Significance
Autophagy impairment is a major hallmark of aging, and any intervention that enhances autophagy is of potential interest to delay aging. However, it was described that the hypothalamus is a brain area with a key role on whole-body aging. In the present study, we show that an endogenous molecule produced by the hypothalamus, the neuropeptide Y (NPY), stimulates autophagy in rodent hypothalamus. Because both hypothalamic autophagy and NPY levels decrease with age, a better understanding of hypothalamic neuronal autophagy regulation by NPY may provide new putative therapeutic strategies to ameliorate age-related deteriorations and delay aging.
Keywords: neuropeptide Y, hypothalamus, autophagy, aging
Abstract
Aging is characterized by autophagy impairment that contributes to age-related disease aggravation. Moreover, it was described that the hypothalamus is a critical brain area for whole-body aging development and has impact on lifespan. Neuropeptide Y (NPY) is one of the major neuropeptides present in the hypothalamus, and it has been shown that, in aged animals, the hypothalamic NPY levels decrease. Because caloric restriction (CR) delays aging, at least in part, by stimulating autophagy, and also increases hypothalamic NPY levels, we hypothesized that NPY could have a relevant role on autophagy modulation in the hypothalamus. Therefore, the aim of this study was to investigate the role of NPY on autophagy in the hypothalamus. Using both hypothalamic neuronal in vitro models and mice overexpressing NPY in the hypothalamus, we observed that NPY stimulates autophagy in the hypothalamus. Mechanistically, in rodent hypothalamic neurons, NPY increases autophagy through the activation of NPY Y1 and Y5 receptors, and this effect is tightly associated with the concerted activation of PI3K, MEK/ERK, and PKA signaling pathways. Modulation of hypothalamic NPY levels may be considered a potential strategy to produce protective effects against hypothalamic impairments associated with age and to delay aging.
Aging is associated with accumulation of specific cellular proteins within neurons, a pathologic hallmark of many neurodegenerative diseases. Because average human life expectancy has increased, but also the prevalence of cognitive decline and dementia, aging research is now focused in finding strategies that increase both lifespan and healthspan.
Autophagy is a highly regulated intracellular process involved in the turnover of most cellular constituents and in the maintenance of cellular homeostasis (1, 2). It is well described that basal autophagic activity decreases with age, contributing to the accumulation of altered macromolecules (3). In addition, autophagy impairment contributes to different aspects of aging phenotype and to aggravation of age-related diseases (4).
Caloric restriction (CR), the reduced intake of calories without malnutrition, extends lifespan of many organisms, from yeast to mammals, and delays the progression of age-related diseases, at least in part, by stimulating autophagy (5–8). One major neuroendocrine effect of CR is the increase of neuropeptide Y (NPY) in the hypothalamus (9–12). The hypothalamus has a key role in the control of body homeostasis, neuroendocrine outputs, and feeding behavior. Recently, it was described that this brain area is critical for the development of whole-body aging and has impact on lifespan (13, 14). In the hypothalamus, NPY is involved in the regulation of different physiological functions, such as regulation of food intake, body temperature, circadian rhythms, memory processing, and cognition (15–19). These diverse actions of NPY are mediated by G protein-coupled receptor subtypes named NPY Y1, Y2, Y4, and/or Y5 (20, 21), all of which have been reported to be present in the hypothalamus (22).
Others showed that CR does not increase lifespan in NPY knockout mice (23), and aging decreases NPY levels in rodent hypothalamus (24–26). However, transgenic rats overexpressing hypothalamic NPY have improved stress resistance and increased mean lifespan (27). Although these observations suggest that NPY may play a relevant role in aging and lifespan, the effect of NPY on autophagy remains unknown.
The aim of the present study was, therefore, to investigate the role of NPY, and NPY receptors, on autophagy regulation in the hypothalamus and the mechanisms underlying this process.
Results
CR Induces Autophagy in Hypothalamic Neurons.
To investigate whether CR regulates autophagy in hypothalamic neurons, we monitored autophagy in two cellular models of rodent hypothalamic neurons exposed to a CR mimetic medium (referred as CR hereafter) by measuring the protein levels of the transient autophagosomal membrane-bound form of LC3B (LC3B-II) and sequestosome 1 (SQSTM1, also known as p62), widely used as markers for monitoring the autophagic process (28, 29).
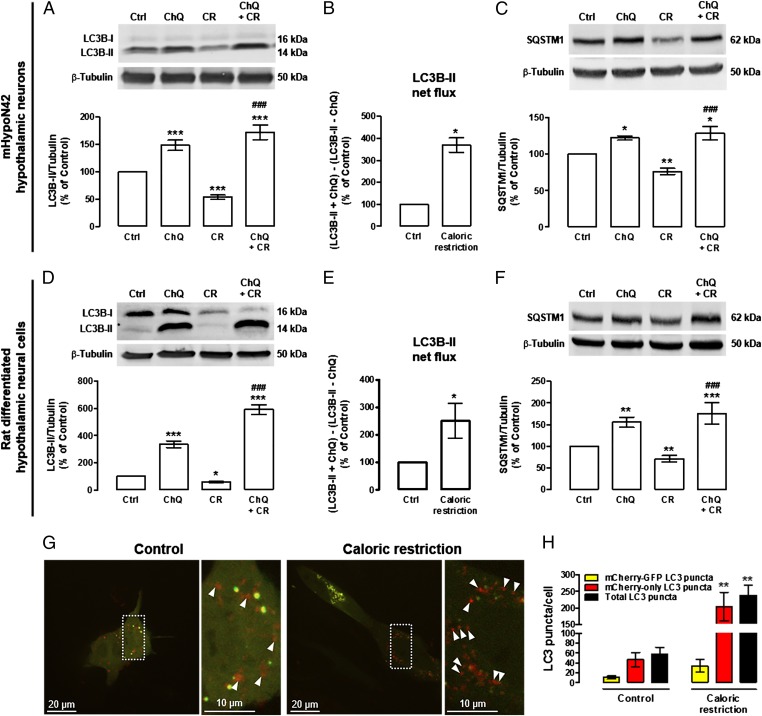
CR decreased LC3B-II (Fig. 1 A and D) and SQSTM1 (Fig. 1 C and F) levels in both mHypoN42 hypothalamic neurons and rat differentiated hypothalamic neural cells, suggesting an increase of autophagic clearance in these conditions. To confirm that CR was in fact enhancing autophagy, we evaluated LC3B-II and SQSTM1 levels in the absence and presence of chloroquine, an inhibitor of autolysosomal degradation (28, 29). Both proteins are degraded at the final stages of autophagy and, in the presence of chloroquine, lysosomal degradation will be impaired, resulting in the accumulation of LC3B-II and SQSTM1. As a consequence of chloroquine-induced autolysosomal degradation inhibition, both autophagy substrates accumulated upon CR (LC3B-II: Fig. 1 A and D; SQSTM1: Fig. 1 C and F), suggesting that their autolysosomal degradation is inhibited. We next determined autophagic flux by the LC3B-II turnover assay, which measures the amount of LC3B-II delivered to the lysosomes by comparing the LC3-II amounts between samples in the presence and absence of lysosomal inhibitors (LC3B-II net flux) (28, 30). We observed that CR increased LC3B-II net flux in both mHypoN42 hypothalamic neurons and rat differentiated hypothalamic neural cells (Fig. 1 B and E). We also measured autophagic flux by using the tandem mCherry-GFP-LC3 cell-based assay (28, 31). In this assay, the activation of autophagy is characterized by the redistribution of mCherry-GFP-LC3 reporter to autophagosomes (yellow puncta; due to fluorescence of both mCherry and GFP) and autolysosomes (red-only puncta; due to mCherry fluorescence alone because GFP fluorescence decays in acidic lysossomal environment). CR increased the number of autolysosomes (red-only LC3 puncta) in mHypoN42 hypothalamic neurons, indicating an increase of autophagic flux (Fig. 1 G and H). Overall, the results from both the Western blot analysis of endogenous LC3B, showing an increase of the LC3B turnover and LC3B net flux, and the real-time image-based quantification of autophagic flux using the tandem mCherry-GFP-LC3 reporter, showing an increase in both LC3 puncta and autophagic flux, clearly demonstrate that CR enhances autophagic flux in hypothalamic neurons.
