Significance
Bovine tuberculosis is a chronic infectious disease that affects a broad range of mammalian hosts. It is a serious threat to agriculture in many less-developed countries. In this study, we introduced a mutation to the FokI of the right hand of wild-type transcription activator-like effector nuclease and established a transcription activator-like effector nickase system that creates single-strand breaks in the genome. Then we used this system to add the mouse gene SP110 to a specific location in the bovine genome and created transgenic cattle with increased resistance to tuberculosis. Our results contribute to the control and prevention of bovine tuberculosis and provide a previously unidentified insight into breeding animals for disease resistance.
Keywords: TALEN, homologous recombination, single-strand break, tuberculosis, disease resistance
Abstract
Transcription activator-like effector nuclease (TALEN)-mediated genome modification has been applied successfully to create transgenic animals in various species, such as mouse, pig, and even monkey. However, transgenic cattle with gene knockin have yet to be created using TALENs. Here, we report site-specific knockin of the transcription activator-like effector (TALE) nickase-mediated SP110 nuclear body protein gene (SP110) via homologous recombination to produce tuberculosis-resistant cattle. In vitro and in vivo challenge and transmission experiments proved that the transgenic cattle are able to control the growth and multiplication of Mycobacterium bovis, turn on the apoptotic pathway of cell death instead of necrosis after infection, and efficiently resist the low dose of M. bovis transmitted from tuberculous cattle in nature. In this study, we developed TALE nickases to modify the genome of Holstein–Friesian cattle, thereby engineering a heritable genome modification that facilitates resistance to tuberculosis.
Gene targeting by homologous recombination can modify the genome precisely and has been widely used to study gene function and produce transgenic animals (1–5). Transcription activator-like effector nuclease (TALEN) is a programmable nuclease that contains a FokI nuclease domain and a DNA-binding domain known as “transcription activator-like effector” derived from the plant pathogenic bacteria Xanthomonas spp. TALEN induces a double-strand break (DSB) at a precise, defined position in the genome, resulting in unpredictable gene mutations when the DSBs are repaired erroneously by nonhomologous end joining (NHEJ) (6, 7). However, TALENs also can be used in conjunction with specially designed exogenous donor DNA to generate large-scale deletions, gene disruptions, DNA additions, or single-nucleotide changes (8, 9). Numerous cases of TALEN-mediated gene knockouts have been reported in the last 2 y (9–12), but successful knockins are rare (7, 13). TALEN-mediated site-specific transgenesis has been applied successfully to model animals (7, 14–16) and even in large livestock, such as pigs and cattle (17–21). However, to the best of our knowledge, transgenic cattle with gene knockin have yet to be created using TALENs (19).
Tuberculosis is a zoonotic disease caused by the transmission of Mycobacterium bovis from animals to human beings and from human to human (22). It is a serious threat to global public health and agriculture (23, 24). Bovine tuberculosis is widely distributed worldwide, and no effective programs currently exist to eliminate or control the disease in many less-developed areas of Africa and Asia (24, 25). Therefore more extensive and effective studies on the control of bovine tuberculosis are urgently required in these regions. The mouse SP110 gene is emerging as a promising candidate in the control of Mycobacterium tuberculosis (MTB) infections (26). SP110 can control MTB growth in macrophages and induce apoptosis in infected cells. In this study, we developed transcription activator-like effector (TALE) nickase technology to insert a mouse SP110 gene into the genome of Holstein–Friesian cattle. Therefore, TALEN represents a validated tool for the targeted genetic modification of this important livestock species. Moreover, the results of the present study could contribute to the control of tuberculosis.
Results
Construction of TALEN Plasmids and Activity Assessment.
