Significance
The opportunistic pathogen Pseudomonas aeruginosa thrives in cystic fibrosis (CF) lung sputum. Here, we define the essential genome of two P. aeruginosa strains in laboratory media and in CF sputum. We also use genomic methods to profile P. aeruginosa genetic requirements for fitness in both natural and synthetic CF sputum. Finally, we show that the essential genomes of different strains of P. aeruginosa are distinct, suggesting that the architecture of genetic networks is a primary determinant of a gene’s role in fitness. This has implications for the development of strain-independent therapeutics and underscores the importance of functional studies in pathogenic strains of interest.
Keywords: Pseudomonas aeruginosa, cystic fibrosis, Tn-seq, sputum, essential genes
Abstract
Defining the essential genome of bacterial pathogens is central to developing an understanding of the biological processes controlling disease. This has proven elusive for Pseudomonas aeruginosa during chronic infection of the cystic fibrosis (CF) lung. In this paper, using a Monte Carlo simulation-based method to analyze high-throughput transposon sequencing data, we establish the P. aeruginosa essential genome with statistical precision in laboratory media and CF sputum. Reconstruction of the global requirements for growth in CF sputum compared with defined growth conditions shows that the latter requires several cofactors including biotin, riboflavin, and pantothenate. Comparison of P. aeruginosa strains PAO1 and PA14 demonstrates that essential genes are primarily restricted to the core genome; however, some orthologous genes in these strains exhibit differential essentiality. These results indicate that genes with similar molecular functions may have distinct genetic roles in different P. aeruginosa strains during growth in CF sputum. We also show that growth in a defined growth medium developed to mimic CF sputum yielded virtually identical fitness requirements to CF sputum, providing support for this medium as a relevant in vitro model for CF microbiology studies.
The opportunistic pathogen Pseudomonas aeruginosa is a common cause of chronic cystic fibrosis (CF) lung infection. In the CF lung, P. aeruginosa grows to high densities (107–109 cfu/mL) within airway sputum, which likely serves as the nutritional source during infection (1, 2). Sputum is a complex mixture of airway mucus, inflammatory substances, and bacterial products. The inflammatory components include large numbers of polymorphonuclear leukocytes, dead host cells, and serum components that enter the airway due to vascular leakage and pulmonary hemorrhage (1). The generation time of P. aeruginosa in CF sputum is variable but can be as short as 40 min, suggesting that sputum provides a robust growth environment for P. aeruginosa (3, 4). Long-term colonization of the CF lung leads to the evolution of numerous presumed adaptive phenotypes including mucoidy, amino acid auxotrophy, loss of acute virulence factors, and antibiotic resistance (5–7). Despite the intense interest in P. aeruginosa CF lung infections, very little is currently known regarding the genetic requirements for P. aeruginosa survival and proliferation in sputum. The goal of this study was to address this knowledge gap using high-throughput genomics.
High-throughput genomic approaches such as transposon sequencing (Tn-seq) have been used to identify genetic elements required for in vitro and in vivo fitness (8). Tn-seq allows for simultaneous assessment of the abundance of tens or hundreds of thousands of individual transposon mutants after growth in a selective condition (e.g., in vivo infection model) (9–11). Comparing the abundance of mutants before and after growth in the selective condition allows for rapid identification of mutants with reduced fitness in that condition. Our laboratory recently used Tn-seq to reveal fitness requirements for P. aeruginosa during acute and chronic murine wound infection (12). A major finding of this study was that transcriptome-based approaches such as RNA sequencing cannot be used to predict fitness requirements (12, 13), thus calling into question the utility of previous P. aeruginosa CF sputum transcriptome results (3, 14) for elucidating fitness requirements in the CF lung.
Analysis of bacterial mutant fitness in an experimental condition can reveal many key features of bacterial physiology compared with appropriate controls. For example, the relative lack of mutants in a particular genetic element in a high-density transposon library can indicate the essentiality of that element. Previous studies have used several criteria to determine the immutable regions of a bacterial genome from Tn-seq data in the absence of control conditions, including the prevalence of transposon insertions detected per genetic element or the probability of encountering a DNA segment with no insertions given that segment’s length (15–17). However, these methods do not always account for two types of information available in Tn-seq data: (i) the abundance of each transposon mutant and (ii) the variability observed in Tn-seq biological replicates. These are important criteria to consider when making statistically sound declarations about the absence of transposon insertions in a particular gene. For example, if a few insertions interrupting a gene are tolerated, yet drastically impair fitness, that gene may inappropriately fail to be identified as essential without considering the expected abundance of mutants in that gene. Recently, more sophisticated methods using hidden Markov models have incorporated mutant abundance data successfully but do not consider biological variability (18, 19).
In this work, Tn-seq and a Monte Carlo simulation-based approach was used to determine the essential genome of two P. aeruginosa strains in laboratory medium and CF sputum with statistical precision. The results show that although essential genes are contained in both the core and accessory genomes they are enriched in the core genome. However, the essentiality of these core genes can differ between strains, suggesting that the mere presence or absence of a gene does not necessarily predict how its function integrates into the networks that define fitness in CF sputum. Finally, we show that growth in a defined growth medium developed to mimic CF sputum yielded fitness requirements virtually identical to CF sputum, providing evidence that this medium is a valid in vitro model to study P. aeruginosa CF colonization and persistence.
Results
A Monte Carlo Approach to Define the P. aeruginosa Essential Genome.