Fig. 1.
CR increases autophagic flux in mHypoN42 hypothalamic neurons and in rat differentiated hypothalamic neural cells. mHypoN42 hypothalamic neurons and rat differentiated hypothalamic neural cells were exposed to CR medium for 30 min or 6 h, respectively, in the absence or presence of chloroquine (100 μM). (A–F) Western blotting analysis of LC3B and SQSTM1. n ≥ 5. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from control; ###P < 0.001, significantly different from CR. LC3B-II net flux (B and E) was determined by subtracting the densitometric value of LC3B-II amount in samples nontreated with chloroquine (LC3B-II − ChQ) from the corresponding sample treated with chloroquine (LC3B-II + ChQ). *P < 0.05, significantly different from control. (G) Representative images of autophagosome (yellow puncta) and autolysosome (red puncta; arrowheads) formation in mHypoN42 hypothalamic neurons transfected with mCherry-GFP-LC3 plasmids. Right images are a higher magnification from the boxed areas in Left. (H) Quantification of the number of mCherry-GFP-LC3 (yellow) and mCherry-only LC3 (red-only) puncta per cell in each condition (>20 cells per group). **P < 0.01, significantly different from control.
NPY Receptors Antagonists Inhibit Autophagy Stimulation Induced by CR in Hypothalamic Neurons.
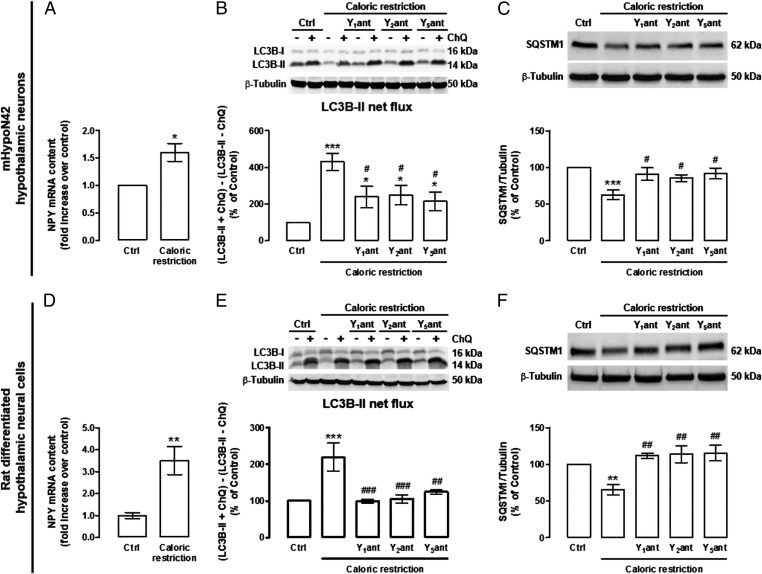
As we observed that CR increased the NPY mRNA content in hypothalamic neurons (Fig. 2 A and D), we hypothesized that an increase of autocrine stimulation of NPY receptors could mediate CR-induced autophagy. For that purpose, we blocked the NPY receptors, using NPY receptors selective antagonists, and evaluated the stimulatory effect of CR on autophagic flux (in the presence or absence of chloroquine). In both mHypoN42 hypothalamic neurons and rat differentiated hypothalamic neural cells, NPY Y1, Y2, and Y5 receptors antagonists inhibited, in part, the autophagic flux increase (Fig. 2 B and E) and SQSTM1 decrease (Fig. 2 C and F) induced by CR.
Fig. 2.
NPY receptors antagonists inhibit CR-induced autophagy in mHypoN42 hypothalamic neurons and in rat differentiated hypothalamic neural cells. (A and D) qPCR analysis of NPY mRNA levels in mHypoN42 hypothalamic neurons and rat differentiated hypothalamic neural cells exposed to CR medium for 30 min or 6 h, respectively. n = 4. *P < 0.05, **P < 0.01, significantly different from control. (B, C, E, and F) Cells were exposed to CR in the presence of NPY Y1 receptor antagonist (Y1ant; BIBP3226; 1 μM); Y2 receptor antagonist (Y2ant; BIIE0246; 1 μM) or Y5 receptor antagonist (Y5ant; l-152 804; 1 μM) in the absence or presence of ChQ (100 μM). Western blotting analysis of LC3B and SQSTM1. LC3B-II net flux (B and E) was determined by subtracting the densitometric value of LC3B-II amount in samples nontreated with chloroquine (LC3B-II − ChQ) from the corresponding sample treated with chloroquine (LC3B-II + ChQ). n ≥ 5. *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from control; #P < 0.05, ##P < 0.01, ###P < 0.001, significantly different from CR.
NPY Induces Autophagy in Hypothalamic Neurons Through NPY Y1 and Y5 Receptors Activation.
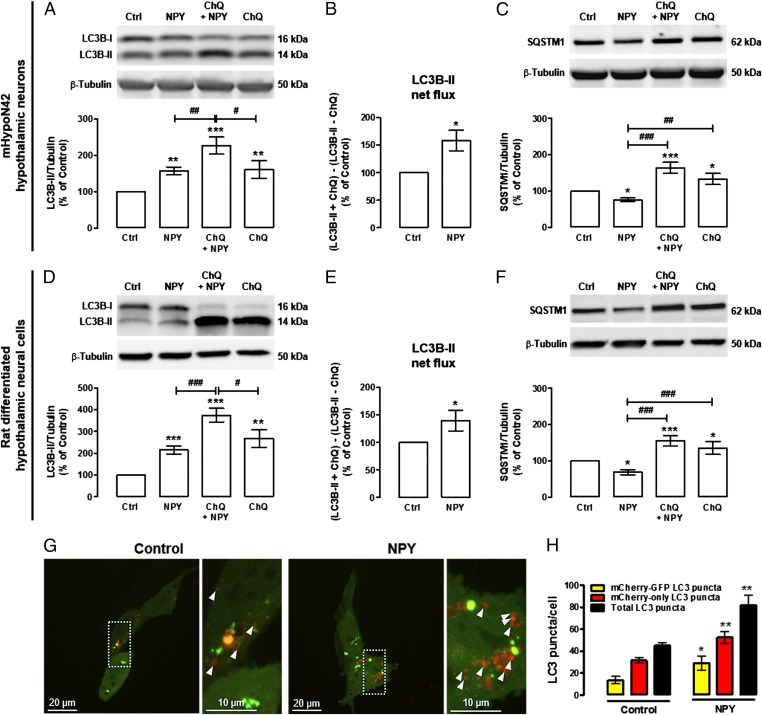
We then investigated the effect of NPY per se on autophagy in hypothalamic neurons. As shown in Fig. 3, NPY increased LC3B-II levels in both mHypoN42 hypothalamic neurons (Fig. 3A) and rat differentiated hypothalamic neural cells (Fig. 3D). It is known that LC3B-II can accumulate due to an enhanced autophagosome formation or impaired autolysosomal degradation. To rule out the possibility that the increase of LC3B-II was due to inhibited autolysosomal degradation, rather than autophagy stimulation and the respective autophagosome formation, we evaluated LC3B-II net flux, as described before. For this purpose, mHypoN42 hypothalamic neurons or rat differentiated hypothalamic neural cells were incubated with NPY in the presence or absence of the lysosomal protein degradation inhibitor chloroquine. The increase of LC3B-II induced by NPY in the presence of chloroquine was significantly higher than in cells treated with NPY only (Fig. 3 A and D). In addition, the LC3B-II turnover analysis showed an increased LC3B-II net flux in NPY-treated cells (Fig. 3 B and E). As expected, the decrease of SQSTM1 levels in NPY-treated cells was not observed in the presence of chloroquine (Fig. 3 C and F), supporting that SQSTM1 autophagic degradation is inhibited and that NPY increased autophagic flux in mHypoN42 hypothalamic neurons and rat differentiated hypothalamic neural cells. To further support that NPY enhances autophagy in hypothalamic neurons, we measured autophagic flux by using the tandem mCherry-GFP-LC3 cell-based assay (28, 31) in mHypoN42 hypothalamic neurons. NPY increased the number of autophagosomes (yellow puncta), autolysosomes (red-only puncta), and consequently, it also increased the total number of LC3 puncta (yellow+ red-only puncta) in mHypoN42 hypothalamic neurons (Fig. 3 G and H). Overall, these results show that NPY enhances autophagic flux in hypothalamic neurons.