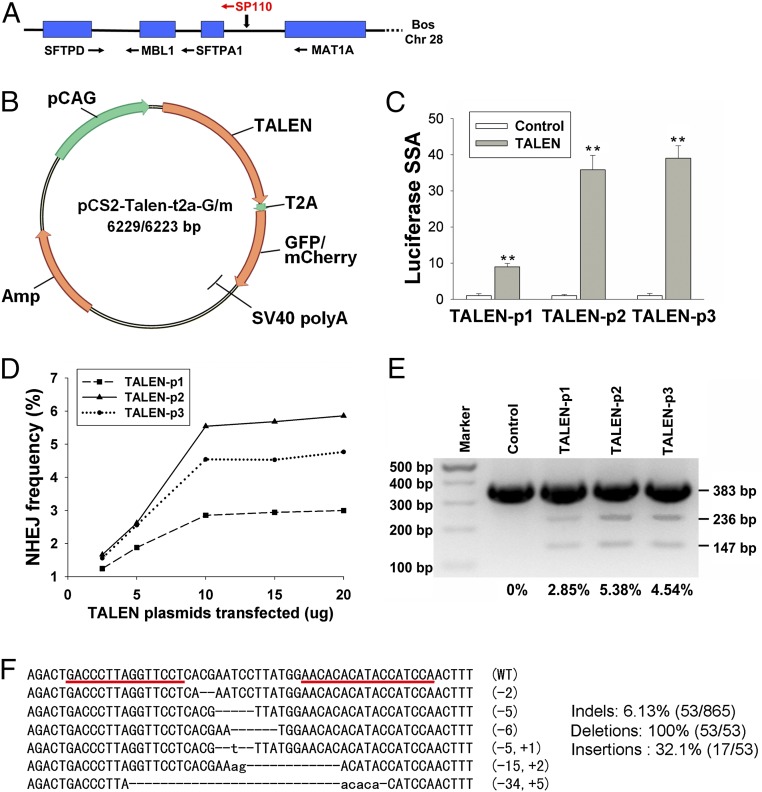
In consideration of potential synergistic effects of neighboring genes, we designed three active TALENs specific to the intergenic region between surfactant protein A1 (SFTPA1) and methionine adenosyltransferase I alpha (MAT1A) on chromosome 28 (Fig. 1A and SI Appendix, Table S1). Because the transfection efficiency of TALEN plasmid and mRNA into bovine fetal fibroblasts (BFFs) is extremely low, EGFP or mCherry was added to the TALEN vectors with a self-cleaving T2A peptide to sort transfected cells via flow cytometry (Fig. 1B).
Fig. 1.
Activity assessment of TALENs. (A) Schematic representation of the targeting locus. (B) Schematic representation of the TALEN constructs. The lengths of constructs were calculated based on TALEN-encoding plasmids with 17 modules. (C) Cleavage activity of each TALEN was measured by luciferase SSA assay in human 293-FT cells. Data are presented as mean ± SD and are derived from three independent experiments. **P < 0.01. (D) Frequency of allelic mutation as determined by Surveyor nuclease assays. Different amounts of each TALEN transfected are shown as indicated. (E) The M-S locus was PCR amplified, and cleavage of the locus was measured using a Surveyor nuclease assay. The degree of cleavage was quantified and is shown below each lane. (F) Some of the representative sequences revealed distinct TALEN-induced insertions and deletions at the targeted locus. The binding site of TALEN is underlined in red. Occurrences of deletions and insertions are listed on the right. The lowercase letters represent inserted bases.
The activity of TALENs in human 293-FT cells was screened with a luciferase single-strand annealing (SSA) assay [pair 1 (9.2 ± 0.86) vs. pair 2 (35.8 ± 3.75), P = 0.000; pair 1 (9.2 ± 0.86) vs. pair 3 (39.7 ± 3.17), P = 0.000; pair 2 (35.8 ± 3.75) vs. pair 3 (39.7 ± 3.17), no significance] (Fig. 1C). The frequency of TALEN-mediated disruption at the target site in BFFs then was determined by Surveyor nuclease assays (27). Of the three pairs of TALENs developed, pair 2 cleaved the target site most efficiently, as demonstrated by the increased incidence of allelic mutations (NHEJ frequency) (Fig. 1 D and E). Therefore, pair 2 was used for subsequent experiments. To confirm further the presence of nuclease-induced insertions and deletions at the targeted locus, the targeted locus was PCR amplified from the genomic DNA and transformed into Escherichia coli by TA cloning. We randomly picked 865 bacterial colonies for sequencing. Deletions or insertions were detected in 6.13% of the colonies generated from TALEN-transfected cells (SI Appendix, Table S2; representative sequences are shown in Fig. 1F).
