Significance
Dendritic cells (DCs) are pivotal for immune responses as they present antigens to T cells. Different DC subsets have different functions and are associated with different diseases. For example, plasmacytoid DCs (pDCs) produce type 1 interferons and are associated with the autoimmune disease, systemic lupus erythematosus. Understanding control of survival/apoptosis in different DC subsets may not only provide a molecular basis for their homeostasis but also guide therapeutic intervention of immunopathology. We revealed that two major DC subsets (pDCs and conventional DCs) express distinct BCL-2 family proteins at different levels and this correlated with their survival requirements. Accordingly, clinically applicable antagonist drugs killed the appropriate DC subsets, informing on the future use of these compounds for treating immune-mediated damage.
Keywords: apoptosis, dendritic cells, BH3-mimetic
Abstract
Dendritic cells (DCs) are heterogeneous, comprising subsets with functional specializations that play distinct roles in immunity as well as immunopathology. We investigated the molecular control of cell survival of two main DC subsets: plasmacytoid DCs (pDCs) and conventional DCs (cDCs) and their dependence on individual antiapoptotic BCL-2 family members. Compared with cDCs, pDCs had higher expression of BCL-2, lower A1, and similar levels of MCL-1 and BCL-XL. Transgenic overexpression of BCL-2 increased the pDC pool size in vivo with only minor impact on cDCs. With a view to immune intervention, we tested BCL-2 inhibitors and found that ABT-199 (the BCL-2 specific inhibitor) selectively killed pDCs but not cDCs. Conversely, genetic knockdown of A1 profoundly reduced the proportion of cDCs but not pDCs. We also found that conditional ablation of MCL-1 significantly reduced the size of both DC populations in mice and impeded DC-mediated immune responses. Thus, we revealed that the two DC types have different cell survival requirements. The molecular basis of survival of different DC subsets thus advocates the antagonism of selective BCL-2 family members for treating diseases pertaining to distinct DC subsets.
Dendritic cells (DCs) are pivotal antigen-presenting cells for induction of an adaptive immune response. Homeostasis of DCs is therefore paramount for induction of effective immunity to microbes as well as maintenance of immune tolerance to self-antigens. For example, removal of DCs abrogates the initiation of immune responses to exogenous antigens (1, 2). Conversely, perturbation of the DC compartment by prolonging DC survival in the mouse leads to autoimmunity (3, 4).
DCs are composed of several subsets that fulfill different roles in immune responses. Broadly, the two major subsets of DCs in mice and humans are the conventional DCs (cDCs) and plasmacytoid DCs (pDCs). The former can present antigen under both inflammatory and steady-state conditions (5) and CD8+ cDCs express TLR-3, abundant IL-12, and IFN-λ (6). Conversely, pDCs express TLR-7 and abundant type 1 interferons but lack the ability to present antigen in noninflammatory conditions (7, 8). pDCs have been implicated in the pathogenesis of systemic lupus erythematosus (9, 10), whereas cDCs are critical for activating killer T cells (CTLs) against Listeria and Plasmodia (1) and immune responses to proteinaceous vaccines (2). The DC subsets have different lifespans (11, 12). Little is known about the molecular basis of how the homeostasis of DC subsets is regulated during physiological and pathological conditions.
The intrinsic (also called BCL-2 regulated) pathway of apoptosis is a key process by which immune cells die (13). This pathway is controlled by the interaction between the members of the antiapoptotic and the two proapoptotic subgroups of this BCL-2 protein family. The five antiapoptotic BCL-2 family members, BCL-2, BCL-XL, BCL-W, A1 (called BFL1 in humans) and MCL-1, share four BCL-2 homology (BH) domains. The BH3-only proteins, which share only the BH3 domain, include BIM, PUMA, BID, NOXA, BMF, BIK, HRK, and BAD. They act either by binding/antagonizing the antiapoptotic BCL-2 family members or by direct binding and activation of the proapoptotic multi-BH domain BCL-2 family members BAX/BAK (14). It has been reported that different antiapoptotic BCL-2 family members are critical for the survival of distinct immune cell subsets: e.g., BCL-2 for naïve B cells (15), BCL-XL for erythroid progenitors and platelets (16), and MCL-1 for germinal center B cells and plasma cells (17, 18). It would therefore seem worthwhile to determine which antiapoptotic proteins maintain the survival of the different DC subsets.
BH3 small molecule mimetics (molecular weight < 1,000) have been developed for cancer therapy and are currently undergoing clinical trials. ABT-737 and navitoclax/ABT-263 bind with high affinity to BCL-2, BCL-XL, and BCL-W, but not A1 or MCL-1 (19), whereas venetoclax/ABT-199 only binds BCL-2 (20). These reagents may not only be valuable tools to probe the role of the antiapoptotic proteins in DCs, but may also be used for selective killing of DC subsets associated with certain disease states, including autoimmune ones.