Fig. 3.
NPY increases autophagic flux in mHypoN42 hypothalamic neurons and in rat differentiated hypothalamic neural cells. mHypoN42 hypothalamic neurons and rat differentiated hypothalamic neural cells were exposed to NPY (100 nM) for 30 min or 6 h, respectively, in the absence or presence of ChQ (100 μM). (A–F) Western blotting analysis of LC3B and SQSTM1. n ≥ 4. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from control; #P < 0.05, ##P < 0.01, ###P < 0.001, significantly different from NPY. LC3B-II net flux (B and E) was determined by subtracting the densitometric value of LC3B-II amount in samples nontreated with chloroquine (LC3B-II – ChQ) from the corresponding sample treated with ChQ (LC3B-II + ChQ). *P < 0.05, significantly different from control. (G) Representative images of autophagosome (yellow puncta) and autolysosome (red puncta; arrowheads) formation in mHypoN42 hypothalamic neurons transfected with mCherry-GFP-LC3 plasmids. Right images are a higher magnification from the boxed areas in Left. (H) Quantification of the number of mCherry-GFP-LC3 (yellow) and mCherry-only LC3 (red-only) puncta per cell in each condition (>30 cells per group). *P < 0.05, **P < 0.01, significantly different from control.
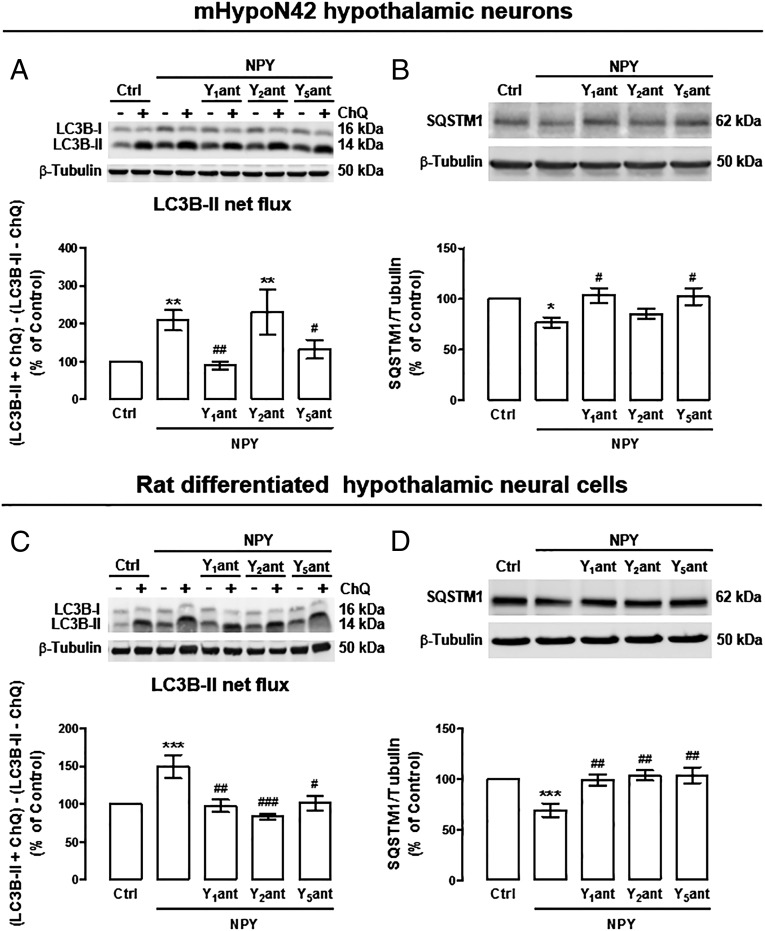
We then investigated which NPY receptors were involved in NPY-induced autophagic flux. In mHypoN42 hypothalamic neurons, blocking NPY Y1 or Y5 receptors, but not the NPY Y2 receptor, inhibited the stimulatory effect of NPY on autophagic flux (Fig. 4A). NPY Y1 and Y5 receptors antagonists also inhibited SQSTM1 decrease in NPY-treated mHypoN42 hypothalamic neurons (Fig. 4B). In rat differentiated hypothalamic neural cells, blocking NPY Y1, Y2, or Y5 receptors inhibited both autophagic flux (Fig. 4C) and SQSTM1 decrease upon NPY treatment (Fig. 4D). These results suggest that NPY increases autophagic flux in hypothalamic neurons through NPY Y1 and Y5 receptors activation. NPY Y2 receptor may be relevant for autophagy regulation in other hypothalamic neural cells.
Fig. 4.
NPY enhances autophagy through NPY Y1, Y2, or Y5 receptors activation. mHypoN42 hypothalamic neurons and rat differentiated hypothalamic neural cells were exposed to NPY (100 nM) for 30 min or 6 h, respectively, in the presence of NPY Y1 receptor antagonist (Y1ant; 1 μM); Y2 receptor antagonist (Y2ant; 1 μM), or Y5 receptor antagonist (Y5ant; 1 μM), in the absence or presence of chloroquine (100 μM; ChQ). (A–D) Western blotting analysis of LC3B and SQSTM1. LC3B-II net flux (A and C) was determined by subtracting the densitometric value of of LC3B-II amount in samples nontreated with chloroquine (LC3B-II − ChQ) from the corresponding sample treated with chloroquine (LC3B-II + ChQ). n ≥ 5. *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from control; #P < 0.05, ##P < 0.01, ###P < 0.001, significantly different from NPY.
NPY Induces Autophagy in Hypothalamic Neurons Through PI3K, ERK, and PKA Activation.