TALE Nickase Restricts Repair to the Homology-Directed Repair Pathway.
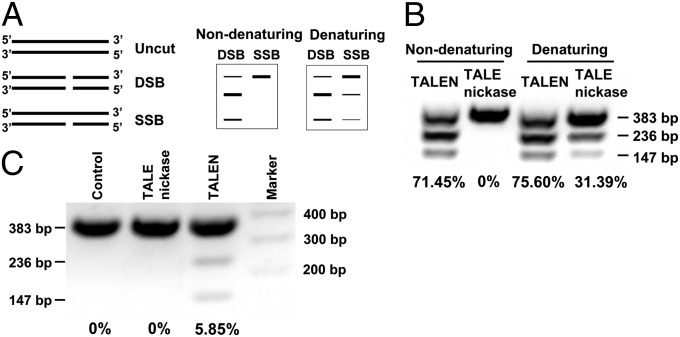
A targeted single-strand break (SSB) has the potential to restrict repair to the homology-directed repair (HDR) pathway (28, 29). Zinc-finger nickases (ZFNs) are well established for generating SSBs; more recently, this strategy even has been reported in TALENs (5, 29–32). Therefore, a mutation at the active site (D450A) that abolishes catalytic activity without affecting protein dimerization or DNA recognition was introduced to FokI of the right hand of TALENs (28).
An in vitro DNA cleavage assay was performed to assess the activity of TALENs bearing the D450A mutation. A linear 383-bp PCR fragment containing an off-center target site for specific TALENs was digested with in vitro-synthesized TALENs. The expected digestion patterns following strand-specific cleavage when resolved under nondenaturing and denaturing conditions are shown in Fig. 2A. Thus, provision of wild-type TALEN synthesized in vitro resulted in efficient double-strand cleavage of the template DNA, regardless of whether the products were resolved under nondenaturing or denaturing conditions (Fig. 2B, lanes 1 and 3, >71% cleavage efficiency). In contrast, introduction of D450A to the wild-type TALEN (TALE nickase) eliminated double-strand cleavage (Fig. 2B, lane 2). The cleavage products also were resolved under denaturing conditions to confirm the strand-specific nicking activity of TALE nickase. As expected, one strand of the double-stranded DNA was cleaved into two smaller fragments, but the other strand was uncleaved and persisted as the full-length template (Fig. 2B, lane 4). These results demonstrate that the D450A mutation of FokI resulted in the generation of a potent, strand-specific TALEN.
Fig. 2.
TALE nickase induced an SSB at the M-S locus and eliminated the NHEJ repair pathway. (A) Illustration of the expected digestion patterns following TALE nickase cleavage when resolved under nondenaturing and denaturing conditions. (B) Actual results of SSB when resolved under nondenaturing and denaturing conditions. (C) Cleavage activity of TALE nickase was measured by Surveyor nuclease assay. TALE nickase decreased DNA cleavage activity, but no NHEJ events were detected.
Then we further confirmed that a TALE nickase-mediated SSB had the potential to restrict repair to the HDR pathway. Wild-type TALEN or TALE nickase was transfected into BFFs, and genomic DNA was extracted after 72 h. Surveyor nuclease assays were performed. As shown in Fig. 2C, TALE nickase dramatically decreased the DNA-cleaving ability, but no NHEJ events were detected. The targeted locus then was PCR amplified and transformed into E. coli, and 823 bacterial colonies were picked and sent for sequencing. Compared with the 6.13% of deletions or insertions in the wild-type TALEN group, none was found in the colonies generated from TALE nickase-transfected cells (SI Appendix, Table S2). These results suggest that a targeted strand-specific nick could be repaired by HDR and that such nicks do not generate the indels characteristic of the NHEJ repair pathway.
Selection of Transgene.