We found herein that pDCs and cDCs each have distinct requirements for sustained survival, displaying a distinguishing expression profile of antiapoptotic BCL-2 family members. This pattern of expression corresponded with their dependence on survival. Thus, pDCs express more BCL-2 and rely for survival more heavily on BCL-2 than cDCs. Conversely, cDCs express more A1 and rely more heavily on A1 than pDCs. The two DC subsets express similar levels of MCL-1 and both require MCL-1 for survival. These findings provide guidance for which BH3 mimetics might be used for treating inflammatory and autoimmune diseases that have differential DC subset involvement.
Results
pDCs and cDCs Exhibit Distinct Expression Profiles of the Antiapoptotic BCL-2 Family Members.
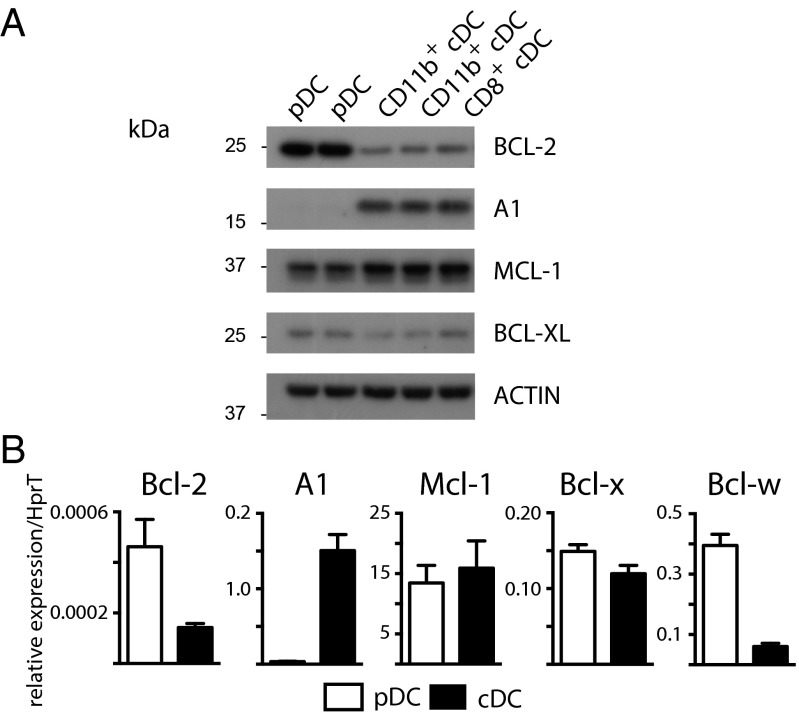
The expression of the antiapoptotic BCL-2 members was examined in sorted pDCs (Siglec H+ or CD317+ CD11cint CD11b−) and cDCs (CD11chi Siglec H−; further subdivided into CD11b−CD8+ and CD11b+CD8−) from wild-type C57BL/6 (WT) spleen. Protein and mRNA expression were measured by Western blotting (Fig. 1A) and RT-quantitative PCR (RT-qPCR), respectively (Fig. 1B). We found a striking difference between cDCs and pDCs. There was an obverse pattern of expression of BCL-2 and A1 between pDCs and cDCs, in other words, pDCs had abundant BCL-2 and scant A1, whereas cDCs had moderate levels of BCL-2 but abundant A1. The modest expression of BCL-XL and high expression of MCL-1 were similar in both subsets (Fig. 1). In cDCs, the pattern of expression was comparable for the CD8+ and CD8− lineages. At the mRNA level, Bcl-w was consistently expressed more highly in pDCs compared with cDCs. However, at the protein level, both pDCs and cDCs expressed only little BCL-W (Fig. S1A). We also observed a similar pattern of expression of BCL-2 vs. A1 for lymph node (LN) pDCs and cDCs (Fig. S1B). Migratory DCs (identified as MHC IIhi and CD11cint, relative to lymph resident cDCs) within LNs expressed moderate levels of BCL-2 but high levels of A1, even compared with cDCs (Fig. S1B).
Fig. 1.
Dendritic cell subsets differ in their expression of antiapoptotic BCL-2 family proteins. Splenic cDCs and pDCs were purified by sorting from 8 to 12 wk C57BL/6 (WT) mice. (A) Western blot analysis of BCL-2, A1, MCL-1, and BCL-XL with β-actin loading control. Data are representative of three independent experiments. (B) RT-qPCR of Bcl-2, A1, Mcl-1, Bcl-x, or Bcl-w using HprT for normalization. Data represent mean ± SEM of n = 3–9 experiments in duplicate.
Next we examined the expression of these antiapoptotic molecules by DCs after activation by CpG in vivo. Overall the expression patterns are similar to those in DCs at steady state (unstimulated). For example, expression of A1 protein by pDCs remained very low even after stimulation with CpG, although a minor increase in the levels of A1 mRNA was observed (Fig. S1C). Activation with CpG also caused an increase in A1 in cDCs and BCL-2 in pDCs.
pDCs Are Preferentially Affected by Transgenic BCL-2 Overexpression.
Preferential expression of BCL-2 in pDCs prompted us to investigate its contribution to the survival and homeostasis of this cell type. BCL-2 is massively overexpressed in hematopoietic cells from vav-Bcl-2 transgenic (Tg) mice (21). We found that both the proportion and absolute number of pDCs in spleen were more than twofold higher in vav-Bcl-2 Tg mice (P < 0.0001) compared with C57BL/6 WT mice (Fig. 2A). The proportion of cDCs in spleen was modestly reduced but their absolute numbers were comparable between vav-Bcl-2 Tg and control mice. Thus, whereas BCL-2 overexpression can significantly increase pDC numbers, it has little influence on cDC numbers in vivo.
