Significance
A brief exposure to a novel environment was recently shown to enhance the extinction of contextual fear probably through a protein synthesis-dependent process of synaptic tagging and capture in the hippocampus. Here we report that this finding can be generalized to the extinction of another fear-motivated task, one-trial inhibitory avoidance. This generalization is important because extinction is used in exposure therapy to treat posttraumatic stress disorder in humans, and this may derive from various forms of fear-related stress. In addition, here we show that the effect of novelty on fear extinction is dependent on dopamine D1 but not D5 receptors in the hippocampus. These findings could be applicable to the exposure therapy of fear memory disorders.
Keywords: dopamine, inhibitory avoidance, modulation of extinction, novelty, behavioral tagging and capture
Abstract
Extinction is the learned inhibition of retrieval. Recently it was shown that a brief exposure to a novel environment enhances the extinction of contextual fear in rats, an effect explainable by a synaptic tagging-and-capture process. Here we examine whether this also happens with the extinction of another fear-motivated task, inhibitory avoidance (IA), and whether it depends on dopamine acting on D1 or D5 receptors. Rats were trained first in IA and then in extinction of this task. The retention of extinction was measured 24 h later. A 5-min exposure to a novel environment 30 min before extinction training enhanced its retention. Right after exposure to the novelty, animals were given bilateral intrahippocampal infusions of vehicle (VEH), of the protein synthesis inhibitor anisomycin, of the D1/D5 dopaminergic antagonist SCH23390, of the PKA inhibitor Rp-cAMP or of the PKC inhibitor Gö6976, and of the PKA stimulator Sp-cAMP or of the PKC stimulator PMA. The novelty increased hippocampal dopamine levels and facilitated the extinction, which was inhibited by intrahippocampal protein synthesis inhibitor anisomysin, D1/D5 dopaminerdic antagonist SCH23390, or PKA inhibitor Rp-cAMP and unaffected by PKC inhibitor Gö6976; additionally, the hippocampal infusion of PKA stimulator Sp-cAMP reverts the effect of D1/D5 dopaminergic antagonist SCH 23390, but the infusion of PKC stimulator PMA does not. The results attest to the generality of the novelty effect on fear extinction, suggest that it relies on synaptic tagging and capture, and show that it depends on hippocampal dopamine D1 but not D5 receptors.
Frey and Morris (1, 2) described the enhancing influence of neuronal plastic processes [long-term potentiation (LTP) or long-term depression (LTD)] generated at one set of hippocampal synapses on LTP and LTD generated at other synapses. This influence is explainable by interactions between new proteins, called plasticity-related proteins (PRPs), at the two sets of synapses; the PRPs that tag one of them can be captured by those of others and enhance their responsiveness (3–5). Many memories rely on hippocampal LTP and LTD (1, 2, 6–11), and the “synaptic tagging-and-capture” process has been applied to the explanation of interactions between concurrent memories (11–13), among which are novelty and fear acquisition (12, 14) and novelty and fear extinction (15, 16). Exposure to novelty [an open field (OF) in which they had never been before] involves two consecutive processes: its detection, which is very brief (seconds), and the immediately ensuing habituation (17), which lasts much longer; both rely on hippocampal LTD (18). With a relatively restricted time window before and/or after an extinction trial, novelty can enhance the extinction of contextual fear conditioning (CFC) lastingly (15, 16). This is obviously of potential importance for exposure therapy (18–21).
In rodents, the exploration of a novel environment, object, or learning task increases the firing of dopaminergic neurons in the Ventral Tegmental Area (22–24), the release of dopamine in hippocampus (25), and the transcription of the Arc gene (26)—all purportedly critical events for memory consolidation. Dopamine acts on D1- or D2-family dopamine receptors and is a neuromodulator of fear and anxiety circuits (27). The D1 family is composed of D1 and D5 receptors. D1 uses adenylyl cyclase as a second messenger and modulates protein kinase A (PKA), and D5 uses the phosphatidylinositol-3-kinase system and modulates protein kinase C (PKC) (28, 29). Discrimination between the two types of D1-family receptors is possible by the simultaneous administration of drugs acting on PKA and PKC (29). Both D1-family receptor types are expressed in brain regions important for learning and memory, such as the hippocampus and amygdala (30, 31), and affect learning and memory (29, 32–34). The time-honored D1-family antagonist SCH23390 (SCH) does not discriminate between D1 and D5 receptors, and drugs acting on enzymes downstream of them have to be used to see if a given effect blocked by this drug is due to one or the other (29). It has been recently found to control the enhancing effect of novelty on spatial alimentary conditioning and its accompanying LTP, which was also attributed to a tagging-and-capture mechanism (35).
D1 and D5 dopamine receptors are expressed in the CA1 region of the hippocampus and play a role in hippocampal neuroplasticity and memory, but they can have different roles in different tasks and situations (28, 32, 33, 36). Also, both PKA and PKC participate in the modulation of hippocampal synaptic plasticity and memory consolidation (6).
To test the generality of the novelty effect [attributable to hippocampal LTD (15–18) during the time of exposure to the OF], here we investigate the influence of exposure to a novel environment on extinction of another form of fear learning, one-trial inhibitory avoidance (IA). For this, we studied the role of hippocampal protein synthesis on the extinction facilitation by novelty through bilateral infusion into the CA1 region of the hippocampus of the protein synthesis inhibitor anisomycin (ANI) (12, 13) after exposing the animals to the novelty. In addition, we examined the influence of hippocampal dopamine on this effect, in particular whether it is mediated by D1 or D5 receptors. Both the extinction of CFC, another widely used fear-motivated task (12, 13, 19, 37), and the acquisition of IA (14) have been shown to be enhanced by novelty, the latter in a D1-family receptor-dependent way.
Results
Exposure to Novelty Facilitates IA Extinction.