Based on the analysis of the tuberculosis-susceptible strain C3HeB/FeJ and the tuberculosis-resistant strain C57BL/6J, Kramnik and colleagues (26) mapped a genetic locus (sst1) with a major effect on tuberculosis susceptibility on mouse chromosome 1 and found that the SP110 (also called “Ipr1”) gene mediates innate immunity to tuberculosis. SP110 is up-regulated in tuberculosis-resistant macrophages after infection, but it is not expressed in tuberculosis-susceptible macrophages. We obtained five different splice variants of SP110 in cattle using rapid amplification of cDNA ends; however, preliminary experiments showed that none of the five bovine SP110 variants was useful in restricting the multiplication of M. bovis in macrophages (SI Appendix, Fig. S1A). In contrast, the mouse SP110 can control the multiplication of M. bovis (SI Appendix, Fig. S1A; 96 h, control 0.71 ± 0.09% vs. mouse SP110 0.38 ± 0.05%, P = 0.0021) and induce apoptosis in macrophages from infected cattle (SI Appendix, Fig. S1B; control 11.2 ± 1.3% vs. mouse SP110 24.5 ± 3.1%, P = 0.0012). Therefore, we chose to add an SP110 gene derived from mouse to a specific location in the bovine genome.
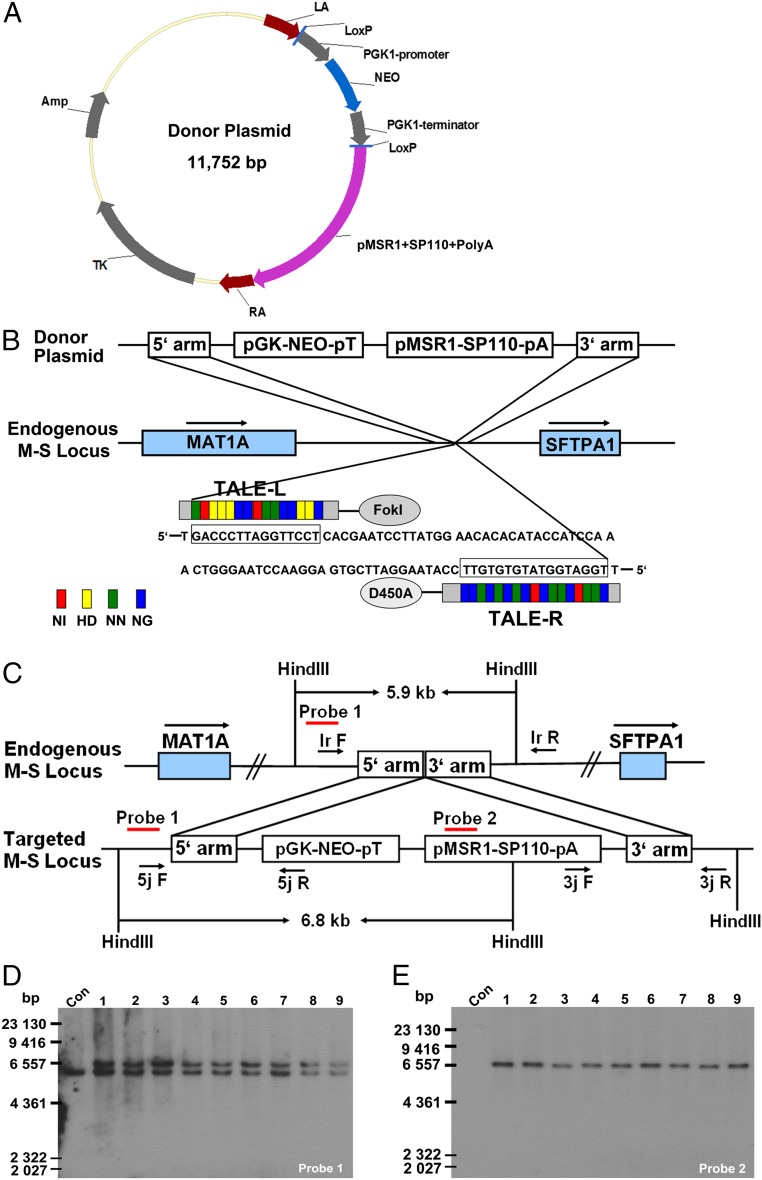
Addition of the TALE Nickase-Mediated Gene at the Specific Locus.