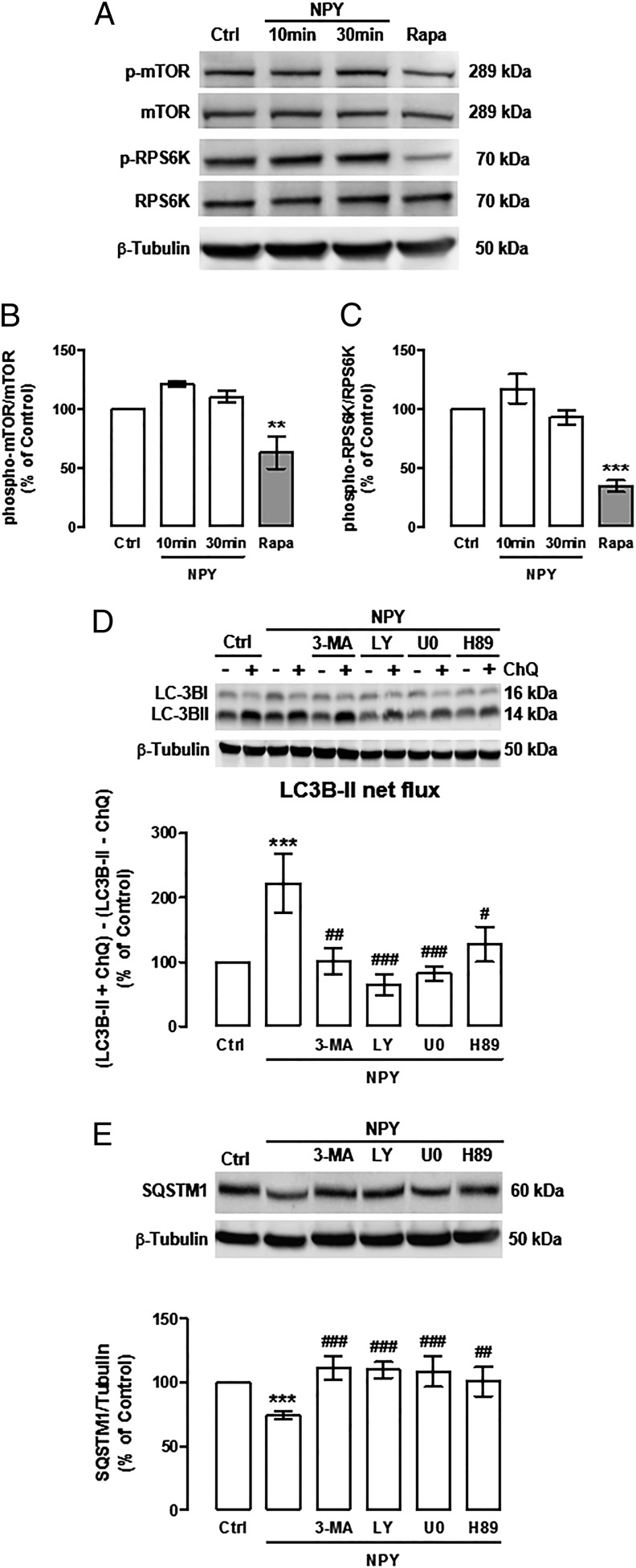
One of the molecular switches for autophagy induction is the inhibition of mechanistic target of rapamycin complex 1 (mTORC1) (32, 33). To evaluate whether NPY was inhibiting mTORC1 activity in NPY-treated mHypoN42 hypothalamic neurons, we analyzed the levels of phosphorylated mTOR (Ser2448), which is the active kinase form, and phosphorylated ribosomal protein S6 kinase (RPS6K) (Thr389), which is a substrate of active mTOR (34). Rapamycin, known to inhibit mTOR activity, was used as positive control (35). Rapamycin significantly decreased both phospho-mTOR (Fig. 5 A and B) and phospho-RPS6K (Fig. 5 A and C) in mHypoN42 hypothalamic neurons. However, NPY did not alter the phosphorylation status of both mTOR and RPS6K proteins (Fig. 5 A–C), suggesting that mTOR inhibition is not involved in NPY-induced autophagy. To investigate other possible pathways involved in NPY-induced autophagic flux, mHypoN42 hypothalamic neurons were treated with NPY in the presence of PI3K inhibitors (3-methyladenine, 5 mM or LY294002, 1 μM), MEK/ERK inhibitor (U0126, 1 μM) or PKA inhibitor (H89, 1 μM), in the presence or absence of chloroquine. The increase of LC3B-II lysosomal accumulation (LC3B-II net flux; Fig. 5D), and the decrease of SQSTM1 protein content (Fig. 5E) induced by NPY were inhibited by all of the protein kinase inhibitors tested. These results suggest that NPY induces autophagy in mHypoN42 hypothalamic neurons through PI3K, MEK/ERK, and PKA signaling pathways.
Fig. 5.
The stimulatory effect of NPY on autophagy in mHypoN42 hypothalamic neurons is mediated by PI3K, MEK/ERK, and PKA signaling pathways. (A–C) mHypoN42 hypothalamic neurons were treated with 100 nM NPY (10 or 30 min) or 10 nM rapamycin (Rapa; 1 h). Whole-cell extracts were assayed for phospho-MTOR (p-MTOR), MTOR, phospho-RPS6K (p-RPS6K), RPS6K, and β-tubulin (loading control) immunoreactivity by Western blotting. n > 5. **P < 0.01 and ***P < 0.001, significantly different from control. (D and E) Cells were treated with NPY in the presence of PI3K inhibitors (3-methyladenine, 3-MA; 5 mM or LY294002; 1 μM); MEK/ERK inhibitor (U0126; 1 μM), or PKA inhibitor (H89; 1 μM), in the absence or presence of ChQ (100 μM). Western blotting analysis of LC3B and SQSTM1. LC3B-II net flux (D) was determined by subtracting the densitometric value of of LC3B-II amount in samples nontreated with chloroquine (LC3B-II − ChQ) from the corresponding sample treated with chloroquine (LC3B-II + ChQ). n ≥ 5. ***P < 0.001, significantly different from control; #P < 0.05, ##P < 0.01, ###P < 0.001, significantly different from NPY.
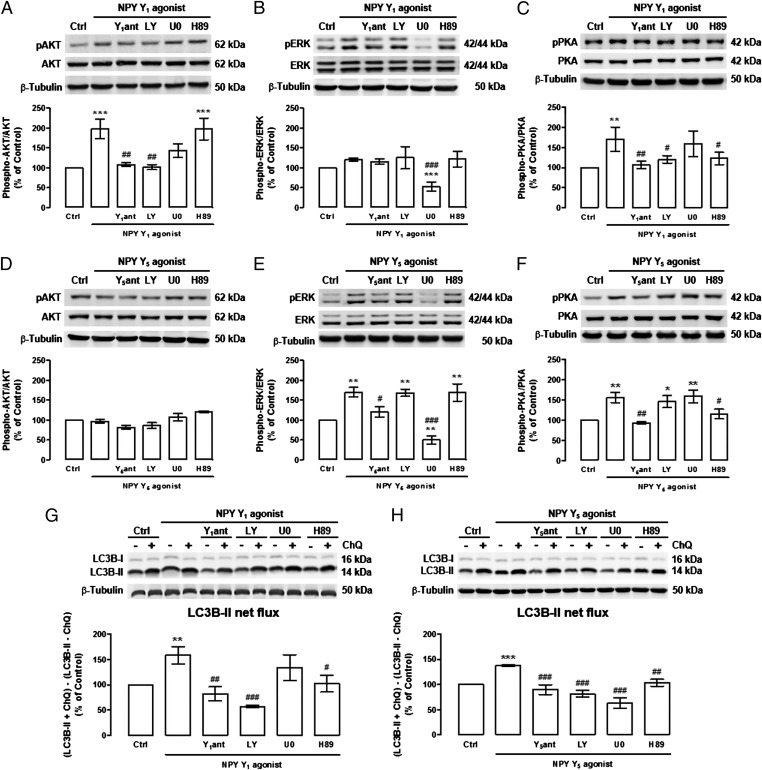
Because NPY increases autophagic flux in mHypoN42 hypothalamic neurons through the activation of NPY Y1 and Y5 receptors (Fig. 4A), to further elucidate the signaling pathways coupled to one or both of these NPY receptors, we evaluated the effect of NPY Y1 and Y5 receptor stimulation, by using the respective receptor agonist, on AKT, a downstream effector of class I PI3K, MEK/ERK, and PKA signal transduction cascades. As shown in Fig. 6A, NPY Y1 receptor agonist increased AKT phosphorylation in mHypoN42 hypothalamic neurons and, as expected, this effect was blocked by the NPY Y1 receptor antagonist, and also by the PI3K inhibitor. NPY Y1 receptor agonist increased the phosphorylation of PKA, and this effect was abolished by the NPY Y1 receptor antagonist, the PI3K inhibitor, or the PKA inhibitor (Fig. 6C). NPY Y1 receptor agonist had no effect on ERK phosphorylation (Fig. 6B). The NPY Y5 receptor agonist increased both ERK (Fig. 6E) and PKA (Fig. 6F) phosphorylation in mHypoN42 hypothalamic neurons, and the NPY Y5 receptor antagonist blocked these effects. The NPY Y5 receptor agonist-mediated ERK and PKA activation was blocked by the MEK/ERK inhibitor and the PKA inhibitor, respectively. The NPY Y5 receptor agonist did not increase AKT phosphorylation (Fig. 6D). These results suggest that whereas the NPY Y1 receptor agonist activates both AKT and PKA signaling pathways in a PI3K-dependent manner, the NPY Y5 receptor agonist activates both MEK/ERK and PKA signaling pathways in mHypoN42 hypothalamic neurons.
