol. 2006;17(4 Suppl 2):S148–52. doi: 10.1681/asn.2005121341. [DOI] [PubMed] [Google Scholar]
- 8.Sirken G, Cheng KS, Rasib R. Decreased erythropoietin requirements in maintenance hemodialysis patients with statin therapy. ASAIOJ. 2003;49:422–5. [PubMed] [Google Scholar]
- 9.Vernaglione L, Cristofano C, Muscogiuri P, Chimienti S. Does atorvastatin influence serum C-reactive protein levels in patients on long-term hemodialysis? Am J Kidney Dis. 2004;43(3):471–8. doi: 10.1053/j.ajkd.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Panichi V, Paoletti S, Mantuano E, Manca-Rizza G, Filippi C, Santi S, et al. In vivo and in vitro effects of simvastatin on inflammatory markers in pre-dialysis patients. Nephrol Dial Transplant. 2006;21(2):337–44. doi: 10.1093/ndt/gfi224. [DOI] [PubMed] [Google Scholar]
- 11.Tsirpanlis G, Boufidou F, Manganas S, Chantzis K, Bleta A, Stamatelou K, et al. Treatment with fluvastatin rapidly modulates, via different pathways, and in dependence on the baseline level, inflammation in hemodialysis patients. Blood Purif. 2004;22(6):518–24. doi: 10.1159/000082166. [DOI] [PubMed] [Google Scholar]
- 12.Judith M. Atorvastatin and Simvastatin in patients on hemodialysis. J Nephrol. 2003;16(2):238–44. [PubMed] [Google Scholar]