The gene-targeting vector pLoxp-SP110-Neo was constructed as shown in Fig. 3A. We used the bovine endogenous macrophage scavenger receptor 1 (MSR1) promoter to direct mouse SP110 expression and express SP110 only in bovine macrophages. The BFFs used for targeting were obtained from three different female Holstein–Friesian dairy cows. These cattle originally were imported from Canada (BFF1), Australia (BFF2), and the United States (BFF3). TALE nickases encoding plasmids were cotransfected with pLoxp-SP110-Neo to introduce an SSB between MAT1A and SFTPA1 (the M-S locus) in BFFs (Fig. 3B). Stably transfected cells were screened by 5′-junction (1.49 kb), 3′-junction (1.67 kb), and long-range (targeted, 5.98 kb; wild-type, 1.64 kb) PCR to confirm that stable genetic modification of cells was targeted to the intended specific site (Fig. 3C and SI Appendix, Table S3). Representative PCR results are shown in SI Appendix, Fig. S2.
Fig. 3.
Targeted and heritable addition of the SP110 gene using TALE nickase. (A) Schematic representation of the gene-targeting vector. (B) Schematic overview depicting the targeting strategy for SP110. D450A, FokI bearing a D450A mutation. (C) Schematic overview screening the individual colonies. 5j F, lr F, and 3j R, lr R are primers for regions outside the homologous arms; 5j R and 3j F are primers for the targeting vector region. Southern blot probes are shown as red lines; Hind III digestion is used in Southern blot analysis. (D and E) Southern blot analysis of the nine heterozygous donor cells used for SCNT. (D) A 6.8-kb band resulting from targeted inclusion of the SP110 cassette was detected in addition to the 5.9-kb wild-type band when probe 1 was used. (E) Only a 6.8-kb targeted band was detected when probe 2 was used.
PCR screening of heterozygous colonies will generate two bands for a single knockin, namely, a 5.98-kb band characteristic of the insertion of the SP110 gene and a 1.64-kb band from the normal chromosome [Fig. 3C, long range (lr) primers]. Thus, the heterozygous colonies were selected for Southern blot confirmation (Fig. 3 D and E; probes 1 and 2). Following confirmation of successful insertion, karyotype analysis of each heterozygous colony was conducted (a typical and representative karyotype is shown in SI Appendix, Fig. S3). A total of 26 heterozygous colonies with normal karyotype, compact spindle-like cell morphology, and rapid growth were considered suitable for somatic cell nuclear transfer (SCNT).