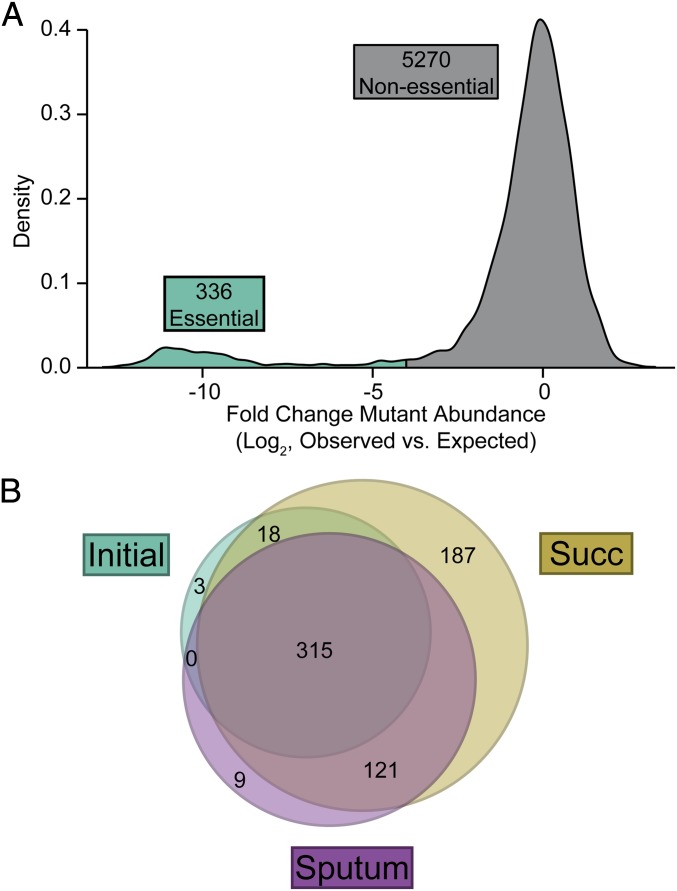
To determine the P. aeruginosa essential genome in a statistically rigorous manner we refined an approach to incorporate experimental variability and mutant abundance into the analysis (Fig. S1) (20). This analysis was performed with data previously generated using a P. aeruginosa strain PAO1 transposon library containing ∼100,000 transposon mutants grown on a complex laboratory medium (21). First, we determined the position of every transposon insertion in the mutant library and tallied the number of reads originating from each insertion to represent the “observed” mutant abundance data. Then, to generate the “expected” mutant abundance data, we simply arranged these ∼100,000 transposon insertions and the associated number of reads per site randomly on the P. aeruginosa chromosome. Here, the null hypothesis is that no gene is essential and all insertions impart no fitness cost. We repeated this simulation process 2,000 times in a Monte Carlo approach, generating an estimate of the variance to be expected from hypothetical, fitness-neutral Tn-seq data with the same insertion numbers and read counts as the authentic data. Then, we tested whether mutant abundance in the observed data were significantly different from the expected pseudodata using the R package DEseq (22). We observed qualitatively that genes typically fall into one of two categories: (i) genes from which a similar number of transposon-derived reads were observed as were expected and the null hypothesis cannot be rejected, or (ii) genes from which significantly less transposon-derived reads were observed than were expected and the null hypothesis is false. To define these categories, we clustered genes according to their fold change mutant abundance versus the expected value using the R package mclust (23). Finally, any gene that had significantly lower mutant abundance than expected (P < 0.01, negative binomial test) and that clustered in the second of the two categories described above (P < 0.01, maximum likelihood estimation) was deemed essential. According to these criteria, 336 of 5,606 PAO1 genes were deemed essential (Fig. 1A and Dataset S1). This number is similar to what is typically seen in studies of the essential genomes of bacteria (24), and to a previously published estimate of 377 essential genes for strain PAO1 based on individually mapping 30,100 transposon mutants (25). As expected, these genes are largely involved in central processes such as transcription, translation, ribosome biogenesis, cell envelope biogenesis, and DNA synthesis and repair.
Fig. 1.
The essential genome of P. aeruginosa strain PAO1 in CF sputum. (A) Density plot of the log2-transformed difference between observed and fitness-neutral pseudodata for strain PAO1. In this initial characterization of the transposon library, 336 genes were deemed essential (cyan). (B) Shown is a Venn diagram of genes declared essential in either the initial PAO1 transposon library, or after subsequent growth of this library in either MOPS-succinate (Succ) or MOPS-sputum. Essentiality of every P. aeruginosa gene in these three conditions is reported in Dataset S1.
The Essential Genomes of Multiple P. aeruginosa Strains in CF Sputum Overlap but Are Distinct.
To determine the P. aeruginosa essential genome in CF sputum the PAO1 transposon library was grown for nine generations in MOPS-buffered defined medium containing 6% authentic sputum (MOPS-sputum) as a sole carbon and energy source. Importantly, we used CF sputum that had a relatively low bacterial load (∼107 cfu/mL), which retains the nutritional potential to support robust growth of P. aeruginosa (3). We then profiled insertion mutant abundance by Tn-seq (Dataset S2) and repeated our essential gene analysis. These analyses yielded an additional 130 essential genes in MOPS-sputum (Fig. 1B and Dataset S1). Many of the essential MOPS-sputum genes are involved in biosynthesis of key nutrients, indicating that CF sputum is nutrient-depleted compared with complex laboratory media.