Fig. 6.
NPY Y1 and Y5 receptors activation increase autophagic flux in mHypoN42 hypothalamic neurons through PI3K, MEK/ERK, and PKA signaling pathways. mHypoN42 hypothalamic neurons were treated with NPY Y1 receptor agonist ([Leu31Pro34]NPY, 100 nM; A–C and G) or NPY Y5 receptor agonist [NPY19–23(Gly1, Ser3,22, Gln4,34.Thr6, Ala31, Aib32)PP; 100 nM; D–F and H), in the absence or presence NPY Y1 receptor antagonist (Y1ant; 1 μM; A–C and G), NPY Y5 receptor antagonist (Y5ant; 1 μM; D–F and H), PI3K inhibitor (LY294002; 1 μM), MEK/ERK inhibitor (U0126; 1 μM), PKA inhibitor (H89; 1 μM) or chloroquine (ChQ; G and H). Western blotting analysis of phospho-AKT (pAKT), AKT, phospho-p44/42 (pERK), p44/42 (ERK), phospho-PKA (pPKA), PKA, and LC3B. LC3B-II net flux (G and H) was determined by subtracting the densitometric value of LC3B-II amount in samples nontreated with chloroquine (LC3B-II − ChQ) from the corresponding sample treated with chloroquine (LC3B-II + ChQ). n ≥ 5. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from control; #P < 0.05, ##P < 0.01, ###P < 0.001, significantly different from NPY Y1 or Y5 receptor agonist.
Because PI3K, MEK/ERK, and PKA signaling pathways were involved in NPY-induced autophagy, as shown in Fig. 5D, we next evaluated the involvement of each signaling pathway on NPY Y1 and Y5 receptor agonists induced autophagic flux. For that, mHypoN42 hypothalamic neurons were exposed to NPY Y1 or NPY Y5 receptor agonists in the presence of PI3K, MEK/ERK, and PKA inhibitors, in the presence or absence of chloroquine. As expected, NPY Y1 (Fig. 6G) or Y5 (Fig. 6H) receptor agonists increased LC3B-II flux in mHypoN42 hypothalamic neurons, and these effects were abolished in the presence of respective NPY receptor antagonists (Fig. 6 G and H). In addition, the stimulatory effect of NPY Y1 receptor activation on autophagic flux was inhibited in the presence of PI3K or PKA inhibitors (Fig. 6G). However, PI3K, MEK/ERK, or PKA inhibitors inhibited the stimulatory effect of NPY Y5 receptor activation on autophagic flux (Fig. 6H). Therefore, whereas NPY Y1 receptor activation increased autophagic flux through PI3K and PKA signaling pathways, NPY Y5 receptor activation increased autophagic flux through PI3K, MEK/ERK, and PKA.
Overall, by using pharmacological tools, we show that, in mHypoN42 hypothalamic neurons, NPY enhances autophagic flux through the activation of NPY Y1 and Y5 receptors, and this effect is mediated by the concerted activation of PI3K, MEK/ERK, and PKA signaling pathways.
NPY Induces Autophagy in Mouse Hypothalamus: An in Vivo Study.
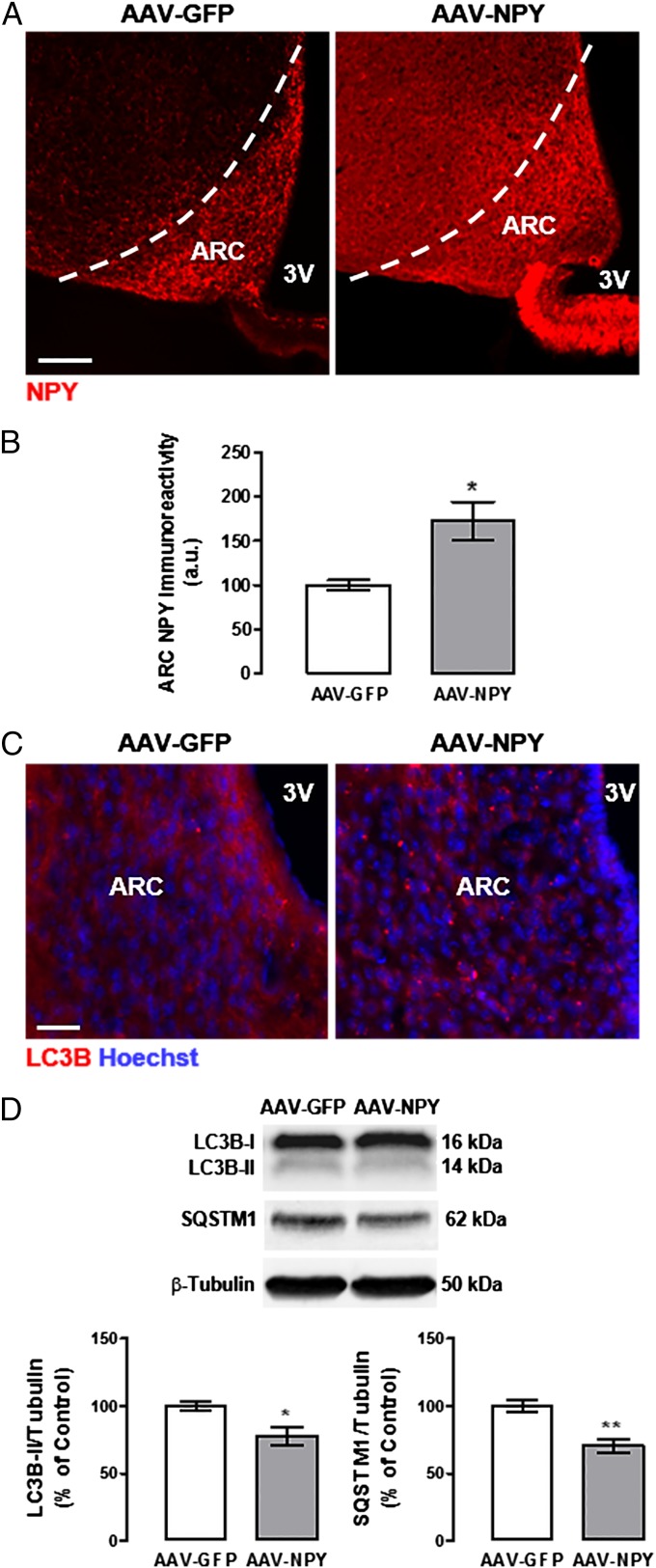
To evaluate the effect of NPY on hypothalamic autophagy modulation in vivo, NPY was overexpressed in hypothalamic arcuate nucleus (ARC) by gene transfer using adeno-associated viral vectors (AAV), in male C57BL/6 mice. Mice were injected with AAV encoding either GFP (AAV-GFP, control group) or NPY (AAV-NPY), under a neuronal-specific promoter (36), by bilateral stereotaxic injection in each ARC. After 4 wk, hypothalamic NPY-overexpressing mice (AAV-NPY mice) brains displayed a stronger, widespread expression of NPY through the several hypothalamic areas, but still more pronounced in the ARC (Fig. 7A). NPY immunoreactivity was quantified through the anterior-posterior length of the ARC and, as shown in Fig. 7B, we observed an increase of ∼70% of NPY immunoreactivity in the ARC in hypothalamic NPY-overexpressing mice compared with control mice (AAV-GFP; 172.5 ± 21.6% of control). We and others previously reported that hypothalamic NPY overexpression led to hyperphagia, increased body weight gain, and several serum alterations, consistent with obesity phenotypes, such as high glucose and high cholesterol (36). To avoid this type of effect that could potentially influence autophagy regulation (37, 38), hypothalamic NPY-overexpressing mice were pair-fed, by giving the same daily amount of food that AAV-GFP mice consumed. Four weeks after AAV infection, hypothalamic NPY-overexpressing mice (AAV-NPY) had a body weight gain similar to control mice (27.8 ± 0.4 g and 26.4 ± 0.6 g, respectively). In addition, the serum levels of glucose, cholesterol, and triglycerides levels of AAV-NPY mice (223.3 ± 28.3 mg/dL, 82.2 ± 2.6 mg/dL, and 100.0 ± 14.2 mg/dL, respectively) were not different from the control group (214.8 ± 11.2 mg/dL, 68.4 ± 3.3 mg/dL, and 89.8 ± 7.6 mg/dL, respectively). We then evaluated the effect of hypothalamic NPY overexpression on autophagy in the mouse hypothalamus. As shown in Fig. 7C, in the control group, LC3B puncta are small and diffusely distributed in the cytoplasm, whereas in the ARC of hypothalamic NPY-overexpressing mice (AAV-NPY), LC3B puncta are larger because of the increase of LC3B recruitment to autophagosomes, resulting in a less diffuse cytoplasmatic staining. Mice overexpressing hypothalamic NPY showed a decrease of LC3B-II (Fig. 7D) and SQSTM1 protein content (Fig. 7D) in the hypothalamus, compared with control mice. The decrease of the LC3B-II and SQSTM1 protein levels may indicate faster protein degradation in these mice, consistent with an increase of the autophagic flux in the hypothalamus.
Fig. 7.