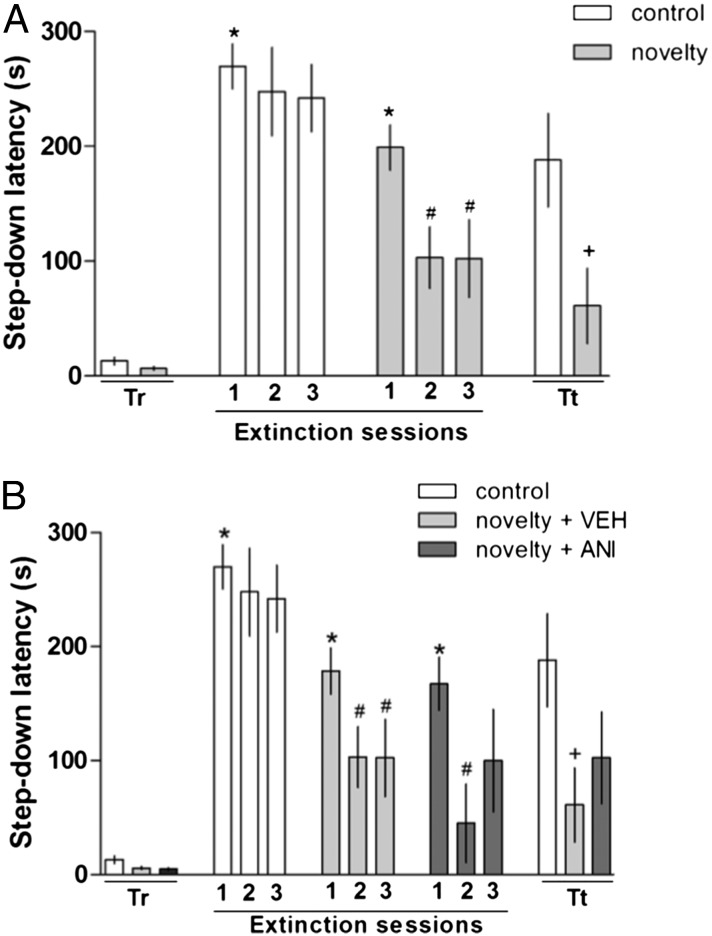
Rats were trained in the IA task (day 1) and 24 h later submitted to extinction training carried out in three sessions 90 min apart (day 2). On the following day, an extinction retention test was performed (day 3). Thirty minutes before the first extinction training session, a group of rats was exposed for 5 min to a novel environment (OF). As shown in Fig. 1, animals trained in the IA task did not present differences on training step-down latencies in a Mann–Whitney test (P = 0.1; Fig. 1A). When comparing the IA training step-down latencies and the step-down latencies observed in the first extinction training, there were significant differences in a Wilcoxon test between the control group and the group exposed to a novel environment (P < 0.03; Fig. 1A). Differences in step-down latencies between the first, second, and third extinction training were significant in the novelty-exposed group (P < 0.01; Fig. 1A). In the retention test, the step-down latency of the novelty group was significantly lower than that of the control group (P = 0.04; Fig. 1A). Thus, exposure to novelty enhanced extinction learning, which corroborates previous findings on the extinction of another fear-motivated task, CFC (15, 16).
Fig. 1.
Effect of exposure to novelty on extinction learning and the role of protein synthesis. Rats were trained in IA (Tr), where all animals showed a low step-down latency. In the subsequent day, the rats were exposed to three sessions of extinction sessions in the IA apparatus without reinforcement (shock)—at 24 h, 25.5 h, and 27 h. The animals in the novelty group were exposed to a novel apparatus 30 min before the first extinction training session, and this improved the extinction learning. On the third day, the animals were exposed to the extinction retention test (Tt). (A) In the retention test, the novelty group exhibited a facilitation of extinction memory. (B) The animals in the novelty groups were exposed to a novel apparatus 30 min before the first extinction training session and received intrahippocampal infusions of VEH or ANI (80 µg/µL–1 µL per side). Both groups exposed to the novelty improved on extinction learning. In the extinction retention test (Tt), the novelty group showed facilitation of extinction memory. Data are presented as medians ± interquartile range. *P < 0.05 (Wilcoxon test) for the IA Training × First Extinction session; #P < 0.05 (Wilcoxon test) for the first extinction session vs. other extinction session; +P < 0.05 (Mann–Whitney test) for the Tt control vs. novelty or (Kruskal–Wallis test post hoc) for the Tt control vs. novelty. n = 8–10 per group. ANI, anisomycin; Tr, training; Tt, extinction retention test; VEH, vehicle.
Protein Synthesis Is Required for Extinction Facilitation by Novelty.
To evaluate whether the facilitation of extinction by novelty depends on hippocampal protein synthesis, as is the case in CFC extinction (15, 16), rats were trained in the IA task and 24 h later exposed to three sessions of extinction training; an extinction retention test was carried out 1 d later, as above. The control group was not exposed to novelty, whereas the groups exposed to novelty 30 min before the first extinction training session received bilateral intrahippocampal infusion of vehicle (VEH) or of the protein synthesis inhibitor ANI (80 µg/µL–1 µL per side) immediately after the exposure to OF.
As shown in Fig. 1B, there were no differences in step-down latency between groups in the IA training session, whereas in the extinction training sessions, both novelty groups (VEH and ANI) showed better extinction learning than the control group (Kruskal–Wallis test, P = 0.05; Fig. 1B). In the retention session, the novelty + VEH group showed better extinction than the novelty + ANI group (P = 0.04; Fig. 1B). This result is in accordance with our previous finding on the enhancement by novelty of CFC extinction and its inhibition by the same dose of intrahippocampal ANI used here (15, 16). This argues in favor of the generality of the effect.
Exposure to Novelty Increases Hippocampal Dopamine Levels.
Rats were trained in the IA task and 24 h later were euthanized, and the bilateral hippocampus was quickly removed and prepared as previously described for high performance liquid chromatography (HPLC) determination of dopamine levels (38). The novelty group was exposed to a novelty 30 min before euthanasia. As shown in Fig. 2, animals exposed to novelty presented significantly higher levels of hippocampal dopamine than the control group (Student’s t test, P < 0.0095; Fig. 2).
Fig. 2.