Using Tn-seq data to determine gene essentiality is often accomplished by directly comparing mutant abundance in the initial library and after growth in a test condition (e.g., MOPS-sputum); thus, we compared our Monte Carlo results to those obtained from this conventional approach. When we directly compared the initial and the final library composition after growth in MOPS-sputum (Dataset S1) mutant abundance decreased in 197 genes (P < 0.05, negative binomial test, fold change greater than or equal to four). Taken together, these data indicate that our Monte Carlo simulation-based approach is more stringent in describing sputum-specific essentiality and that less than 8% of the P. aeruginosa genome is required for growth in CF sputum.
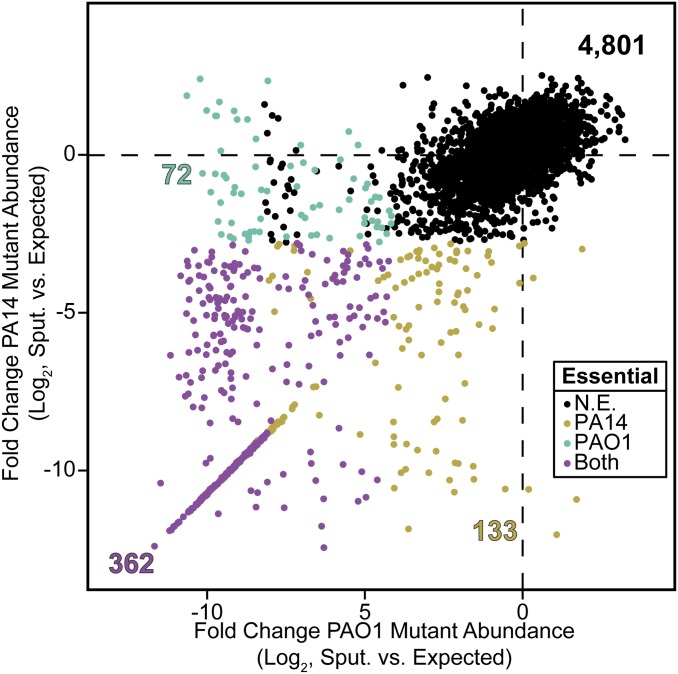
Although the core genome of P. aeruginosa is highly conserved, the accessory genome can vary considerably. Because many P. aeruginosa strains are able to found lifelong CF infections, we sought to determine the essentiality of the core and accessory P. aeruginosa genome. To accomplish this, the essential genome of a second P. aeruginosa strain (PA14) was determined in CF sputum. An ∼300,000 transposon insertion mutant library was created in strain PA14 using a complex laboratory medium and analyzed as described above. By our criteria, 434 of 5,906 PA14 genes were deemed essential (Dataset S3) and growth in MOPS-sputum revealed an additional 122 essential genes (Dataset S4). To determine the conserved essential genes in PAO1 and PA14, precomputed BLAST results and synteny information available at the Pseudomonas Genome Database (26) was used to identify 5,366 orthologous gene pairs between these two strains. Of the 434 PA14 essential genes, 20 have no ortholog in PAO1, which is a disproportionately low share of the PA14-unique genes (P = 3.52 × 10−4, Fisher’s exact test). This suggests that essential genes are more likely to be found in the core rather than the accessory P. aeruginosa genome. Of note, the orthologous essential genes observed in PA14 and not PAO1 were primarily composed of small ORFs (Dataset S3) and likely arise from the fact that the higher-density PA14 transposon library allows for more statistical power when analyzing essentiality of these smaller genes.
Among orthologous gene pairs there were a large number of genes that showed differential sputum essentiality in both PAO1 and PA14 (Fig. 2 and Dataset S4). Some of these genes are involved in efflux: The nalD and nalC efflux pump regulators are required in strain PAO1 and not strain PA14 in MOPS-sputum, whereas the mexAB-oprM efflux pump is required in strain PA14 and not strain PAO1. We also found that mutants in PA4063 and PA4065 were essential in CF sputum in strain PAO1 but not strain PA14. PA4064 and PA4065 are predicted to encode components of an ABC transport system homologous to the SalXY antimicrobial peptide resistance transporter in Streptococcus salivarius, whereas the predicted PA4063 gene product has no known function (27). These data indicate that in P. aeruginosa, an organism well known for its innate efflux capabilities, diverse strains use these capabilities differently during growth in sputum. Most interesting to us are the 46 genes that are essential in both PAO1 and PA14 when grown in sputum but not initially (Dataset S5). Several of these sputum-specific genes include those involved in biosynthesis of biotin, purines, pyrimidines, and pantothenate, which suggests that these metabolites are not present at levels sufficient for maximal growth in sputum.
Fig. 2.
P. aeruginosa strains PAO1 and PA14 have common yet distinct CF sputum essential genomes. Shown is the log2-transformed difference in transposon mutant abundance for every gene identifiable in a one-to-one ortholog mapping of strains PAO1 (x axis) and PA14 (y axis) after growth of these transposon libraries in MOPS-sputum. Gene essentiality calls are colored as shown, and the total number of genes per class is displayed in the appropriate color on the plot. N.E., not essential.