Overexpression of ARC NPY increases autophagy in mouse hypothalamus. NPY expression in mouse ARC was modulated via bilateral injection with AAV. (A) Representative images of NPY immunoreactivity in the mouse ARC 1 mo after AAV injection, in the control group (AAV-GFP) and ARC NPY overexpressing group (AAV-NPY). (B) Quantification of NPY immunoreactivity through the anterior-posterior length of the mouse ARC, 1 mo after AAV injection. n = 4 mice per group. *P < 0.05, significantly different from control group. (C) Representative images of LC3B immunoreactivity in the mouse ARC 1 mo after AAV injection. n = 3 mice per group. (D) Western blotting analysis of LC3B and SQSTM1. n = 6–7 mice per group. *P < 0.05, **P < 0.01, significantly different from control group. (Scale bars: A, 100 μm; C, 20 μm.)
Discussion
Growing evidence show that autophagy activity decreases with age, contributing to aging phenotype and to age-related disease (4). The aging process is also related to alterations in neuroendocrine control of energy homeostasis, namely a decrease of hypothalamic NPY levels (24–26). Therefore, we hypothesized that modulation of hypothalamic NPY levels could regulate autophagy. In the present study, we demonstrate, for the first time to our knowledge, that NPY induces a stimulatory effect on autophagy in rodent hypothalamus.
The ability of hypothalamic neurons to stimulate autophagy in response to nutrient deprivation or NPY is consistent with the roles of hypothalamic neurons in feeding and energy homeostasis (30, 39, 40). Interestingly, the study by Kaushik et al. (30) showed that autophagy in the hypothalamus is impaired in normal-aged mice. Aging reduced hypothalamic Atg7 levels, decreased steady-state levels of LC3B-II, indicating reduced autophagosome content, and decreased lysosomal accumulation of LC3B-II and SQSTM1 (30). Because autophagy declines with aging (4), it is possible that decreased autophagy in the hypothalamus might contribute to the metabolic deregulation observed with aging and that such dysfunction may result from decreased NPY levels.
In the present study, we observed that NPY increased autophagic flux in mHypoN42 hypothalamic neurons through NPY Y1 or Y5 receptors activation, and this effect was associated with the concomitant activation of PI3K, MEK/ERK, and PKA signaling pathways. Because autophagy impairment in NPY/AgRP or POMC hypothalamic neurons has opposite effects on food intake and body weight (30, 39), the contribution of both NPY receptors on autophagy may represent a synergic, redundant, or compensatory mechanism among NPY receptors localized in different neurons. Further studies are needed to dissect this issue, and the use of NPY receptors knockout mice could elucidate which NPY receptor is critical.
Aging is associated with reduced levels of NPY in several cerebral areas, such as hypothalamus, cortex, and hippocampus (24–26). In addition, reduced NPY is associated with neurodegenerative diseases (41–43). However, the increase in NPY can lead to several physiological modifications similar to those induced by CR. Central administration of NPY has been shown to induce hyperphagia (44, 45), lower blood glucose levels in both humans and rats (46–48), and reduce core body temperature (49, 50). Interestingly, transgenic rats overexpressing NPY show improved resistance to stress and increased mean lifespan (27). In addition, it has been shown recently that CR does not increase lifespan in NPY knockout mice, enlightening NPY role as a lifespan and aging regulator (23). In humans, increased NPY levels may also be correlated with lifespan benefits because long-lived female centenarians have higher NPY plasma levels compared with younger women (51). Moreover, our study shows that NPY induces autophagy in the hypothalamus and that the inhibition of NPY receptors blocks CR-induced autophagy in hypothalamic neurons.
Because hypothalamic autophagy and NPY levels decrease with aging and that NPY regulates autophagy in the hypothalamus, as shown in our study, modulation of NPY levels may be manipulated to produce protective effects against hypothalamic impairments associated with age. Therefore, further studies on the role of NPY in the regulation of hypothalamic autophagy are expected to provide new therapeutic avenues to extend longevity and ameliorate age-related deteriorations.
Materials and Methods
Animals.
Female Wistar rats and male C57BL/6 mice were purchased from Charles River Laboratories. Mice were housed two per cage under a 12-h light/dark cycle in a temperature/humidity controlled room with ad libitum access to water and a standard chow diet. All experimental procedures were performed in accordance with the European Union Directive 86/609/EEC for the care and use of laboratory animals. Moreover, all of the people working with animals have received appropriate education [Federation of Laboratory Animal Science Associations (FELASA) course] as required by the Portuguese authorities. In addition, animals are housed in our licensed animal facility (International Animal Welfare Assurance number 520.000.000.2006). The present study is included in a project approved and financed by the Portuguese Science Foundation that approved the animal experimentation described. CNC - Center for Neuroscience and Cell Biology animal experimentation board approved the utilization of animals for this project (reference PTDC/SAU-FCF/099082/2008).