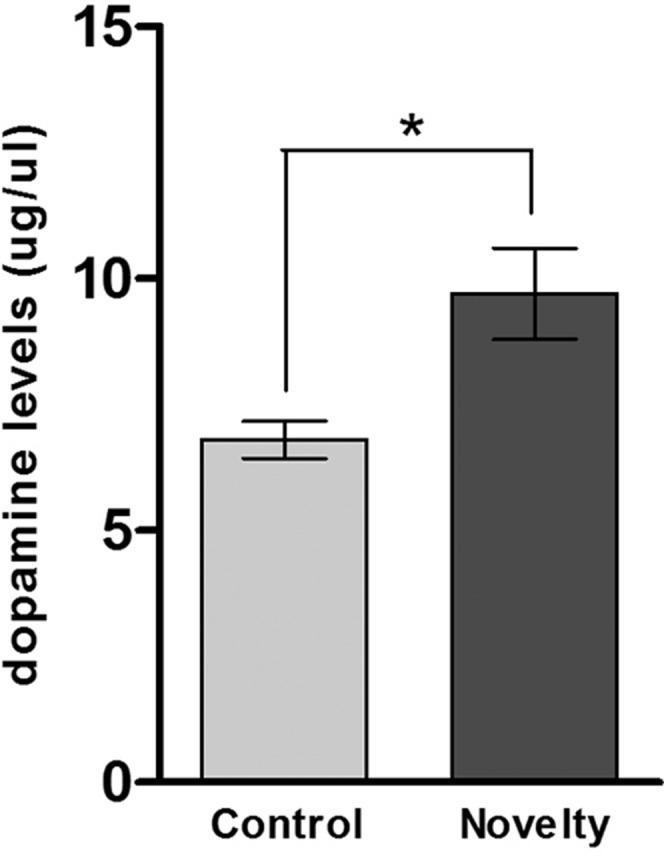
Effect of exposure to novelty on hippocampal dopamine levels. Rats were trained in the IA task and 24 h later were euthanized, and the bilateral hippocampus was removed and prepared as previously described for HPLC determination of dopamine levels. The novelty group was exposed to a novelty 30 min before euthanasia. The tissue content of dopamine in the hippocampus homogenate (µg/µL) was higher in the rats exposed to a novelty. *P < 0.05 (Student’s t test) for control vs. novelty. n = 4 per group analyzed in triplicate.
D1-Family Dopaminergic Receptors Are Required for the Facilitation of Extinction by Novelty.
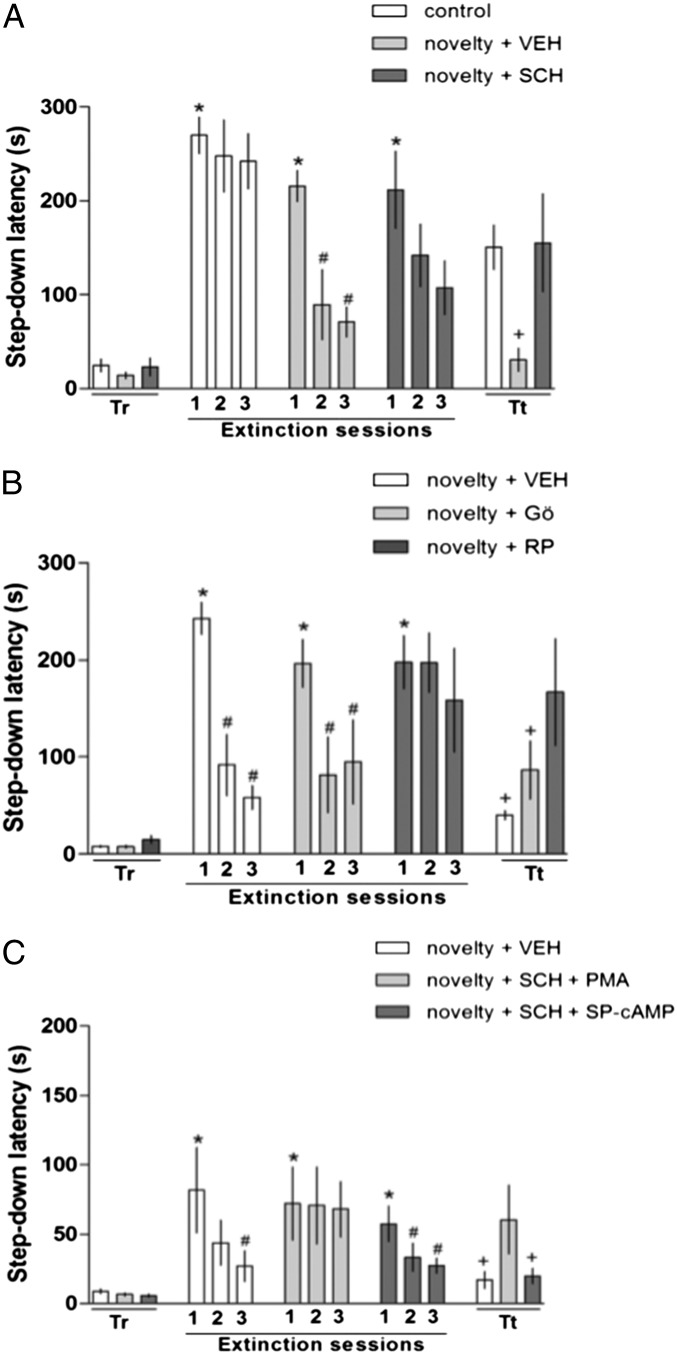
Rats were trained in the IA task and 24 h later submitted to the extinction training, as above. Thirty minutes before the first extinction training session, rats were exposed to novelty (OF) and a group of animals received a bilateral intrahippocampal infusion of the generic D1-family receptor antagonist SCH (1 µg/µL–1 µL per side). On the third day, the animals were submitted to the extinction retention test (Fig. 3A). In the IA training, there were no differences between IA latencies (Kruskal–Wallis test, P = 0.31; Fig. 3A), whereas in the extinction training sessions, the novelty group showed better extinction learning than the control did. In the extinction retention test, the novelty group presented a shorter test latency than the novelty + SCH group and the control group (Kruskal–Wallis test followed by Mann–Whitney tests, P = 0.03 for novelty vs. novelty + SCH, P = 0.03 for control vs. novelty, and P = 1.00 for control vs. novelty + SCH) (Fig. 3A). Thus, these results demonstrate that the facilitation of extinction by novelty requires dopamine receptors of the D1 family in the hippocampus (29).
Fig. 3.