P. aeruginosa Physiology in CF Sputum.
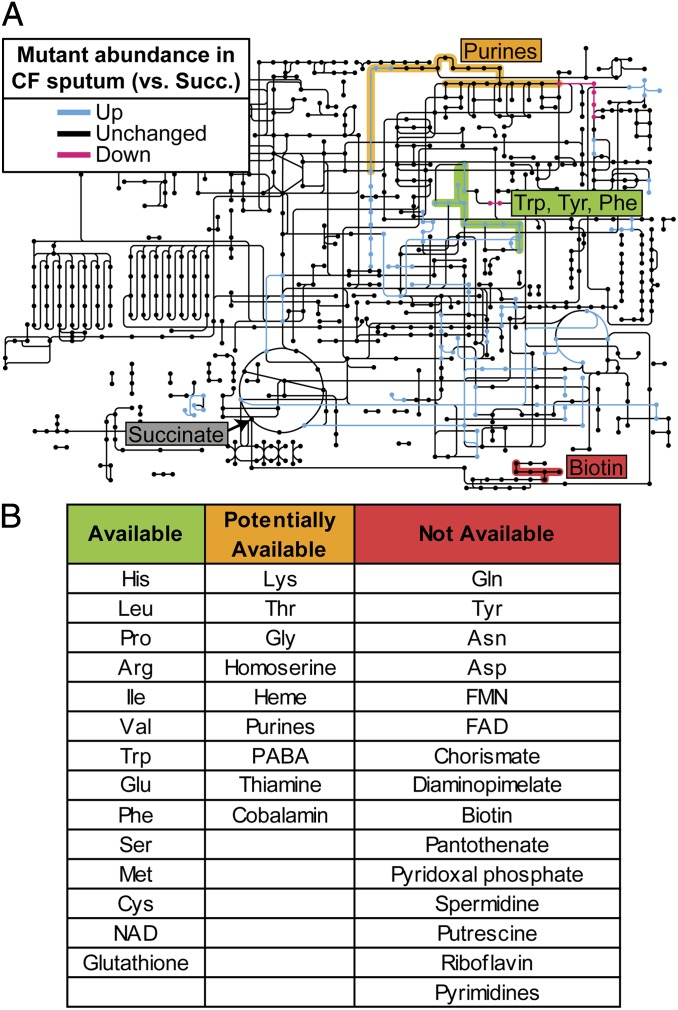
Our laboratory previously showed that metabolic cues present in CF sputum affect P. aeruginosa virulence factor production (14). Direct detection and measurement of the chemical constituents of CF sputum revealed that many nutrients are present and potentially available to infecting pathogens (14); however, which nutrients are available for growth to the high densities commonly observed in the CF lung is not known. To profile nutrient bioavailability in sputum, insertion mutant abundance in MOPS-sputum was compared with that in a defined medium containing succinate (MOPS-succinate) as the sole carbon and energy source (Dataset S6). The goal of this analysis was to identify specific features of primary metabolism in CF including both catabolic and anabolic processes required for growth in CF sputum. No genes involved in catabolism of specific carbon and energy sources were identified by this analysis, supporting our previous assertion that P. aeruginosa uses multiple carbon and energy sources during growth in CF sputum. Although P. aeruginosa is known to up-regulate fatty acid catabolism genes in CF sputum (28), our results show that these genes are not required for fitness in our CF sputum growth conditions. This analysis did identify several genes predicted to be involved in biosynthesis (Fig. 3A and Dataset S6); however, owing to the integrated nature of central metabolism it was not readily apparent which anabolic pathways were essential for CF sputum growth.
Fig. 3.
Metabolite availability in CF sputum. (A) Metabolic reactions identified by KEGG Orthology are colored according to their relative mutant fitness in strain PAO1 grown in MOPS-sputum medium compared with growth in MOPS-succinate minimal medium. Only significant changes (fold change greater than or equal to two, P < 0.05) are shown. Example biosynthetic pathways for metabolites deemed “available” (green), “potentially available” (yellow), and “not available” (red) are colored (see below for details). (B) Full profile of metabolite availability in CF sputum (ordered arbitrarily). If mutants in >33% of genes predicted to be in the biosynthetic pathway of a given metabolite were more fit in MOPS-sputum than in MOPS-succinate (P < 0.05, negative binomial test, fold change greater than or equal to two) that metabolite was said to be “available” to P. aeruginosa in CF sputum. If 5–33% of genes predicted to be in the biosynthetic pathway of a given metabolite were more fit in MOPS-sputum than in MOPS-succinate, that metabolite was said to be “potentially available”. If <5% of genes predicted to be in the biosynthetic pathway of a given metabolite were more fit in MOPS-sputum than in MOPS-succinate, that metabolite was said to be “not available.” Genes predicted to be involved in biosynthesis of necessary metabolites were identified from the PseudoCyc database (see Dataset S7 for details).
To identify anabolic pathways required for CF sputum growth we applied an approach recently used to identify metabolite bioavailability in a murine wound infection model (12). In this approach, if >33% of biosynthetic genes for a particular metabolite contribute more to fitness in MOPS-succinate than in MOPS-sputum (P < 0.05, negative binomial test, fold change greater than or equal to two) that metabolite was deemed “available” (Fig. 3B and Dataset S7). This analysis revealed that that 12 amino acids as well as NAD and glutathione are readily bioavailable for P. aeruginosa growth in sputum. These results are consistent with findings that after long-term colonization of the CF lung many P. aeruginosa strains arise that are unable to biosynthesize particular amino acids (referred to as auxotrophs). Notably, the most common auxotrophs observed are for methionine, leucine, and arginine (7, 29, 30), which are amino acids deemed available by our analysis. Nutrients for which >95% of the biosynthetic genes contribute to fitness in both MOPS-sputum and MOPS-succinate equally were deemed to be “not available.” These include several cofactors such as biotin, pantothenate, and riboflavin, as well as the electron carriers flavin mononucleotide and flavin adenine dinucleotide. These unavailable nutrients are particularly interesting from a therapeutic perspective, because bacteria-specific pathways for biosynthesis of these metabolites may represent novel therapeutic targets (31, 32). Thus, although CF sputum is an amino acid-rich environment our comparative Tn-seq approach reveals that it is relatively cofactor-poor.