Hypothalamic Cell Cultures.
Rat hypothalamic neural cells primary cultures were obtained as we described (52). mHypoN42, an embryonic mouse hypothalamic cell line (CELLutions Biosystems), were maintained in high glucose (4.5 g/L d-glucose) Dulbecco’s Modified Eagle Medium (DMEM; Sigma), supplemented with 10% (vol/vol) heat inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C and 5% CO2/air.
Experimental Conditions.
To mimic a caloric restriction condition, mHypoN42 hypothalamic neurons were exposed to a serum/amino acid-free Earle’s balanced salt solution (EBSS plus 5.5 mM d-glucose) for 30 min, and rat differentiated hypothalamic neural cells were exposed to DMEM plus 5.5 mM d-glucose for 6 h. Cell death was not observed when cells were exposed to these caloric restriction mimetic conditions. To evaluate the effect of NPY on autophagy, cells were treated with recombinant NPY (100 nM; Phoenix Pharmaceuticals), NPY Y1 receptor agonist (Leu31Pro34, 100 nM; Bachem) or NPY Y5 receptor agonist [NPY19–23(Gly1, Ser3,22, Gln4,34.Thr6, Ala31, Aib32)PP, 100 nM; Bachem]. Other drugs used were as follows: chloroquine (100 μM, Sigma-Aldrich); NPYY1 receptor antagonist (BIBP3226; 1 μM), NPY Y2 receptor antagonist (BIIE0246, 1 μM), and NPY Y5 receptor antagonist (l-152,804; 1 μM), all from Tocris; PI3K inhibitors, 3-methyladenine (5 mM) and LY294002 (1 μM), ERK inhibitor (U0126; 1 μM), and PKA inhibitor (H-89; 1 μM), all from Merck Millipore. Chloroquine, NPY receptor antagonists, or protein kinase inhibitors were added to cell culture medium 30 min before the addition of caloric restriction mimetic medium, NPY, or NPY receptor agonists.
Western Blotting.
Western blotting was performed as described in SI Materials and Methods.
Autophagic Flux Measurement.
LC3B turnover assay.
The LC3B turnover assay measures the amount of LC3B-II delivered to the lysosomes by comparing the LC3B-II amounts in the presence and absence of the lysosomal inhibitor chloroquine (ChQ; 100 µM). Autophagic flux, expressed as “LC3B-II net flux,” was determined by subtracting the densitometric value of LC3B-II amount in samples nontreated with chloroquine (LC3B-II − ChQ) from the corresponding sample treated with chloroquine (LC3B-II + ChQ). This calculation was performed for each experimental condition tested. For each independent experiment, results are expressed as percentage of control.
Live-cell imaging of mHypoN42 hypothalamic neurons.
mHypoN42 cells plated in µ-Slide 8 well ibiTreat imaging chambers (Ibidi) were cultured for 2 d at 37 °C in a 5% CO2/air incubator. Cells were then transfected with 0.5 µg of mCherry-GFP-LC3 plasmid DNA by using TorpedoDNA Transfection Reagent (Ibidi) for 24 h, according to the manufacturer’s specifications. For mCherry-GFP-LC3B analysis, imaging was performed on a spinning-disk confocal (Cell Observed SD; Carl Zeiss) with an inverted microscope (Axio Observer Z1; Carl Zeiss) by using an Plan-Apochromat 63×, 1.4 N.A. oil immersion objective (Carl Zeiss) in an environmental chamber at 37 °C. Digital images were acquired with an EM charge-coupled device camera (Rolera EM-C2; QImaging) by using ZEN software (Carl Zeiss). For each condition, a z-stack in three different fields was imaged in each well and images were taken every 1 min for 90 min, upon the treatment with either caloric restriction medium or NPY. Cellular GFP-LC3- and mCherry-LC3-positive puncta were detected and quantified by using the Find Peaks plugin and the Analyze Particles tool in FIJI (Fiji is Just ImageJ) software (National Institutes of Health) and averaged per cell. More than 20 cells were analyzed in each condition, and data are representative of at least three independent experiments.
Quantitative Real-Time PCR.
Total RNA extraction, reverse transcription, and quantitative real-time PCR (qPCR) analysis was performed as described in the SI Materials and Methods.
Neuropeptide Y Overexpression in the Mouse Hypothalamic Arcuate Nucleus by Stereotaxic Injection of AAV.
Recombinant AAV particles were generated as described (36). AAV-1/2 chimerical capsids, containing recombinant plasmids with NPY cDNA under a neuronal-specific promoter, the human synapsin promoter, were injected in mice hypothalamic ARC to induce constitutive NPY overexpression. The human synapsin promoter in the viral vector guarantees that only mature neurons will express the transgene. The proximal region of the synapsin promoter is sufficient for directing neuron-specific gene expression. This proximal region is highly conserved between mouse and human (53). Recombinant plasmids with EGFP cDNA were used as a control for the procedure. Further details on stereotaxic injection procedure, animal follow up, and tissue sample processing are described in SI Materials and Methods.
Statistics.
Results are expressed as mean ± SEM. Data were analyzed by using one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test for multiple comparisons or Student’s unpaired t test with two-tailed P value when comparing two groups only. A value of P < 0.05 was considered significant. Prism 5.0 (GraphPad Software) was used for all statistical analysis.
Supplementary Material
Acknowledgments
We thank José Santos Ramalho (CEDOC, New University of Lisbon) for providing the tandem mCherry-GFP-LC3 plasmid DNA and Henrique Girão and Carla Marques for their help with the large-scale plasmid DNA isolation experiments. This work was supported by the Portuguese Foundation for Science and Technology, Fundo Europeu De Desenvolvimento Regional (FEDER), and COMPETE - Programa Operacional Factores de Competitividade (Grants PTDC/SAU-FCF/099082/2008, SFRH/BPD/73942/2010, SFRH/BD/73004/2010, SFRH/BPD/78424/2011, PEst-C/SAU/LA0001/2013.2014) and Projeto Mais Centro—“Aging, Stress And Chronic Diseases: From Mechanisms To Therapeutics” (CENTRO-07-ST24-FEDER-002006).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416609112/-/DCSupplemental.