Fig. 2.
Enforced BCL-2 expression causes an increase in the numbers of pDCs but not cDCs. (A) Splenic DC subsets from vav-Bcl-2 Tg mice and C57BL/6 (WT) control mice (n = 6–7 each). Representative FACS plots show gates for pDCs and cDCs (Left) and subsets within cDCs (Right). Scatterplots show percentages (Upper) and absolute numbers (Lower) of DC subsets from individual mice. (B) DC subsets in the spleens from reconstituted mixed bone marrow chimera. Scatterplots show percentages of DC subsets from six individual mice. (C) Enforced BCL-2 expression prolongs pDC survival. Sorted pDCs (20,000 per well) from vav-Bcl-2 Tg and WT control mice were cultured for 0 and 20 h. Viable DCs were enumerated. Bar graphs represent mean ± SEM of viable DC number (Left) and percentage relative to input at 0 h (Right). ***P < 0.001 compared with WT.
To verify that the increase in pDCs in vav-Bcl-2 Tg mice is cell intrinsic, we produced mixed bone marrow chimeras by reconstitution of lethally irradiated Ly5.1 mice with Ly5.1/Ly5.2 WT bone marrow (BM) and vav-Bcl-2 Tg (Ly5.2) BM cells (1:1, 2 × 106 cells each). When spleens of recipient animals were analyzed 7 wk after reconstitution, the proportion of pDCs in vav-Bcl-2 Tg (Ly5.2) DCs was twofold higher than that of WT DCs (Fig. 2B).
In accordance with our observation, a recent report showed that pDCs but not cDCs from vav-Bcl-2 Tg mice have markedly prolonged survival in vitro (22). We confirmed that purified pDCs from vav-Bcl-2 Tg mice had profoundly prolonged survival: 80% of WT pDCs died within 20 h in culture, whereas there was no loss of vav-Bcl-2 Tg pDCs over the same duration of culture (Fig. 2C).
pDCs but Not cDCs Are Killed by Pharmacological Antagonism of BCL-2.
Next we tested whether BCL-2 antagonist drugs could selectively kill pDCs. We cultured DC-enriched splenocytes in vitro with increasing doses of ABT-199 over 1–3 d (Fig. 3A). pDC survival was profoundly affected by ABT-199 (Fig. 3A); at 10 nM ABT-199 killed 80% of pDCs and at 100 nM 99% of pDCs after a 1-d exposure. In contrast, cDCs within the same well were unaffected, even at 1,000 nM ABT-199. Although spontaneous death of pDCs and cDCs increased when cells were cultured for a longer time, preferential killing of pDCs by ABT-199 in a dose-dependent manner was observed over 3 d of culture (Fig. 3A). Although ABT-199 also kills T cells and B cells, the killing of pDCs by ABT-199 was more profound (Fig. S2F). Because the BCL-XL–specific inhibitor WEHI-539 did not potently kill pDCs and cDCs and ABT-737 did not show an overt increase in killing over the BCL-2–specific antagonist ABT-199 (Fig. S2G), these results collectively demonstrate that killing of pDCs is mainly mediated by BCL-2 antagonism.
Fig. 3.
Pharmacological antagonism of BCL-2 function impairs pDC but not cDC survival in vitro and in vivo. (A) Sensitivity to ABT-199 in vitro. DC-enriched C57BL/6 splenocytes were treated with the BH3 mimetic compound ABT-199 at the indicated dose. After 1–3 d cells were recovered and survival was measured by propidium iodide (PI) staining and FACS analysis. Data are presented as the proportions (percentage) of the average number of cell subsets isolated from untreated cultures with means ± SEM (Left) and numbers of recovered live DCs (Right). (B–D) Sensitivity to ABT-199 in vivo. Mice were treated daily for 4 d with 100 mg/kg body weight ABT-199 or vehicle. After treatment spleens were recovered and viable DCs remaining in the organs determined by flow cytometry. (B) FACS plots show DC profiles. Bar graphs show percentage of DC subsets. (C) The numbers of spleen DCs and lymphocytes. (D) The proportion (percentage) of the numbers of cell subsets. Data represent mean of n = 6 animals ± SEM *P < 0.05, **P < 0.01, ***P < 0.001.
To confirm and extend our in vitro studies, the effect of ABT-199 was assessed in vivo. C57BL/6 WT mice were treated for 4 consecutive days with either ABT-199 (100 mg/kg body weight) or vehicle, and DC composition was analyzed on day 5. Consistent with our in vitro findings, ABT-199 treatment dramatically reduced the number of pDCs (sixfold reduction; P < 0.01 by two-tailed t test) but not the number of cDCs (including CD8+ cDCs) in the spleen (Fig. 3 B and C). Accordingly, the percentage of pDCs within spleen cells was significantly reduced in treated mice, whereas the percentage of cDCs was increased due to reduction of pDCs and lymphocytes (Fig. 3B).