An Enhanced Synthetic Sputum Medium Closely Approximates Natural CF Sputum.
The results described above show that the nutritional environment of CF sputum is distinct from both rich and minimal laboratory media. Previously, we reported the development of a chemically defined synthetic CF sputum medium (SCFM) based on chemical analyses of expectorated sputum and showed that P. aeruginosa gene expression in SCFM closely approximated that in authentic CF sputum (14). Notably, SCFM lacks several molecules and polymers known to be present in CF sputum including DNA (33, 34), lipids (35–37), N-acetyl glucosamine (GlcNAc) (38), and mucin (39, 40). To more closely approximate the contents of natural CF sputum we supplemented SCFM with concentrations of salmon sperm DNA, GlcNAc, bovine maxillary mucin, and dioleoylphosphatidylcholine (DOPC) similar to those observed in CF sputum to create SCFM2 (Dataset S8) (33–40). We then investigated how closely SCFM2 approximates the nutritional environment of authentic CF sputum using Tn-seq as a test of the utility of this medium for in vitro experimentation.
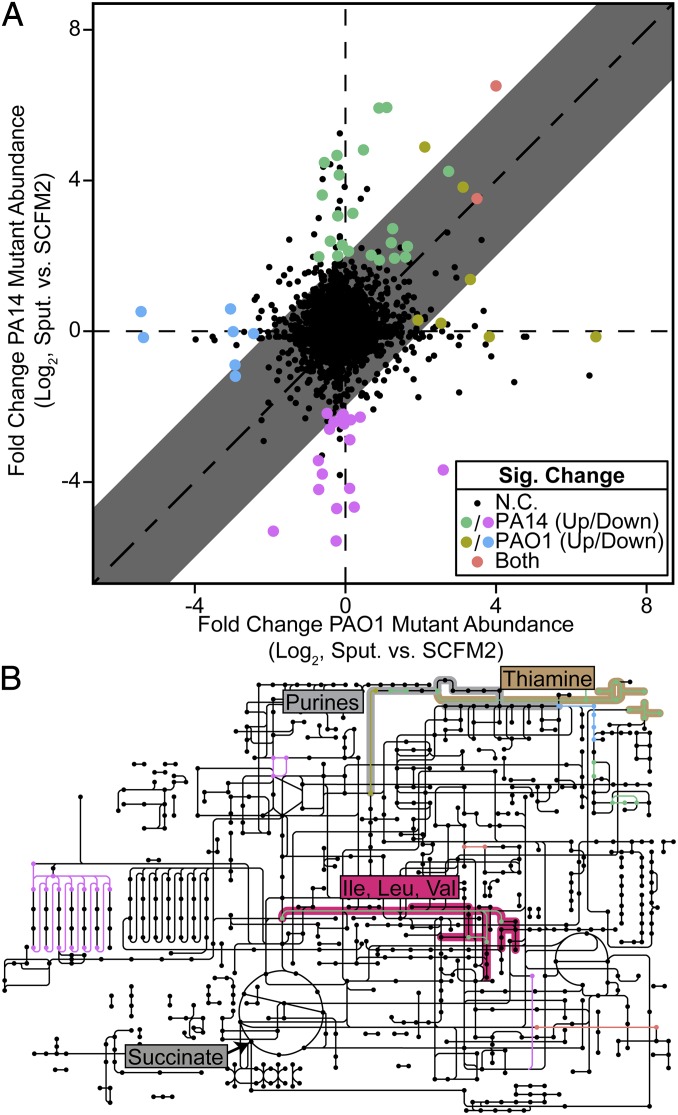
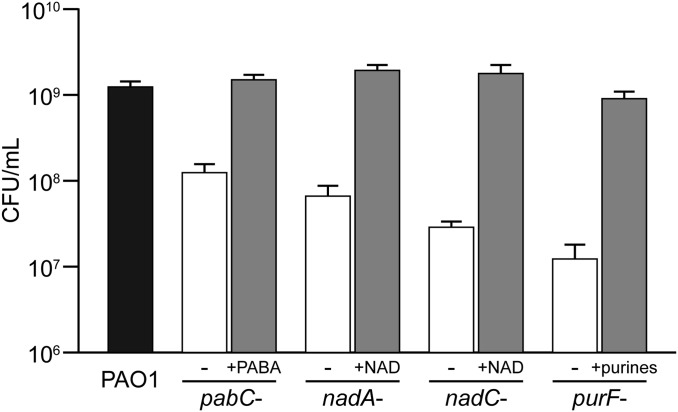
Our results revealed that mutants in only two genes were significantly less fit in SCFM2 than in MOPS-sputum in both PAO1 and PA14 (P < 0.05, negative binomial test, fold change greater than or equal to four), suggesting that SCFM2 presents selective pressures to P. aeruginosa nearly identical to CF sputum (Fig. 4A and Dataset S9). Interrogation of strain-specific genetic requirements for sputum growth revealed 14 PAO1 and 41 PA14 genes; however, many genes were located in the same anabolic pathways, including biosynthesis of thiamine, NAD, purines, folate, branched-chain amino acids, and tryptophan (Fig. 4B). This suggests that these metabolites are more bioavailable in CF sputum than in SCFM2. To test this prediction, we measured the growth yield of mutants in four of these genes (pabC, nadA, nadC, and purF) individually in SCFM2. All four of these mutants grew to lower yields in SCFM2, verifying that our Tn-seq results accurately reflect single mutant fitness (Fig. 5). When SCFM2 was nutritionally complemented with the appropriate nutrient, growth yield was restored to wild-type levels. This indicates that SCFM2 could be further improved by the inclusion of these metabolites. Taken together, these results show that our synthetic SCFM2 approximates the selective pressures encountered by P. aeruginosa in CF sputum, yet as in natural CF sputum different strains require distinct gene sets for fitness.