Effect of exposure to novelty on extinction learning and the role of D1-family dopamine receptors. Rats were trained in the IA task (Tr), where all animals showed a low step-down latency. In the subsequent day, the rats were exposed to three sessions of extinction training in the IA apparatus without reinforcement (shock)—at 24 h, 25.5 h, and 27 h. The animals in the novelty groups were exposed to a novel apparatus 30 min before the first extinction training session. On the third day, the animals were submitted to the extinction retention test (Tt). (A) The animals received intrahippocampal infusions of VEH or SCH (1 µg/µL–1 µL per side) immediately after novelty exposure. The novelty group exhibited a facilitation of extinction memory, whereas the animals that received SCH were not able to extinguish the memory. (B) The animals received intrahippocampal infusions of VEH or Gö (PKC inhibitor; 1.7 ng/µL–1 µL per side) or Rp (PKA inhibitor; 0.5 µg/µL) immediately after novelty exposure. The novelty group exhibited a facilitation of extinction memory, whereas the animals that received Rp were not able to extinguish the memory. (C) The animals received intrahippocampal infusions of VEH or SCH (dopaminergic antagonist; 2 µg/µL–0.5 µL per side) plus PMA (PKC stimulator; 0.5 µg/µL–0.5 µL per side) or SCH (2 µg/µL–0.5 µL per side) plus Sp (PKA stimulator; 0.5 µg/µL–0.5 µL per side) immediately after novelty exposure. The novelty group exhibited a facilitation of extinction memory, as did the animals that received Sp after SCH, whereas the animals that received PMA were not able to extinguish the memory. Data are presented as medians ± interquartile range of step-down latency. *P < 0.05 (Wilcoxon test) for the IA Training × First Extinction session; #P < 0.05 (Wilcoxon test) for first extinction session vs. other extinction session; +P < 0.05 (Kruskal–Wallis test) for Tt control vs. novelty. n = 8–10 per group. SCH, SCH23390; Tr, training; Tt, extinction retention test; VEH, vehicle.
Involvement of Dopamine D1 but Not D5 Receptor Subtypes on Novelty Facilitation Effect on Memory Extinction.
To investigate whether the involvement of dopamine receptors in the effect of novelty on IA extinction occurs through D1 or D5 receptors of the D1 family, we studied the participation of PKA and PKC on this. PKA participates in D1 effects and PKC in D5-mediated effects (29). Rats were trained in IA and in IA extinction and examined for retention of the latter, as above.
In the first experiment, control animals received a bilateral intrahippocampal infusion of VEH right after the novelty, another group received the PKA inhibitor Rp-cAMP (Rp; 0.5 µg/µL–1 µL per side), and the third group received the PKC inhibitor Gö6976 (Gö; 1.7 ng/µL–1 µL per side). Rp blocked the effect of novelty on extinction learning, and Gö did not (Kruskal–Wallis test followed by Mann–Whitney test, P = 0.005 for VEH vs. Rp and P = 0.30 for VEH vs. Gö) (Fig. 3B). The doses of the two enzyme inhibitors used here had been found to have strong effects in previous papers on a number of behavioral parameters (see ref. 6 for references).
In the second experiment, to confirm the role of PKA and D1 receptors, we associated the SCH infusion and the infusion of PKA and PKC stimulators [Sp-cAMP (Sp) and PMA, respectively]. The stimulation of PKC by PMA (0.05 µg/µL–0.5 µL per side) did not revert the effect of SCH (2 µg/µL–0.5 µL per side) and the animals were not able to extinguish, but the stimulation of PKA by Sp (10 µg/µL–0.5 µL per side) reverted the SCH effect and the animals extinguished, as did the VEH group animals (Kruskal–Wallis test followed by Mann–Whitney test, P = 0.05 for VEH vs. SCH + PMA and P = 0.37 for VEH vs. SCH + Sp) (Fig. 3C). Together, these two experiments confirm that the novelty effect on fear extinction depends on hippocampal dopamine D1 but not D5 receptors.
Behavioral Control Experiments.
Rats were exposed to OF, plus maze (PM), and tail flick (TF) tests after the ANI, SCH, Rp, Gö, Sp, or PMA infusions to verify whether exploratory and locomotor activity, anxiety, and pain thresholds, respectively, were affected by the drug infusions. As shown in Table 1, neither the drugs nor the exposure to novelty affected the evaluated parameters.
Table 1.
Effect of the intrahippocampal infusion of ANI, SCH, Gö, Rp, SCH + PMA, or SCH + Sp and the novelty exposure on locomotor and exploratory activities and anxiety and pain thresholds
| Groups | |||||||||
| Behavioral task | Control | Novelty + VEH | Novelty + SCH | Novelty + ANI | Novelty + Rp | Novelty + Gö | Novelty + SCH + PMA | Novelty + SCH + Sp | |
| OF | Crossings, n | 29.1 ± 12.5 | 22.9 ± 10.2 | 20.8 ± 6.2 | 21.9 ± 8.5 | 15.2 ± 7.8 | 34.2 ± 17.8 | 23.4 ± 8.0 | 27.0 ± 10.1 |
| Rearings, n | 20.3 ± 7.9 | 14.6 ± 8.8 | 18.2 ± 6.5 | 14.0 ± 5.2 | 11.4 ± 7.4 | 22.1 ± 9.3 | 13.1 ± 5.4 | 16.6 ± 8.1 | |
| PM | Total entries in open arms, n | 52.0 ± 13.2 | 43.0 ± 9.3 | 42.0 ± 7.0 | 38.7 ± 18.3 | 51.0 ± 29.2 | 50.7 ± 19.8 | 37.6 ± 10.0 | 38.4 ± 13.0 |
| Time in open arms, s | 1.1 ± 0.4 | 1.6 ± 0.9 | 1.2 ± 0.4 | 2.0 ± 0.9 | 2.4 ± 0.9 | 1.5 ± 0.7 | 1.9 ± 1.0 | 1.8 ± 0.8 | |
| TF | Latency, s | 3.3 ± 0.1 | 2.7 ± 0.5 | 2.9 ± 0.7 | 4.0 ± 1.6 | 3.1 ± 0.5 | 3.7 ± 1.0 | 2.9 ± 0.7 | 3.2 ± 1.4 |
Neither the drugs nor the novelty exposure affected the animals’ performance on the OF, PM, and TF test. Data are expressed as mean ± SD of the number of crossings and rearings (OF), the time spent and the number of entries in the open arms (PM), and time latency (TF). There were no differences between the groups (ANOVA; n = 6–10 per group for all tests).