As reported previously for ABT-737 (15), ABT-199 treatment also resulted in a reduction in the numbers of B cells and T cells (Fig. 3C). Nevertheless, when individual populations were compared between mice receiving ABT-199 and vehicle, the reduction in pDCs (13.5% of untreated) was significantly more profound than the reduction in lymphocytes (42% of untreated for B cells; 35% of untreated for T cells) (Fig. 3D). Therefore, we conclude that inhibition of BCL-2 alone was sufficient to kill the majority of pDCs both in vitro and in vivo.
Of note, the expression patterns of the antiapoptotic BCL-2 family members underwent some changes in response to TLR activation (Fig. S1). To test whether these changes in expression affected sensitivity to the BH3 mimetic compounds, we activated purified pDCs and cDCs with 100 nM CpG for 16 h and then treated these cells with ABT-199 or ABT-737 for the next 24 h (Fig. S2). Both pDCs and cDCs up-regulated CD86 following CpG stimulation (Fig. S2B). CpG stimulation also led to increased survival of both pDCs and cDCs in culture, and this increase was more prominent for pDCs (Fig. S2C). Nevertheless, CpG-treated pDCs remained highly sensitive to ABT-199 and ABT-737, whereas cDC remained resistant (Fig. S2 D and E). Resistance to ABT-199 or ABT-737 by cDCs is also reflected by the preservation of cDC function, based on cytokine production in response to CpG (Fig. S2H).
cDCs Are Preferentially Depleted by Reduction of A1.
Due to the overtly higher expression of A1 by cDCs compared with pDCs, we wanted to test the contribution of A1 to DC survival. To do this testing, we used a mouse model of RNA interference (RNAi), enabling stable knockdown of all functional A1 isoforms (A1-a, A1-b, and A1-d) (23) (Fig. 4). Briefly, mice were generated containing a pan A1-targeted shRNA sequence embedded in the context of microRNA 30 (miR30), under control of the tetracycline responsive CMVmin promoter (TRE), followed downstream by eGFP, driven from the human ubiquitin promoter (UbiP) (Fig. 4A). These mice, referred to as TREA1 (TRE-miR30-A1), showed intermediate GFP expression in all hematopoietic cell subsets (as well as other cell types) in flow cytometric analysis (23) (Fig. 4B). TREA1 mice were intercrossed to mice expressing the Tet transactivator (tTa) under control of the Vav2 promoter, selectively directing A1-specific small interfering RNAs (siRNAs) to hematopoietic cells in double transgenic mice (double Tg). We found these cells, marked by high GFP expression due to tTA binding to the upstream TRE promoter, to constitute ∼10–20% of cells in spleen and pooled subcutaneous lymph nodes of double Tg mice (Fig. 4B). Notably, in addition to the GFPhi (A1 deficient) and GFPint (A1 sufficient) populations, double Tg mice also comprised a population of cells that no longer expressed GFP, indicative of transgene silencing, which was shown to be stable over time (23).
Fig. 4.
A1 is critical for the in vivo survival of cDCs but not pDCs. (A) Schematic representation of the construct used to generate the A1 knockdown transgenic mice. (B) Transgenic expression of GFP in cells isolated from LNs from representative tTA single transgenic, TREA1 single transgenic, or double Tg mice. Percentages of eGFP intermediate and GFP high cells are shown. (C and D) Percentages of cDCs and pDCs in spleen and LNs from representative tTA single transgenic, TREA1 single transgenic, or GFP intermediate, or high cells from double Tg mice. DCs were enriched on Nycodenz gradients before analysis. (E) Percentages of cDCs or pDCs from total DCs isolated from spleen and LNs from tTA single transgenic, TREA1 single transgenic, or GFP intermediate or high cells from double Tg mice. Data represent mean of n = 5 (spleen) and n = 3 (LN) animals ± SEM and analyzed using a two-tailed Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
Analysis of double Tg mice revealed that cDCs were profoundly underrepresented in the GFPhi A1-deficient population in both the spleen and LNs, with an approximate fourfold reduction compared with the A1-sufficient GFPint population within the same double Tg mouse, or compared with single transgenic TREA1 or tTA control animals (Fig. 4E). As would be expected with the reduction of cDCs, pDCs were found to be proportionally overrepresented in these A1-deficient cell populations. In an attempt to exclude off-target effects, mice with similar microRNA-based shRNA targeting of an irrelevant gene, Renilla luciferase (TRELuc) were also assessed and found to contain comparable proportions of cDC and pDC to control animals (Fig. S3). Taken together, these data argue for a critical role for A1 in cDC, but not pDC survival in vivo.
Both pDCs and cDCs Are Affected by Loss of MCL-1.
As MCL-1 expression was substantial in both pDCs and cDCs (Fig. 1), we examined its contribution to cell survival. Global deficiency in MCL-1 results in periimplantation lethality in mice but Mcl-1+/− mice are reportedly largely normal (24). Remarkably, even Mcl-1 haploinsufficiency led to a dramatic reduction (more than sevenfold) in the absolute numbers of both cDCs and pDCs in the spleen (Fig. 5A) and LNs (Fig. 5B).