Fig. 4.
SCFM2 closely models natural CF sputum for multiple P. aeruginosa strains. (A) Shown is the log2-transformed difference in transposon mutant abundance between SCFM2 and MOPS-sputum for every gene identifiable in a one-to-one ortholog mapping of strains PAO1 (x axis) and PA14 (y axis). Significant changes in mutant abundance (P < 0.05, negative binomial test, fold change greater than or equal to four) are colored as shown. The shaded area indicates that mutant fitness response in SCFM2 compared with MOPS-sputum differs by less than fourfold in 97.3% of genes. N.C., no change. (B) PAO1 and PA14 exhibit similar pathway-level mutant fitness differences in SCFM2 compared with MOPS-sputum. Metabolic reactions as identified by KEGG Orthology are colored as in A and indicate that unifying pathways underlie the distinct fitness determinants of PAO1 and PA14 in SCFM2 and MOPS-sputum. Biosynthetic pathways discussed in the text are highlighted.
Fig. 5.
Tn-seq reveals metabolites missing from SCFM2. Shown are 5-h growth yields of either wild-type PAO1 or mutant derivatives less fit in SCFM2 than in MOPS-sputum (Dataset S9). Growth yields in SCFM2 is indicated by the black or white bars. The gray bars indicate growth yields in SCFM2 chemically complemented with 200 µM p-aminobenzoate (+PABA), 10 µg/mL nicotinamide adenine dinucleotide (+NAD), or 10 µg/mL each adenine, guanine, xanthine, and hypoxanthine (+purines). Error bars indicate SEM.
Discussion
P. aeruginosa causes an array of infections in immunocompromised hosts, including chronic CF infections that persist for decades. Discerning the genetic requirements essential for growth in CF has proven elusive, in part owing to the difficulty of performing high-throughput genetic analysis in a suitable model system. In this work we used a statistically rigorous high-throughput genetic approach to define the P. aeruginosa essential genome during in vitro growth in CF sputum. We also defined the nutrients available to P. aeruginosa in CF sputum by examining fitness of strains containing mutations in known anabolic pathways. These analyses revealed that CF sputum lacks a number of nutrients, including several amino acids/cofactors, and provide rationale for the development of small-molecule inhibitors of specific biosynthetic pathways as novel therapeutics for treating P. aeruginosa CF infections. Previous work has shown that inhibitors of biosynthetic pathways are identifiable in screens of FDA-approved small molecules (32); thus, there may be opportunities for using known drugs to treat P. aeruginosa CF infections.
Metabolic inhibitors developed to target CF P. aeruginosa infections may also be useful in non-CF P. aeruginosa infections, since Tn-seq revealed that many biosynthetic pathways required in CF sputum are also required in murine surgical wound infections (12). For example, P. aeruginosa requires biosynthetic pathways for diaminopimelate, chorismate, purines, pantothenate, pyridoxal phosphate, polyamines, and riboflavin in both CF sputum and chronic wounds. However, catabolism may not be a universal therapeutic target, because fatty acid catabolism is required in wound infections but not in CF sputum. Generally, chronic wounds seem to be a much harsher selective environment for P. aeruginosa. In a previous report, we found that 992 PAO1 genes contributed to fitness in chronic wounds (12), whereas only 72 genes contributed to fitness in MOPS-sputum (P < 0.05, fold change greater than or equal to four). However, 48 of those genes contributed differentially to fitness in both conditions (Dataset S10). As expected, most of these genes encode biosynthetic proteins for the required nutrients described above.
Our finding that essential genes are concentrated in the core genome has previously been described for related bacterial species (41); however, it was surprising that essentiality of core genes varied significantly between two P. aeruginosa strains. The reason for this difference is not known, but clues may be provided in the future through development of functional gene networks (42). Based on our results, it would be expected that these differentially essential genes may be incorporated into the P. aeruginosa gene network differently. For example, an essential gene in one P. aeruginosa strain may be incorporated into the gene network of a different strain in a manner that makes it nonessential or functionally redundant. In addition, these data suggest that although orthology can be used to predict the molecular function of a gene product it cannot reliably determine the genetic essentiality of that product. Thus, it will be essential to perform perturbation analyses such as Tn-seq to determine genetic essentiality for any P. aeruginosa strain of interest.
A challenge for CF P. aeruginosa researchers is the lack of readily available, relevant in vitro models. Refinement of our previously published synthetic CF sputum medium to include polymers and small molecules known to affect P. aeruginosa physiology provides a relevant, versatile tool for studying CF infection. The observation that less than 0.7% of the genes in PAO1 and PA14 are differentially essential during growth in CF sputum and SCFM2 provides strong rationale for the use of this medium as a relevant in vitro CF sputum model for studying virtually all P. aeruginosa genes. In addition, the fact that most of the differentially essential genes are involved in anabolic pathways indicates that addition of these nutrients to SCFM2 would allow these genes to be studied.