Discussion
Exposure to novelty before extinction training facilitates the extinction of the IA task with a specific time course. The effect depends on ribosomal protein synthesis in the hippocampus. Thus, this extends our previous findings on the memory of CFC (15, 16) to another type of extinction: The facilitation of IA extinction by novelty is also explainable by synaptic tagging and capture. The time course of the effect here is a bit different from that reported for CFC extinction, which may be attributed to differences between the two extinction tasks.
The synaptic tagging-and-capture theory predicts that a weak stimulus can activate synapses and set a tag that provides the capture of the synthesized proteins by a strong stimulus (for more details, see refs. 1, 2). Application of this theory to behavioral interactions, as predicted by the authors of the hypothesis (2, 10, 11, 24, 35), led to the finding of facilitation by a brief exposure to a previously unvisited environment (OF, hereafter called “novelty”) on the learning of a variety of tasks, including IA (10, 12, 13, 15, 35), and very particularly, including the extinction of CFC. The behavioral tagging predicts the induction of synaptic tagging by behavioral manipulations (10, 12, 13, 15). This hypothesis has been used to explain the effects of novelty exposure on memory facilitation by several authors (13, 15, 35).
Different times of novelty exposure, pre- and postmemory formation, were previously tested, and these data show that a temporal window exists to allow the novelty exposure effects in the memory (13, 15). In this work, we chose to expose the rats to the novelty 30 min before retrieval, considering the results obtained by Wang et al. (35) that demonstrated that the novelty exposure in this time facilitates the persistence of a weak spatial memory. The authors also determined that the novelty effect depends on D1-family dopamine receptors, as the intrahippocampal SCH injection prevented the novelty effects.
Kandel and coworkers (18) comment that both the detection and the habituation to the novelty rely on hippocampal LTD, which here would be the form of hippocampal plasticity whose PRPs would interact with those of IA extinction (which involves depotentiation of preexisting LTP) (7). According to Ballarini et al. (13) and de Carvalho Myskiw et al. (15), the enhancing effects of exposure to a novel environment on IA learning and on CFC extinction, respectively, depend on the novel nature of the stimulus, as the exposure to a familiar environment did not produce effects.
Exposure to novelty immediately posttraining or postinduction can hinder the retention of IA (39) and the development of LTP (39), respectively. These effects have been attributed to the presumably arousing detection of novelty alone (39), not to the ensuing habituation to it. The effect of novelty exposure on IA retrieval was also studied (40–42). Those other effects of exposure to a novel environment are unrelated to the one described here or in previous papers on IA acquisition (13), spatial learning (35), or CFC extinction (15, 16), which are explainable quite neatly by a synaptic tagging-and-capture process, dependent on ribosomal and nonribosomal protein synthesis.
The present findings strongly suggest that the effect of novelty on extinction depends specifically on dopaminergic D1-family receptors, which agrees generically with the findings of Frey, Viola, and coworkers (10) and Wang et al. (39), who showed that the enhancement of IA learning and spatial alimentary learning and LTP, respectively, can be blocked by intrahippocampal SCH. So the present findings attest to the generality of the dependence of hippocampal synaptic tagging-and-capture effects resulting in memory changes (“behavioral tagging”) (10, 12, 13) on dopaminergic modulation. Lisman and Grace (23) suggested in 2005 that the dopaminergic fibers acting on D1 receptors in the hippocampus come from the Ventral Tegmental Area.
The results may also suggest a possible application of the effect of novelty on extinction to exposure therapy (15, 16). The modulation of memory by novelty is not a novel concept. One of us (42) found 11 y ago that exposure to a novel environment can enhance retrieval of IA acquired months before. Novelty enhances the phosphorylation state of CREB in the hippocampus (43) and activates hippocampal MAPKs (44). Salvetti et al. (45) showed that novelty presented after weak memory training can promote the memory consolidation of a spatial task.
The main previously unidentified findings of this study are the generality of the enhancing effect of novelty on fear extinction and the involvement of dopaminergic D1 rather than D5 receptors of the D1 family in that effect.
Materials and Methods
Animals.
We purchased 148 male Wistar rats (3 mo old, 300–350 g) from the Federal University of Santa Maria Central Vivarium. They were housed four per cage and maintained under controlled light and environmental conditions (12 h light/12 h dark cycle at a temperature of 23 ± 2 °C and 50 ± 10% humidity), with food and water ad libitum. All experiments were conducted in accordance with the Principles of Laboratory Animal Care of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the Federal University of Pampa (institutional review board 0252012).
To study the influence of novelty on extinction and the role of hippocampal protein synthesis and D1-family dopaminergic receptors in this process, we performed six experiments using 148 rats, 120 of which were implanted with chronic bilateral guide cannulae in the CA1 region of the hippocampus.
IA Training, Extinction Training, and Extinction Retention Test.
The IA memory was evaluated in a 50 cm × 25 cm × 25 cm Plexiglas box, with a 5-cm high, 7-cm wide, and 25-cm long formica platform at the left end of a series of 0.3-cm caliber bronze bars spaced 1.0 cm apart that made up the floor of the box. For the IA training (day 1), the animals were placed on the platform facing the rear left corner of the training box. When they stepped down, placing their four paws on the grid, they received a 2-s, 0.5-mA scrambled footshock and then withdrew immediately from the apparatus and returned to their cage. Twenty-four hours later (day 2), the animals were placed again on the platform as described, and when they stepped down, they were allowed to explore the box freely for 300 s without a footshock. This extinction training was repeated three times, at 90-min intervals, 24–27 h after the IA training. Twenty-four hours later (day 3), the animals were submitted to an extinction retention test. Step-down latencies were measured in the IA training session, extinction training session, and extinction retention test with a stopwatch.
Influence of Exposure to Novelty on Fear Extinction.