Fig. 5.
Loss of one allele of Mcl-1 substantially reduces the numbers of both pDCs and cDCs. Flow cytometric analysis of DC subsets in spleen (A) and (B) and LNs (C) from 8- to 14-wk-old mice deficient in one allele of Mcl-1 (Mcl-1+/−) or C57BL/6 (WT) controls. Representative FACS profiles are shown for Mcl-1+/− and WT controls. Plots are gated on total spleen cells with boxes showing total DCs (Upper) and total CD11c+ DCs with boxes showing the percentages pDCs (CD317+ CD11cint) and cDCs (CD11chi) (Middle) with cDCs further gated and defined by expression of CD8 (CD8+ CD11b−, CD8− CD11b+) (Lower). Bar graphs show mean of n = 7 (spleen) and n = 3 (LN) animals ± SEM and were analyzed using a two-tailed Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we tested CD11c-Cre Mcl-1fl/fl mice, in which Mcl-1 is conditionally deleted only in DCs. For an unbiased comparison, CD11c-Cre+Mcl-1fl/fl mice (Cre+Mcl-1fl/fl) were compared with WT C57BL/6 mice, rather than Mcl-1fl/fl littermates, as the floxed locus generates an abnormally stabilized form of MCL-1 protein (due to a 13 amino acid N-terminal extension), which could potentially enhance DC survival (25). Recombination of a floxed allele of Mcl-1 places a human CD4 cassette under the control of Mcl-1 regulatory elements and thus human CD4 serves as a reporter of Mcl-1 deletion (26). In CD11c-Cre+Mcl-1fl/+ mice human CD4 was detected on both pDCs and cDCs (Fig. S4A), confirming that Mcl-1 could be deleted efficiently by CD11c-Cre in both cell types.
Conditional deletion of Mcl-1 dramatically reduced (by ∼10-fold) the numbers of pDCs and cDCs in spleens, compared with WT mice (Fig. 6A). To demonstrate that loss of DC in this model is cell intrinsic, and not due to a drop in a peripheral cell population required for DC survival, lethally irradiated congenic mice (Ly5.1) reconstituted with 1:1 mixed Cre+Mcl-1fl/fl BM (Ly5.2) and WT BM (Ly5.1/Ly5.2) were generated. Strikingly, Cre+Mcl-1fl/fl BM essentially did not contribute to the DC population, with the overwhelming majority of cDCs and pDCs present in spleen and LNs found to be of WT origin (Fig. 6 B and C).
Fig. 6.
MCL-1 is critical for the survival of both pDCs and cDCs. (A) DC subsets were assessed in spleen (Upper) or LNs (Lower) from 6- to 8-wk-old lethally irradiated Ly5.1 mice reconstituted with BM from either WT or Cre+Mcl-1fl/fl donors. Data represent mean of n = 3 animals ± SEM. (B and C) BM chimera (Ly5.1) from 1:1 mix of WT (Ly5.1/Ly5.2):Cre+Mcl-1fl/fl (Ly5.2) donors. (B) Representative FACS profiles of DC-enriched spleen cells from mixed bone marrow chimera showing pDC (Siglec H+ CD11cint) and cDC (CD11chi) populations (boxes) derived from either WT (Ly5.1/Ly5.2) or Cre+Mcl-1fl/fl (Ly5.2) gate. (C) Percentages and numbers of DC subsets. Data represent mean of n = 6 animals ± SEM. (D) Proliferation of transferred OT-I T cells. (E) Induction of endogenous OVA-specific CD8+ T cells. (F) IFN-α production by spleen cells. Spleen cells (5 × 105 per well) from six individual WT and Cre+Mcl-1fl/fl mice were stimulated with CpG for 20 h. Data represent mean of n = 6 animals ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared with WT mice.
The functional consequence of DC reduction due to loss of MCL-1 was then investigated. First, cross-priming of CD8+ T cells was compared between WT and Cre+Mcl-1fl/fl mice in two ways: proliferation of transferred OVA-specific OT-I T cells and enumeration of endogenous OVA-Kb tetramer cells after immunization with cell-associated OVA. In both tests, expansion of antigen-specific CD8+ T cells was significantly reduced in Cre+Mcl-1fl/fl mice (Fig. 6 D and E). Second, CpG-stimulated IFN-α production by spleen cells was compared between WT and Cre+Mcl-1fl/fl mice. IFN-α production was also significantly reduced in Cre+Mcl-1fl/fl mice (Fig. 6F). Furthermore, in vivo IFN-α production following an acute LMCV infection was tested. Infection induced a significantly lower IFN-α production in Cre+Mcl-1fl/fl mice (Fig. S4B). Thus, antigen presentation and cytokine production were impaired when DC survival and numbers were reduced due to loss of MCL-1.