Materials and Methods
Bacterial Strains and Growth Conditions.
P. aeruginosa strain PAO1 and the transposon mutant library and individual transposon mutants derived from it were obtained from Colin Manoil, University of Washington, Seattle (21, 25). Transposon insertions were confirmed by PCR. The P. aeruginosa PA14 transposon mutant library was generated with the Tn5-based transposon T8 (ISlacZhah-tc) by conjugation as described (21) in strain PA14 on BHI agar. MOPS-succinate is described in ref. 12. Sputum with low bacterial load was collected from a single patient as described previously (3), and SCFM2 is described in detail in SI Materials and Methods. All cultures were grown shaking at 250 rpm at 37 °C in specified media.
Essential Gene Analysis.
Frozen aliquots of the PAO1 and PA14 transposon insertion libraries were washed twice with 1 mL MOPS-buffered media base, inoculated into SCFM2 or MOPS-sputum at 106 cfu/mL, and grown for approximately nine generations (to ∼109 cfu/mL). Tn-seq libraries were prepared as previously described (12), except for the initial sequencing of the PA14 transposon library, which is described in SI Materials and Methods. Gene essentiality was determined using a custom Unix, Perl, and R pipeline (available at github.com/khturner/Tn-seq), and the analysis approach is detailed in SI Materials and Methods. All sequences are deposited with the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA) under accession number PRJNA265367. Tn-seq reads for the initial PAO1 library were reported previously (21), obtained from the NCBI SRA with accession number SRX031647, and used in analysis after collapsing sites that were 1 bp apart (see ref. 21 for details). Tn-seq data for outgrowth of the PAO1 library in MOPS-succinate or murine chronic wounds were reported previously (12) and are available at the NCBI SRA under accession number SRP033652.
Nutritional Complementation of Mutants.
Wild-type PAO1 and individual transposon mutants with growth defects in SCFM2 were grown overnight in LB. Fresh cultures were then grown to exponential phase in LB, washed twice with MOPS-buffered media base (38), and starved in MOPS-buffered media base for 1.5–2 h at 37 °C. Cells were inoculated into SCFM2 (with or without additional nutrients as indicated in Fig. 5) at OD600 0.005, grown for 5 h, shaking at 37 °C, and assayed for colony-forming units per milliliter by plate counting.
Supplementary Material
Acknowledgments
We thank M.W. laboratory members for critical discussions of the manuscript. This work was supported by a grant from the Cystic Fibrosis Foundation (to M.W.) K.H.T. is a Cystic Fibrosis Foundation Postdoctoral Research Fellow. M.W. is a Burroughs Wellcome Investigator in the Pathogenesis of Infectious Disease.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the National Center for Biotechnology Information Sequence Read Archive (accession no. PRJNA265367).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419677112/-/DCSupplemental.
References
- 1.Hoiby N. Pseudomonas in Cystic Fibrosis: Past, Present, and Future. Cystic Fibrosis Trust; Berlin: 1998. [Google Scholar]
- 2.Ohman DE, Chakrabarty AM. Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect Immun. 1982;37(2):662–669. doi: 10.1128/iai.37.2.662-669.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer KL, Mashburn LM, Singh PK, Whiteley M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol. 2005;187(15):5267–5277. doi: 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kragh KN, et al. Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Infect Immun. 2014;82(11):4477–4486. doi: 10.1128/IAI.01969-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkesson A, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat Rev Microbiol. 2012;10(12):841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 6.Smith EE, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103(22):8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barth AL, Pitt TL. Auxotrophic variants of Pseudomonas aeruginosa are selected from prototrophic wild-type strains in respiratory infections in patients with cystic fibrosis. J Clin Microbiol. 1995;33(1):37–40. doi: 10.1128/jcm.33.1.37-40.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Opijnen T, Camilli A. Transposon insertion sequencing: A new tool for systems-level analysis of microorganisms. Nat Rev Microbiol. 2013;11(7):435–442. doi: 10.1038/nrmicro3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman AL, et al. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe. 2009;6(3):279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Opijnen T, Bodi KL, Camilli A. Tn-seq: High-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods. 2009;6(10):767–772. doi: 10.1038/nmeth.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langridge GC, et al. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 2009;19(12):2308–2316. doi: 10.1101/gr.097097.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner KH, Everett J, Trivedi U, Rumbaugh KP, Whiteley M. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet. 2014;10(7):e1004518. doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutschbauer A, et al. Evidence-based annotation of gene function in Shewanella oneidensis MR-1 using genome-wide fitness profiling across 121 conditions. PLoS Genet. 2011;7(11):e1002385. doi: 10.1371/journal.pgen.1002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189(22):8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moule MG, et al. Genome-wide saturation mutagenesis of Burkholderia pseudomallei K96243 predicts essential genes and novel targets for antimicrobial development. MBio. 2014;5(1):e00926–e13. doi: 10.1128/mBio.00926-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christen B, et al. The essential genome of a bacterium. Mol Syst Biol. 2011;7:528. doi: 10.1038/msb.