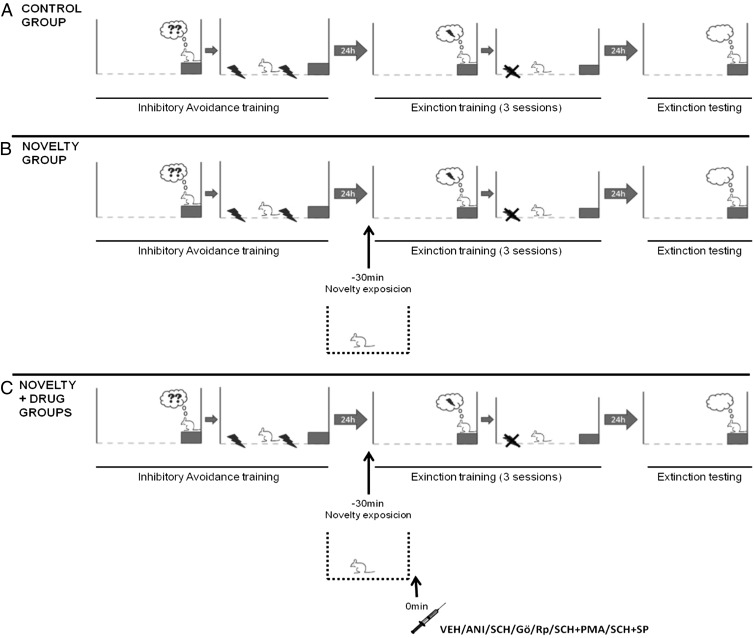
Twenty animals were divided into two groups: (i) For the control group, rats submitted to IA followed at 24 h by extinction training (n = 10) (Fig. 4A), and (ii) for the novelty-exposed group, rats submitted to IA training and were exposed 30 min before extinction training to a novel environment for 5 min (a 50 × 50 × 60 cm OF with a frontal glass wall) (15, 16) (Fig. 4B). The interval between the novelty and extinction was established in pilot experiments and was found to be shorter than that described as optimum by de Carvalho Myskiw et al. (15–17) in another task, the CFC. In an alimentary spatial task, Morris and coworkers found an enhancement by exposure to novelty 30 min before the task (35). IA extinction retention tests were conducted 24 h after extinction training.
Fig. 4.
Schematic drawing of the behavioral experiments. (A) Animals were submitted to IA training (0.5-mA, 2-s footshock). They were submitted 24 h later to three extinction training sessions with an interval of 90 min between sessions. Twenty-four hours after this, they were submitted to an extinction retention test to measure the retention of extinction. (B) Exposure to a novel environment 30 min before the first extinction training session was investigated regarding its influence in the acquisition of extinction studied on day 2 and in the retention of extinction studied on day 3. (C) The drugs ANI, SCH, Gö, Rp, PMA, and Sp (see Material and Methods) were injected into the CA1 region of the hippocampus immediately after exposure to novelty for additional investigations. Control animals received an equal volume of VEH (2% DMSO in saline).
Need of Hippocampal Protein Synthesis for the Influence of Novelty on IA Extinction.
Thirty animals were divided into three groups: (i) For the control group, rats were trained in IA and 24 h later submitted to extinction training (n = 10) (Fig. 4A); (ii) for the novelty-exposed group, rats were trained in IA and before extinction training were exposed to the novelty (OF) (Fig. 4B); and (iii) for the novelty + ANI group, rats were trained in IA and before extinction training were exposed to the novelty (OF), followed by intrahippocampal infusion of the protein synthesis inhibitor ANI (n = 10) (Fig. 4C). The retention test was conducted 24 h after extinction training. Afterward, animals from all groups were euthanized with excess anesthesia for posterior verification of cannula placements.
Influence of Novelty on Hippocampal Dopamine Levels.
Eight animals were divided in two groups: (i) For the control group, rats were trained in IA and 24 h later were euthanized for hippocampus dissection (n = 4), and (ii) for the novelty-exposed group, rats were trained in IA, 23.5 h later were exposed to the novelty, and 30 min later were euthanized for hippocampus dissection (n = 4). Both hippocampi were homogenized and prepared for determination of dopamine levels by HPLC (38).
Need of Hippocampal D1-Family Receptors for the Effect of Novelty on Fear Extinction.
Three groups of animals were studied: (i) For the control group, rats were trained in IA, followed by extinction training 24 h later (Fig. 4A); (ii) for the novelty-exposed group, rats were trained in IA and 30 min before extinction training were exposed to the novelty (n = 10) (Fig. 4B); and (iii) for the novelty + SCH group, rats were trained in IA and 30 min before extinction training were exposed to the novelty, followed by intrahippocampal infusion of SCH (D1 receptor antagonist) (n = 10) (Fig. 4C). The extinction retention test was conducted 24 h after extinction training. Afterward, animals from all groups were euthanized for posterior verification of cannula placements.
Participation of Hippocampal D1- and D5-Receptor Subtype in the Effect of Novelty on Fear Extinction.
In the fifth experiment, 30 animals were divided into three groups: (i) for the novelty-exposed group, rats were trained in IA and 30 min before extinction training were exposed to a novelty (OF) (n = 10) (Fig. 4B); (ii) for the novelty + Gö group, rats were trained in IA and 30 min before extinction training were exposed to a novelty, followed by intrahippocampal infusion of Gö (PKC inhibitor) (Fig. 4C); and (iii) for the novelty + Rp group, rats were trained in IA and 30 min before extinction training were exposed to a novelty, followed by intrahippocampal infusion of Rp (PKA inhibitor) (n = 10) (Fig. 4C). Retention tests were conducted 24 h after extinction training. Afterward, animals from all groups were euthanized for posterior verification of cannula placements.
In the sixth experiment, 30 animals were divided into three groups: (i) for the novelty-exposed group, rats were trained in IA and 30 min before extinction training were exposed to a novelty (OF) (n = 10) (Fig. 4B); (ii) for the novelty + SCH + PMA group, rats were trained in IA and 30 min before extinction training were exposed to a novelty, followed by intrahippocampal infusion of SCH (D1-family antagonist) and PMA (PKC stimulator) (n = 10) (Fig. 4C); and (iii) for the novelty + SCH + Sp group, rats were trained in IA and 30 min before extinction training were exposed to a novelty, followed by intrahippocampal infusion of SCH (D1-family antagonist) and Sp (PKA stimulator) (n = 10) (Fig. 4C). Retention tests were conducted 24 h after extinction training. Afterward, animals from all groups were euthanized for posterior verification of cannula placements.
Surgery.
Indwelling cannulae were implanted in the animals to be used in experiments 2, 4, 5, and 6 under deep anesthesia with ketamine and xylazine (i.p., 75 mg/kg and 10 mg/kg, respectively). The cannulae were 27-gauge stainless steel tubes stereotaxically aimed at the CA1 region of the dorsal hippocampus (A, –4.2; L, ±3.0; and V, –2.0 mm) [coordinates according to Paxinos and Watson (46)]. The cannulae were affixed with dental cement. Animals were allowed to recover from surgery for 4 d before submitting them to any other procedure. Surgery was performed at least 1 wk after the arrival of the animals to the laboratory.