To investigate whether loss of pDCs and cDCs was peculiar to the spleen, DC composition in the thymus, skin-draining LNs and gut-draining mesenteric LNs of intact Cre+Mcl-1fl/fl mice and WT mice were evaluated. Reduction of pDCs and cDCs occurred in all lymphoid tissues examined (Fig. S5). LNs contain various types of migratory DCs (with higher expression of MHC class II and lower expression of CD11c, compared with lymphoid-resident cDCs). Conditional deletion of Mcl-1 also dramatically reduced the percentages and numbers of migratory DCs (Fig. S5B). Of note, the reduction of migratory DC subsets was not uniform. In skin-draining LNs, three main migratory DC types can be identified: CD326+CD11bint Langerhans cells, CD11b+ dermal DCs, and CD103-equivalent DCs (CD24+CD11b−). As CD103 expression on “CD103 DCs” is subject to up-regulation by GM-CSF (and hence inflammation) (27, 28), we have used CD24 as a surrogate marker that is not affected by GM-CSF (28). In Cre+Mcl-1fl/fl mice the reduction of CD326+CD11bint Langerhans cells (57% of WT) was more moderate, compared with the profound reduction in CD11b+ dermal DCs (16% of WT) and CD103-equivalent DCs (13% of WT).
Collectively, our results highlight an indispensable role of MCL-1 in the survival of both the cDC and pDC subsets in vivo.
Discussion
We found that pDCs and cDCs have a “cell survival identity” ascribable to the expression levels of the different antiapoptotic BCL-2 family members with high BCL-2 and low A1 in pDCs and the converse in cDCs. This pattern of differential expression is largely preserved after DC activation through TLRs. Importantly, this pattern of expression predicted the differential reliance by the DC subsets for their survival, namely, pDCs are more reliant on BCL-2, whereas cDCs are more dependent on A1. Accordingly, and of therapeutic relevance, BCL-2 antagonist drugs (ABT-737 antagonizing BCL-2, BCL-XL, and BCL-W and ABT-199 antagonizing BCL-2 alone) preferentially killed pDCs, although sparing cDCs.
pDCs are thought to have longer lifespans than cDCs (12, 29). Notably, BCL-2 has a 40-fold longer half-life than A1 (∼10 h compared to ∼15 min) (30) and pDCs rely more on BCL-2, whereas cDCs rely more on A1. Nevertheless, it remains speculative whether the protein turnover rate of the different antiapoptotic BCL-2 family member has a causal relationship with the lifespan of the DC subsets.
Several earlier studies examined the role of BCL-2 in the regulation of DC survival and lifespan (31, 32). For example, CD11c-Bcl-2 transgenic mice had increased numbers of DCs. Unfortunately, these studies did not investigate any differential effects on DC subsets. Relatively high levels of BCL-2 in pDCs have also previously been reported (4). Of note, both pDCs and cDCs express BCL-XL at the mRNA and protein level. However, ABT-737 targeting BCL-2, BCL-XL, as well as BCL-W did not increase killing potency, compared with BCL-2 targeting alone with ABT-199 (Fig. S2G). The BCL-XL–specific inhibitor WEHI-539 (33) also did not potently kill pDCs and cDCs (Fig. S2G). Thus, BCL-XL may only have a limited role in regulating DC survival. Similarly, although some BCL-W mRNA was seen in both cDCs and pDCs, no BCL-W protein could be detected. Comparison of DC killing by ABT-737 vs. ABT-199 suggests that BCL-W has only a limited role although the precise contribution of BCL-W to DC survival remains to be examined by conditional deletion of its gene in DCs.
The role of neither MCL-1 nor A1 in DC survival had been addressed previously. This in part may be due to the periimplantation embryonic lethality associated with global MCL-1 deficiency (24) and the quadruplication of A1 genes in mice. Our studies using conditional Mcl-1 gene deletion and Mcl-1 haploinsufficiency indicated that MCL-1 was critical for the maintenance of both pDCs and cDCs. Nevertheless, steady-state levels of MCL-1 were not sufficient at protecting pDCs from death induced by BCL-2 antagonist drugs. It remains moot whether in steady state, constitutive A1 expression, rather than MCL-1, may be more predictive of resistance to BCL-2 antagonist drugs.
Importantly, we also demonstrated that perturbation of DC survival has significant functional consequence. When DC numbers were reduced due to MCL-1 deficiency, T-cell priming and cytokine production were profoundly affected. Implications of these findings are twofold: first, it informs us that manipulation of DC survival may have potential to curtail an unwanted immune response and to provide an avenue for intervention in autoimmune diseases; second, it reveals that drugs targeting DC survival may impede certain immune function and this may constitute a significant “side effect” of such drugs. Our dissection of the survival requirements of DC subsets provides useful information regarding which immune responses are likely to be affected by these type of drugs. In this context, we found that ABT-199 or ABT-737 does not impede in vitro cDC function, based on production of several cytokines (Fig. S2H). Conversely, ABT-199 significantly reduces IFN-α production by pDCs, including pDCs from lupus-prone (NZB/NZW F1) mice (34).
Despite relatively high steady-state expression of endogenous BCL-2, enforced expression of additional BCL-2 further increased pDC numbers in transgenic mice, suggesting the level of BCL-2 protein was an important determinant of the survival and numbers of these cells. However, enforced expression of BCl-2 in cDCs only moderately enhance in vitro survival of cDC subsets (22). Similarly, despite high steady-state levels of A1 and MCL-1 in cDCs, and similar specificity for the apoptosis initiating BH3-only proteins, these prosurvival proteins were not functionally redundant in cDCs, with loss of either substantially reducing peripheral cDC numbers.