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, et al. Genome-scale metabolic network validation of Shewanella oneidensis using transposon insertion frequency analysis. PLOS Comput Biol. 2014;10(9):e1003848. doi: 10.1371/journal.pcbi.1003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao MC, et al. High-resolution definition of the Vibrio cholerae essential gene set with hidden Markov model-based analyses of transposon-insertion sequencing data. Nucleic Acids Res. 2013;41(19):9033–9048. doi: 10.1093/nar/gkt654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeJesus MA, Ioerger TR. A Hidden Markov Model for identifying essential and growth-defect regions in bacterial genomes from transposon insertion sequencing data. BMC Bioinformatics. 2013;14:303. doi: 10.1186/1471-2105-14-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zomer A, Burghout P, Bootsma HJ, Hermans PW, van Hijum SA. ESSENTIALS: Software for rapid analysis of high throughput transposon insertion sequencing data. PLoS ONE. 2012;7(8):e43012. doi: 10.1371/journal.pone.0043012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher LA, Shendure J, Manoil C. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. MBio. 2011;2(1):e00315–e10. doi: 10.1128/mBio.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraley C, Raftery AE. MCLUST: Software for model-based cluster analysis. J Classif. 1999;16(2):297–306. [Google Scholar]
- 24.Juhas M, Eberl L, Glass JI. Essence of life: Essential genes of minimal genomes. Trends Cell Biol. 2011;21(10):562–568. doi: 10.1016/j.tcb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs MA, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2003;100(24):14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winsor GL, et al. Pseudomonas Genome Database: Improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res. 2011;39(Database issue):D596–D600. doi: 10.1093/nar/gkq869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upton M, Tagg JR, Wescombe P, Jenkinson HF. Intra- and interspecies signaling between Streptococcus salivarius and Streptococcus pyogenes mediated by SalA and SalA1 lantibiotic peptides. J Bacteriol. 2001;183(13):3931–3938. doi: 10.1128/JB.183.13.3931-3938.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Son MS, Matthews WJ, Jr, Kang Y, Nguyen DT, Hoang TT. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun. 2007;75(11):5313–5324. doi: 10.1128/IAI.01807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor RF, Hodson ME, Pitt TL. Auxotrophy of Pseudomonas aeruginosa in cystic fibrosis. FEMS Microbiol Lett. 1992;71(3):243–246. doi: 10.1016/0378-1097(92)90716-2. [DOI] [PubMed] [Google Scholar]
- 30.Fothergill JL, Mowat E, Ledson MJ, Walshaw MJ, Winstanley C. Fluctuations in phenotypes and genotypes within populations of Pseudomonas aeruginosa in the cystic fibrosis lung during pulmonary exacerbations. J Med Microbiol. 2010;59(Pt 4):472–481. doi: 10.1099/jmm.0.015875-0. [DOI] [PubMed] [Google Scholar]
- 31.Gerdes SY, et al. From genetic footprinting to antimicrobial drug targets: Examples in cofactor biosynthetic pathways. J Bacteriol. 2002;184(16):4555–4572. doi: 10.1128/JB.184.16.4555-4572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zlitni S, Ferruccio LF, Brown ED. Metabolic suppression identifies new antibacterial inhibitors under nutrient limitation. Nat Chem Biol. 2013;9(12):796–804. doi: 10.1038/nchembio.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchner KK, Wagener JS, Khan TZ, Copenhaver SC, Accurso FJ. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5):1426–1429. doi: 10.1164/ajrccm.154.5.8912759. [DOI] [PubMed] [Google Scholar]
- 34.Brandt T, Breitenstein S, von der Hardt H, Tümmler B. DNA concentration and length in sputum of patients with cystic fibrosis during inhalation with recombinant human DNase. Thorax. 1995;50(8):880–882. doi: 10.1136/thx.50.8.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hull J, South M, Phelan P, Grimwood K. Surfactant composition in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156(1):161–165. doi: 10.1164/ajrccm.156.1.9609090. [DOI] [PubMed] [Google Scholar]
- 36.Meyer KC, et al. Function and composition of pulmonary surfactant and surfactant-derived fatty acid profiles are altered in young adults with cystic fibrosis. Chest. 2000;118(1):164–174. doi: 10.1378/chest.118.1.164. [DOI] [PubMed] [Google Scholar]
- 37.Griese M, Birrer P, Demirsoy A. Pulmonary surfactant in cystic fibrosis. Eur Respir J. 1997;10(9):1983–1988. doi: 10.1183/09031936.97.10091983. [DOI] [PubMed] [Google Scholar]
- 38.Korgaonkar AK, Whiteley M. Pseudomonas aeruginosa enhances production of an antimicrobial in response to N-acetylglucosamine and peptidoglycan. J Bacteriol. 2011;193(4):909–917. doi: 10.1128/JB.01175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung C, et al. Gene expression of Pseudomonas aeruginosa in a mucin-containing synthetic growth medium mimicking cystic fibrosis lung sputum. J Med Microbiol. 2010;59(Pt 9):1089–1100. doi: 10.1099/jmm.0.019984-0. [DOI] [PubMed] [Google Scholar]
- 40.Henke MO, John G, Germann M, Lindemann H, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007;175(8):816–821. doi: 10.1164/rccm.200607-1011OC. [DOI] [PubMed] [Google Scholar]
- 41.Jordan IK, Rogozin IB, Wolf YI, Koonin EV. Essential genes are more evolutionarily conserved than are nonessential genes in bacteria. Genome Res. 2002;12(6):962–968. doi: 10.1101/gr.87702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deutschbauer A, et al. Towards an informative mutant phenotype for every bacterial gene. J Bacteriol. 2014;196(20):3643–3655. doi: 10.1128/JB.01836-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.