References
- 1.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Klionsky DJ. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 3.Mariño G, López-Otín C. Autophagy and aging: New lessons from progeroid mice. Autophagy. 2008;4(6):807–809. doi: 10.4161/auto.6478. [DOI] [PubMed] [Google Scholar]
- 4.Cuervo AM. Autophagy and aging: Keeping that old broom working. Trends Genet. 2008;24(12):604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masoro EJ. Dietary restriction-induced life extension: A broadly based biological phenomenon. Biogerontology. 2006;7(3):153–155. doi: 10.1007/s10522-006-9015-0. [DOI] [PubMed] [Google Scholar]
- 6.Blagosklonny MV. Linking calorie restriction to longevity through sirtuins and autophagy: any role for TOR. Cell Death Dis. 2010;1:e12. doi: 10.1038/cddis.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen M, et al. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008;4(2):e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donati A. The involvement of macroautophagy in aging and anti-aging interventions. Mol Aspects Med. 2006;27(5-6):455–470. doi: 10.1016/j.mam.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Minor RK, Chang JW, de Cabo R. Hungry for life: How the arcuate nucleus and neuropeptide Y may play a critical role in mediating the benefits of calorie restriction. Mol Cell Endocrinol. 2009;299(1):79–88. doi: 10.1016/j.mce.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1030–R1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- 11.Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52(5):441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- 12.de Rijke CE, Hillebrand JJ, Verhagen LA, Roeling TA, Adan RA. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. J Mol Endocrinol. 2005;35(2):381–390. doi: 10.1677/jme.1.01808. [DOI] [PubMed] [Google Scholar]
- 13.Satoh A, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang G, et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature. 2013;497(7448):211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck B, Pourié G. Ghrelin, neuropeptide Y, and other feeding-regulatory peptides active in the hippocampus: role in learning and memory. Nutr Rev. 2013;71(8):541–561. doi: 10.1111/nure.12045. [DOI] [PubMed] [Google Scholar]
- 16.Decressac M, Barker RA. Neuropeptide Y and its role in CNS disease and repair. Exp Neurol. 2012;238(2):265–272. doi: 10.1016/j.expneurol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen AD, Herzog H, Sainsbury A. Neuropeptide Y and peptide YY: important regulators of energy metabolism. Curr Opin Endocrinol Diabetes Obes. 2011;18(1):56–60. doi: 10.1097/MED.0b013e3283422f0a. [DOI] [PubMed] [Google Scholar]
- 18.Wettstein JG, Earley B, Junien JL. Central nervous system pharmacology of neuropeptide Y. Pharmacol Ther. 1995;65(3):397–414. doi: 10.1016/0163-7258(95)98598-k. [DOI] [PubMed] [Google Scholar]
- 19.Wiater MF, et al. Circadian integration of sleep-wake and feeding requires NPY receptor-expressing neurons in the mediobasal hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1569–R1583. doi: 10.1152/ajpregu.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel MC, et al. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50(1):143–150. [PubMed] [Google Scholar]
- 21.Silva AP, Xapelli S, Grouzmann E, Cavadas C. The putative neuroprotective role of neuropeptide Y in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2005;4(4):331–347. doi: 10.2174/1568007054546153. [DOI] [PubMed] [Google Scholar]
- 22.Acuna-Goycolea C, van den Pol AN. Peptide YY(3-36) inhibits both anorexigenic proopiomelanocortin and orexigenic neuropeptide Y neurons: Implications for hypothalamic regulation of energy homeostasis. J Neurosci. 2005;25(45):10510–10519. doi: 10.1523/JNEUROSCI.2552-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiba T, et al. A key role for neuropeptide Y in lifespan extension and cancer suppression via dietary restriction. Sci Rep. 2014;4:4517. doi: 10.1038/srep04517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in neuropeptide-Y gene expression in the arcuate nucleus of the male rat brain is independent of testicular feedback. Endocrinology. 1994;134(6):2383–2389. doi: 10.1210/endo.134.6.8194464. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi H, Yang HY, Costa E. Age-related bidirectional changes in neuropeptide Y peptides in rat adrenal glands, brain, and blood. J Neurochem. 1988;50(6):1879–1886. doi: 10.1111/j.1471-4159.1988.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 26.Vela J, Gutierrez A, Vitorica J, Ruano D. Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J Neurochem. 2003;85(2):368–377. doi: 10.1046/j.1471-4159.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- 27.Michalkiewicz M, Knestaut KM, Bytchkova EY, Michalkiewicz T. Hypotension and reduced catecholamines in neuropeptide Y transgenic rats. Hypertension. 2003;41(5):1056–1062. doi: 10.1161/01.HYP.0000066623.64368.4E. [DOI] [PubMed] [Google Scholar]
- 28.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaushik S, et al. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep. 2012;13(3):258–265. doi: 10.1038/embor.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3(5):452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 32.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584(7):1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: Signalling inputs, substrates and feedback mechanisms. Cell Signal. 2009;21(6):827–835. doi: 10.1016/j.cellsig.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Ravikumar B, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 36.Sousa-Ferreira L, et al. Moderate long-term modulation of neuropeptide Y in hypothalamic arcuate nucleus induces energy balance alterations in adult rats. PLoS ONE. 2011;6(7):e22333. doi: 10.1371/journal.pone.0022333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Las G, Serada SB, Wikstrom JD, Twig G, Shirihai OS. Fatty acids suppress autophagic turnover in β-cells. J Biol Chem. 2011;286(49):42534–42544. doi: 10.1074/jbc.M111.242412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ost A, et al. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol Med. 2010;16(7-8):235–246. doi: 10.2119/molmed.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaushik S, et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011;14(2):173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan W, et al. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. 2012;153(4):1817–1826. doi: 10.1210/en.2011-1882. [DOI] [PubMed] [Google Scholar]
- 41.Decressac M, et al. Neuroprotection by neuropeptide Y in cell and animal models of Parkinson’s disease. Neurobiol Aging. 2012;33(9):2125–2137. doi: 10.1016/j.neurobiolaging.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Decressac M, Wright B, Tyers P, Gaillard A, Barker RA. Neuropeptide Y modifies the disease course in the R6/2 transgenic model of Huntington’s disease. Exp Neurol. 2010;226(1):24–32. doi: 10.1016/j.expneurol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Rose JB, et al. Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer’s disease. J Neurosci. 2009;29(4):1115–1125. doi: 10.1523/JNEUROSCI.4220-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck B, Stricker-Krongrad A, Nicolas JP, Burlet C. Chronic and continuous intracerebroventricular infusion of neuropeptide Y in Long-Evans rats mimics the feeding behaviour of obese Zucker rats. Int J Obes Relat Metab Disord. 1992;16(4):295–302. [PubMed] [Google Scholar]
- 45.Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: A powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7(6):1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- 46.Ahlborg G, Lundberg JM. Inhibitory effects of neuropeptide Y on splanchnic glycogenolysis and renin release in humans. Clin Physiol. 1994;14(2):187–196. doi: 10.1111/j.1475-097x.1994.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 47.Bischoff A, Michel MC. Neuropeptide Y lowers blood glucose in anaesthetized rats via a Y5 receptor subtype. Endocrinology. 1998;139(6):3018–3021. doi: 10.1210/endo.139.6.6127. [DOI] [PubMed] [Google Scholar]
- 48.Marks JL, Waite K. Intracerebroventricular neuropeptide Y acutely influences glucose metabolism and insulin sensitivity in the rat. J Neuroendocrinol. 1997;9(2):99–103. doi: 10.1046/j.1365-2826.1997.00554.x. [DOI] [PubMed] [Google Scholar]
- 49.Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol. 1991;260(2 Pt 2):R321–R327. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- 50.Kotz CM, Wang CF, Briggs JE, Levine AS, Billington CJ. Effect of NPY in the hypothalamic paraventricular nucleus on uncoupling proteins 1, 2, and 3 in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R494–R498. doi: 10.1152/ajpregu.2000.278.2.R494. [DOI] [PubMed] [Google Scholar]
- 51.Baranowska B, et al. Neuroendocrine control of metabolic homeostasis in Polish centenarians. J Physiol Pharmacol. 2006;57(Suppl 6):55–61. [PubMed] [Google Scholar]
- 52.Sousa-Ferreira L, et al. Proliferative hypothalamic neurospheres express NPY, AGRP, POMC, CART and Orexin-A and differentiate to functional neurons. PLoS ONE. 2011;6(5):e19745. doi: 10.1371/journal.pone.0019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoch S, Cibelli G, Thiel G. Neuron-specific gene expression of synapsin I. Major role of a negative regulatory mechanism. J Biol Chem. 1996;271(6):3317–3323. doi: 10.1074/jbc.271.6.3317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.