Although mouse pDCs express very little A1, even after TLR stimulation, and although A1 inhibition impacts little on mouse pDCs, A1 has been identified as a direct transcriptional target of Spi-B in human pDCs, based on expression of its mRNA and a reporter (35). Furthermore, inhibition of A1 impaired in vitro differentiation of human pDC but not non-pDCs from CD34+ hematopoietic progenitor cells (35). It remains to be determined whether mouse pDCs and human pDCs differ in their cell survival requirements.
Two studies showed that perturbation of BH3 proteins variably affect DC homeostasis (36, 37). Neither study enumerated pDCs and cDCs separately. We found that DC numbers were increased in Bim−/− mice; all DC subsets were increased proportionally in these animals (Fig. S6). We also found that NOXA deficiency had only minimal impact on DC numbers (Fig. S6). Thus, whereas it still remains unclear exactly how BCL-2 family members regulate DC subset-specific survival programs, we contend that differential expression and dependence on antiapoptotic BCL-2 proteins by DC subsets, rather than differences in proapoptotic BH3-only proteins, are the dominant determinants.
We reasoned that different dependencies of distinct DC subsets for cell survival may make it possible to use highly selective BH3 mimetics for the treatment of particular diseases. As pDCs have been linked to the pathogenesis of immunopathological diseases, such as systemic lupus erythematosus (9, 10, 38), our findings suggest that ABT-737 and ABT-199 may be useful in treating such pDC-associated diseases. Indeed, ABT-737 was shown to ameliorate morbidity in exogenous IFN-α accelerated lupus in NZB/W mice (39). Moreover, pDCs are likely to exert critical roles in immunity to some infections (40). As BCL-2 antagonists are currently undergoing clinical trials for the treatment of certain hematopoietic cancers (20), our findings alert that monitoring reductions in pDCs and possible susceptibility to certain pathogens may be imperative.
Although ABT-737 and ABT-199 can kill T cells and B cells (15), we found that pDCs are more sensitive to BCL-2 antagonists than T cells and B cells both in vitro and in vivo. Furthermore, activated and memory B and T cells were shown to be resistant to these BH3 mimetics (15, 41, 42). Thus, BCL-2 antagonism may have selectivity to treat pDC-involved diseases.
In conclusion, we show that pDC survival is regulated by expression of BCL-2 and MCL-1, whereas cDCs require A1 and MCL-1. This differential dependence on BCL-2 advocates the use of BCL-2 selective antagonist BH3 mimetic drugs for treating pDC-associated diseases (34). Refined understanding of how the survival of the distinct DC subsets is regulated is therefore expected to provide valuable insight into the pathogenesis of inflammatory diseases and guide the development of effective therapeutic interventions for such diseases.
Materials and Methods
Mice.
Mice were housed under specific pathogen-free conditions. For detailed descriptions of mouse strains used in this study, see SI Materials and Methods. For in vivo use ABT-199 (Abbott Laboratories) or vehicle control was prepared and administered at 100 mg/kg body weight as a daily i.p injection for 4–6 d as described (43).
Cell Preparation, Antibodies, and Flow Cytometry.
Cell samples were analyzed as described in SI Materials and Methods.
Detection of Antiapoptotic Molecules.
Protein was detected by Western blot analysis and mRNA was detected by RT-PCR analysis. For detailed descriptions of mouse strains used in this study, see SI Materials and Methods.
Evaluation of DC Survival and DC Functions.
For detailed descriptions of mouse strains used in this study, see SI Materials and Methods.
Statistical Analysis.
Statistical comparisons were made using a two-tailed Student’s t test with Prism v.5.0 software (GraphPad). Data are shown as means ± SE with P values of <0.05 considered statistically significant.
Supplementary Material
Acknowledgments
We thank C. Yates, M. Dayton, and M. Hancock for technical assistance. This work was supported by National Health and Medical Research Council (NHMRC) Australia Program Grants 1037321 (to A.M.L.), 1016701 (to A.S.), and 1016647 (to J.-G.Z.); Project Grants 637324 and 1007703 (to Y.Z.), 1043414 (to A.M.L.), and 104610 (to A.S.); Fellowships 1020363 (to A.S.), 1024620 (to E.F.L.), and 1080321 (to A.M.L.); the Juvenile Diabetes Research Foundation; the Rebecca L. Cooper Foundation; the Leukemia and Lymphoma Society Special Center of Research Grant 7001-13 (to A.S.); NHMRC Independent Research Institutes Infrastructure Support Scheme Grant 361646; Victorian State Government Operational Infrastructure Support; and the Australian government.
Footnotes
Conflict of interest statement: All authors are employees of the Walter and Eliza Hall Institute of Medical Research. This institute receives milestone payments for the development of BH3 mimetic drugs for therapy from Genentech and AbbVie.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417620112/-/DCSupplemental.
References
- 1.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anandasabapathy N, et al. 2014. Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J Exp Med 211(9):1875–1891.