Exposure to Novelty.
Exposure to novelty was carried out 30 min before the first extinction session (15, 16). It consisted of placing the rat in a novel environment, a 50 × 50 × 60 cm wooden OF box painted white, with a frontal glass wall. The rat was removed from the home cage and taken to the field, where it was left to explore freely for 5 min, after which it was returned to its home cage. Thirty minutes later, the rat was submitted to the first extinction training session.
Drugs and Drug Treatments.
All of the drugs used were purchased from Sigma-Aldrich Brazil and were dissolved in 2% (vol/vol) DMSO in saline (VEH) to a total infusion volume of 1 µL per side. Infusions were carried out into the dorsal CA1 region of the hippocampus on both sides. At the time of drug delivery, 30-gauge infusion cannulas were tightly fitted into the guides. Infusions of drug or VEH (0.5 or 1 µL per side in the CA1 region of the dorsal hippocampus) were carried out over 60 s with an infusion pump, and the cannulas were left in place for an 60 additional seconds to minimize backflow.
The drugs and doses used were as follows: ANI, 80 µg/µL; SCH, 1 µg/µL or 2 µg/µL; Gö, 1.7 ng/µL; Rp, 0.5 µL/µL; PMA, 0.05 µg/µL; and Sp, 10 µg/µL. These are the doses found to be effective previously (6, 29).
Behavioral Control Experiments.
To analyze exploratory and locomotor activities and ensure that the drug infusion did not impair such behaviors, altering the results of the memory tests, after drug infusion rats were placed in the left quadrant of a 50 × 50 × 39 cm OF made with wood that was painted white, with a frontal glass wall. Black lines were drawn on the floor to divide it into 12 equal quadrants. Crossing and rearing, as measures for locomotor and exploratory activities, respectively, were measured over 5 min (47). To evaluate the animals’ anxiety state, 24 h after drug infusion rats were exposed to an elevated PM as described by Pellow et al. (48). The number of entries and the time spent into the open arms were recorded over a 5-min session. To ensure the IA test efficacy, nociception was measured by using the TF test (49). For the assay, pain was induced by placing infrared light on the tail of the rat 5 cm away from the tip of the tail. The reaction time (TF latency) was noted by observing the interval between placing the tail on the infrared light source and the withdrawal of the tail.
Homogenate Preparation for HPLC Determination of Dopamine.
Rats in the control (n = 4) and novelty (n = 4) groups trained in IA were euthanized by decapitation. The brain was removed, and the bilateral hippocampus was quickly dissected out in an iced surface and homogenized in 50 mM Tris⋅HCl, pH 7.4 (1/10, wt/vol). Afterward, samples were centrifuged at 2,400 × g for 20 min, and supernatants were filtered and then stored at –80 °C until use (38).
HLPC Determination of Hippocampal Dopamine Levels.
Levels of dopamine in homogenates prepared from the hippocampus were determined using a reverse-phase HPLC system (YL9100, Young Lin) with a Diode Array Detector. The HPLC system consisted of a Vacuum Degasser (YL9101) and quaternary pump (YL9110) connected to a reversed phase column (KINETEX 2.6u HILIC 100 × 100 × 4.60 mm; Phenomenex) on a Column Compartment (YL9131) coupled to a Diode Array Detector (YL9160). The mobile phase consisted of acetonitrile and water at pH 3 (phosphoric acid 1:1, vol/vol). To separate dopamine, we used the following gradient program: 90% at 0 min to 10% at 10 min, with a flow rate of 0.7 mL/min. The sample was filtered through 0.22-µm syringe filters. We injected 20-µL samples into the HPLC system by an auto sampler device (YL9150). Chromatograms were recorded and integrated by PC integration software (YL-Clarity). All analyses were run in triplicate.
The analytical parameters were as follows: linear range, 0.5–30 μg/mL; determination coefficient, 0.9955; and calibration equation, y = 414.46x – 126.22. Dopamine HCl was supplied by Sigma-Aldrich Brazil. Other reagents used in this experiment were of analytical grades and obtained from standard commercial suppliers.
Statistics.
For IA results, a ceiling of 300 s was imposed on step-down latencies during the retention test (latencies equal to or higher than 300 s were counted as 300 s). So this variable did not follow a normal distribution, and these data were analyzed by a Kruskal–Wallis nonparametric ANOVA. Comparisons between groups were determined by Mann–Whitney U tests (two-tailed). To compare step-down latency differences between the training and test in each group, a Wilcoxon test was used. IA data were expressed as medians ± interquartile range. In the OF, PM, and TF tests, the data were analyzed using parametric ANOVA and were expressed as means ± SD. In HPLC results, the data of the two groups were compared using Student’s t test and were expressed as mean ± SD. The sample size (n, number of animals in each group) for each experiment is stated in the figure legends. The differences were considered statistically significant at P < 0.05.
Acknowledgments
This work was supported by research grants and a fellowship from Fundação de Amparo à Pesquisa do Rio Grande do Sul, the Federal University of Pampa, and the National Council of Research of Brazil.
Footnotes
The authors declare no conflict of interest.
References
- 1.Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385(6616):533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 2.Frey U, Morris RG. Weak before strong: Dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology. 1998;37(4-5):545–552. doi: 10.1016/s0028-3908(98)00040-9. [DOI] [PubMed] [Google Scholar]
- 3.Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44(1):59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82(1):12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Sajikumar S, Morris RG, Korte M. Competition between recently potentiated synaptic inputs reveals a winner-take-all phase of synaptic tagging and capture. Proc Natl Acad Sci USA. 2014;111(33):12217–12221. doi: 10.1073/pnas.1403643111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29(9):496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 8.Gruart A, Muñoz MD, Delgado-García JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26(4):1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-García JM. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci USA. 2010;107(6):2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moncada D, Ballarini F, Martinez MC, Frey JU, Viola H. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci USA. 2011;108(31):12931–12936. doi: 10.1073/pnas.1104495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almaguer-Melian W, et al. Novelty exposure overcomes foot shock-induced spatial-memory impairment by processes of synaptic-tagging in rats. Proc Natl Acad Sci USA. 2012;109(3):953–958. doi: 10.1073/pnas.1114198109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: Evidence for behavioral tagging. J Neurosci. 2007;27(28):7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. Behavioral tagging is a general mechanism of long-term memory formation. Proc Natl Acad Sci USA. 2009;106(34):14599–14604. doi: 10.1073/pnas.0907078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moncada D, Viola H. Phosphorylation state of CREB in the rat hippocampus: A molecular switch between spatial novelty and spatial familiarity? Neurobiol Learn Mem. 2006;86(1):9–18. doi: 10.1016/j.nlm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.de Carvalho Myskiw J, Benetti F, Izquierdo I. Behavioral tagging of extinction learning. Proc Natl Acad Sci USA. 2013;110(3):1071–1076. doi: 10.1073/pnas.1220875110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Carvalho Myskiw J, Furini CR, Benetti F, Izquierdo I. Hippocampal molecular mechanisms involved in the enhancement of fear extinction caused by exposure to novelty. Proc Natl Acad Sci USA. 2014;111(12):4572–4577. doi: 10.1073/pnas.1400423111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Carvalho Myskiw J, Furini CRG, Schmidt B, Ferreira F, Izquierdo I. Extinction learning, which consists of the inhibition of retrieval, can be learned without retrieval. Proc Natl Acad Sci USA. 2015;112(2):E230–E233. doi: 10.1073/pnas.1423465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etkin A, et al. A role in learning for SRF: Deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron. 2006;50(1):127–143. doi: 10.1016/j.neuron.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Furini C, Myskiw J, Izquierdo I. The learning of fear extinction. Neurosci Biobehav Rev. 2014;47:670–683. doi: 10.1016/j.neubiorev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sher L, Vilens A. Neurobiology of Post-Traumatic Stress Disorder. Nova Science Publishers; Hauppauge, NY: 2010. [Google Scholar]
- 22.Frey U, Morris RGM. Synaptic tagging: Implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21(5):181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- 23.Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Navakkode S, Sajikumar S, Frey JU. Synergistic requirements for the induction of dopaminergic D1/D5-receptor-mediated LTP in hippocampal slices of rat CA1 in vitro. Neuropharmacology. 2007;52(7):1547–1554. doi: 10.1016/j.neuropharm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Ihalainen JA, Riekkinen P, Jr, Feenstra MGP. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neurosci Lett. 1999;277(2):71–74. doi: 10.1016/s0304-3940(99)00840-x. [DOI] [PubMed] [Google Scholar]
- 26.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2(12):1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 27.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 28.Felder CC, Jose PA, Axelrod J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther. 1989;248(1):171–175. [PubMed] [Google Scholar]
- 29.Furini CRG, Myskiw JC, Schmidt BE, Marcondes LA, Izquierdo I. D1 and D5 dopamine receptors participate on the consolidation of two different memories. Behav Brain Res. 2014;271:212–217. doi: 10.1016/j.bbr.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Nagai T, et al. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem. 2007;14(3):117–125. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA. 2009;106(12):4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: A convergence of theory with fear and reward circuitry. Neurobiol Learn Mem. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bethus I, Tse D, Morris RG. Dopamine and memory: Modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J Neurosci. 2010;30(5):1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1995;92(7):2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang SH, Redondo RL, Morris RG. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc Natl Acad Sci USA. 2010;107(45):19537–19542. doi: 10.1073/pnas.1008638107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26(29):7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232(1):210–216. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Wilson CB, Ebenezer PJ, McLaughlin LD, Francis J. Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS ONE. 2014;9(2):e89104. doi: 10.1371/journal.pone.0089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izquierdo I, Schröder N, Netto CA, Medina JH. Novelty causes time-dependent retrograde amnesia for one-trial avoidance in rats through NMDA receptor- and CaMKII-dependent mechanisms in the hippocampus. Eur J Neurosci. 1999;11(9):3323–3328. doi: 10.1046/j.1460-9568.1999.00742.x. [DOI] [PubMed] [Google Scholar]
- 40.Izquierdo I, McGaugh JL. Effect of a novel experience prior to training or testing on retention of an inhibitory avoidance response in mice: Involvement of an opioid system. Behav Neural Biol. 1985;44(2):228–238. doi: 10.1016/s0163-1047(85)90240-7. [DOI] [PubMed] [Google Scholar]
- 41.Izquierdo LA, Barros DM, Medina JH, Izquierdo I. Novelty enhances retrieval of one-trial avoidance learning in rats 1 or 31 days after training unless the hippocampus is inactivated by different receptor antagonists and enzyme inhibitors. Behav Brain Res. 2000;117(1-2):215–220. doi: 10.1016/s0166-4328(00)00286-2. [DOI] [PubMed] [Google Scholar]
- 42.Izquierdo LA, Barros DM, Medina JH, Izquierdo I. Exposure to novelty enhances retrieval of very remote memory in rats. Neurobiol Learn Mem. 2003;79(1):51–56. doi: 10.1016/s1074-7427(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 43.Viola H, et al. Phosphorylated cAMP response element-binding protein as a molecular marker of memory processing in rat hippocampus: Effect of novelty. J Neurosci. 2000;20(23):RC112. doi: 10.1523/JNEUROSCI.20-23-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izquierdo LA, et al. Novelty enhances retrieval: Molecular mechanisms involved in rat hippocampus. Eur J Neurosci. 2001;13(7):1464–1467. doi: 10.1046/j.0953-816x.2001.01530.x. [DOI] [PubMed] [Google Scholar]
- 45.Salvetti B, Morris RGM, Wang SH. The role of rewarding and novel events in facilitating memory persistence in a separate spatial memory task. Learn Mem. 2014;21(2):61–72. doi: 10.1101/lm.032177.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (Academic, Sydney) 2nd Ed. 1986. [DOI] [PubMed] [Google Scholar]
- 47.Bonini JS, et al. Angiotensin II disrupts inhibitory avoidance memory retrieval. Horm Behav. 2006;50(2):308–313. doi: 10.1016/j.yhbeh.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 49.Tjølsen A, Lund A, Berge OG, Hole K. An improved method for tail-flick testing with adjustment for tail-skin temperature. J Neurosci Methods. 1989;26(3):259–265. doi: 10.1016/0165-0270(89)90124-6. [DOI] [PubMed] [Google Scholar]