- 3.Chen M, et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311(5764):1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Huang L, Shabier Z, Wang J. Regulation of the lifespan in dendritic cell subsets. Mol Immunol. 2007;44(10):2558–2565. doi: 10.1016/j.molimm.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM. Decisions about dendritic cells: Past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 6.Lauterbach H, et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207(12):2703–2717. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YJ. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 8.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29(3):352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8(8):594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 10.Baccala R, et al. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc Natl Acad Sci USA. 2013;110(8):2940–2945. doi: 10.1073/pnas.1222798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamath AT, et al. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165(12):6762–6770. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- 12.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113(15):3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 14.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 15.Carrington EM, et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci USA. 2010;107(24):10967–10971. doi: 10.1073/pnas.1005256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kile BT. The role of apoptosis in megakaryocytes and platelets. Br J Haematol. 2014;165(2):217–226. doi: 10.1111/bjh.12757. [DOI] [PubMed] [Google Scholar]
- 17.Pierson W, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat Immunol. 2013;14(9):959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vikstrom I, et al. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330(6007):1095–1099. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 20.Souers AJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 21.Ogilvy S, et al. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96(26):14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vremec D, et al. Maintaining dendritic cell viability in culture. Mol Immunol. 2015;63(2):264–267. doi: 10.1016/j.molimm.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Ottina E, et al. Targeting antiapoptotic A1/Bfl-1 by in vivo RNAi reveals multiple roles in leukocyte development in mice. Blood. 2012;119(25):6032–6042. doi: 10.1182/blood-2011-12-399089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14(1):23–27. [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto T, et al. Enhanced stability of Mcl1, a prosurvival Bcl2 relative, blunts stress-induced apoptosis, causes male sterility, and promotes tumorigenesis. Proc Natl Acad Sci USA. 2014;111(1):261–266. doi: 10.1073/pnas.1321259110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peperzak V, et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol. 2013;14(3):290–297. doi: 10.1038/ni.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelson BT, et al. Batf3-dependent CD11b(low/-) peripheral dendritic cells are GM-CSF-independent and are not required for Th cell priming after subcutaneous immunization. PLoS ONE. 2011;6(10):e25660. doi: 10.1371/journal.pone.0025660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao Z, et al. The closely related CD103+ dendritic cells (DCs) and lymphoid-resident CD8+ DCs differ in their inflammatory functions. PLoS ONE. 2014;9(3):e91126. doi: 10.1371/journal.pone.0091126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Keeffe M, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J Exp Med. 2002;196(10):1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herold MJ, et al. The stability and anti-apoptotic function of A1 are controlled by its C terminus. J Biol Chem. 2006;281(19):13663–13671. doi: 10.1074/jbc.M600266200. [DOI] [PubMed] [Google Scholar]
- 31.Nopora A, Brocker T. Bcl-2 controls dendritic cell longevity in vivo. J Immunol. 2002;169(6):3006–3014. doi: 10.4049/jimmunol.169.6.3006. [DOI] [PubMed] [Google Scholar]
- 32.Hou WS, Van Parijs L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat Immunol. 2004;5(6):583–589. doi: 10.1038/ni1071. [DOI] [PubMed] [Google Scholar]
- 33.Lessene G, et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013;9(6):390–397. doi: 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- 34.Zhan Y, et al. 2014. BCL-2 antagonists kill plasmacytoid dendritic cells of lupus-prone mice and dampen IFN-α production. Arthritis Rheum (Munch) 2014(Nov):21 10.1002/art.38966.
- 35.Karrich JJ, et al. The transcription factor Spi-B regulates human plasmacytoid dendritic cell survival through direct induction of the antiapoptotic gene BCL2-A1. Blood. 2012;119(22):5191–5200. doi: 10.1182/blood-2011-07-370239. [DOI] [PubMed] [Google Scholar]
- 36.Chen M, Huang L, Wang J. Deficiency of Bim in dendritic cells contributes to overactivation of lymphocytes and autoimmunity. Blood. 2007;109(10):4360–4367. doi: 10.1182/blood-2006-11-056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuertes Marraco SA, et al. Type I interferon drives dendritic cell apoptosis via multiple BH3-only proteins following activation by PolyIC in vivo. PLoS ONE. 2011;6(6):e20189. doi: 10.1371/journal.pone.0020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rönnblom L, Alm GV. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J Exp Med. 2001;194(12):F59–F63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bardwell PD, et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J Immunol. 2009;182(12):7482–7489. doi: 10.4049/jimmunol.0802813. [DOI] [PubMed] [Google Scholar]
- 40.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33(6):955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cippà PE, et al. Resistance to ABT-737 in activated T lymphocytes: Molecular mechanisms and reversibility by inhibition of the calcineurin-NFAT pathway. Cell Death Dis. 2012;3:e299. doi: 10.1038/cddis.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koenen P, et al. Mutually exclusive regulation of T cell survival by IL-7R and antigen receptor-induced signals. Nat Commun. 2013;4:1735. doi: 10.1038/ncomms2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandenberg CJ, Cory S. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood. 2013;121(12):2285–2288. doi: 10.1182/blood-2013-01-475855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.