Nuclear Transfer to Produce SP110 Transgenic Cattle.
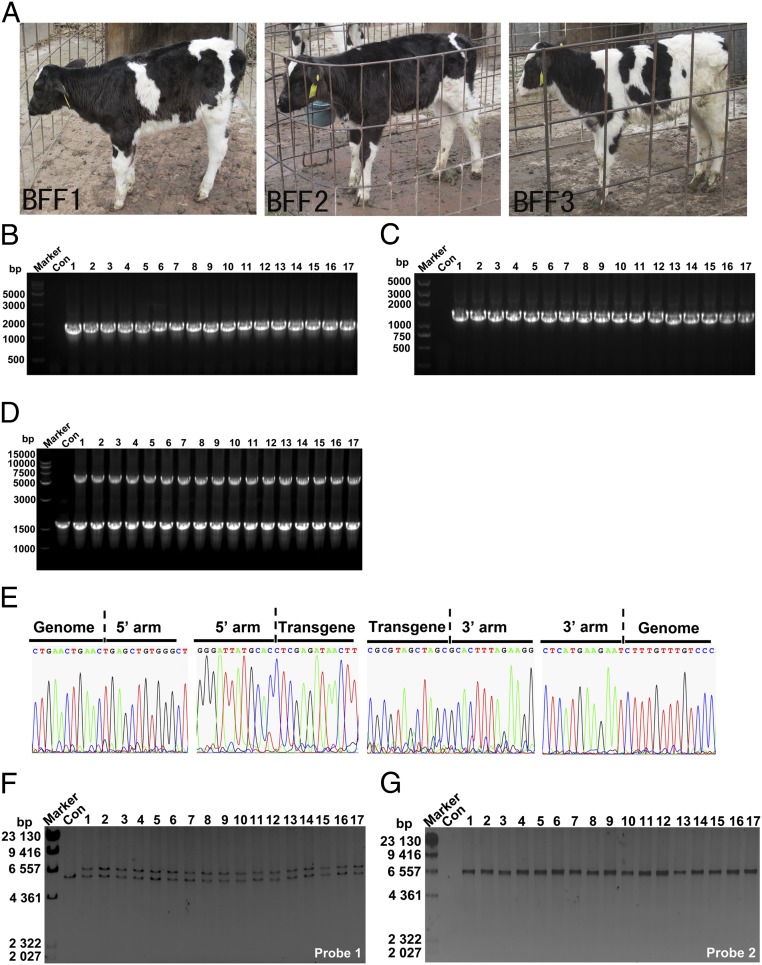
Nine of the transgenic cell colonies were used as donor cells to produce cloned transgenic cattle. A total of 1,580 reconstructed embryos were cultured in vitro; of these, 465 were developed into blastocysts. No significant difference in the blastocyst formation rate was observed among the different nuclear donor cell lines (Table 1). These transgenic blastocysts were transferred into the oviducts of 147 recipient heifers. A total of 23 calves were born, and 13 survived longer than 6 mo (Table 1 and Fig. 4A). As shown by junction PCR and genome-walking analysis, SP110 was integrated at the expected site in all 13 calves (Fig. 4 B–E and Dataset S1). Southern blot demonstrated that the transgenic cattle were heterozygous for SP110 knockin (Fig. 4 F and G).
Table 1.
In vivo development of cloned embryos from different transgenic cells
| Nuclear donor | BFF1 | BFF2 | BFF3 | Total | ||||||
| Cell clone | SC178 | SC208 | SC364 | SC504 | SC598 | SC671 | SC821 | SC892 | SC973 | — |
| Embryos cultured | 166 | 175 | 182 | 161 | 173 | 204 | 182 | 170 | 167 | 1,580 |
| Blastocysts (%) | 58 (34.9) | 49 (28.0) | 54 (29.7) | 42 (26.1) | 47 (27.2) | 51 (25.0) | 61 (33.5) | 54 ( 31.8) | 49 (29.3) | 465 (29.5) |
| Recipients | 18 | 15 | 15 | 16 | 15 | 17 | 19 | 16 | 16 | 147 |
| Pregnancies (%) | 9 (50.0) | 7 (46.7) | 5 (33.3) | 4 (25.0) | 2 (13.3) | 4 (23.5) | 8 (42.1) | 6 (37.5) | 5 (31.3) | 50 (34.0) |
| Calves at birth | 5 | 3 | 1 | 3 | 0 | 1 | 3 | 3 | 4 | 23 |
| Calves surviving at 6 mo | 3 | 2 | 0 | 2 | 0 | 1 | 2 | 2 | 1 | 13 |
Fig. 4.
Assessment of transgenic cattle. (A) Photographs of SP110 gene-targeted calves that lived longer than 6 mo. The legends in the photographs identify the origin of donor cell lines. (B–D) 5′-junction (B, 1.49 kb), 3′-junction (C, 1.67 kb), and long-range (D, wild type: 1.64 kb; targeted: 5.98 kb) PCR to confirm site-specific targeting in transgenic cattle. Templates for PCR were genomic DNA extracted from cattle peripheral blood. Con, control normal cattle. Lanes 1–13 represent the 13 live calves. Lanes 14–17 represent four randomly selected dead transgenic calves. (E) Nucleotide sequence between endogenous and exogenous DNA corresponding to homologous recombination in transgenic cattle. (F and G) Southern blot analysis of the genomic DNA extracted from transgenic cattle. Lanes 1–13 represent the 13 live calves. Lanes 14–17 represent four randomly selected dead transgenic calves.
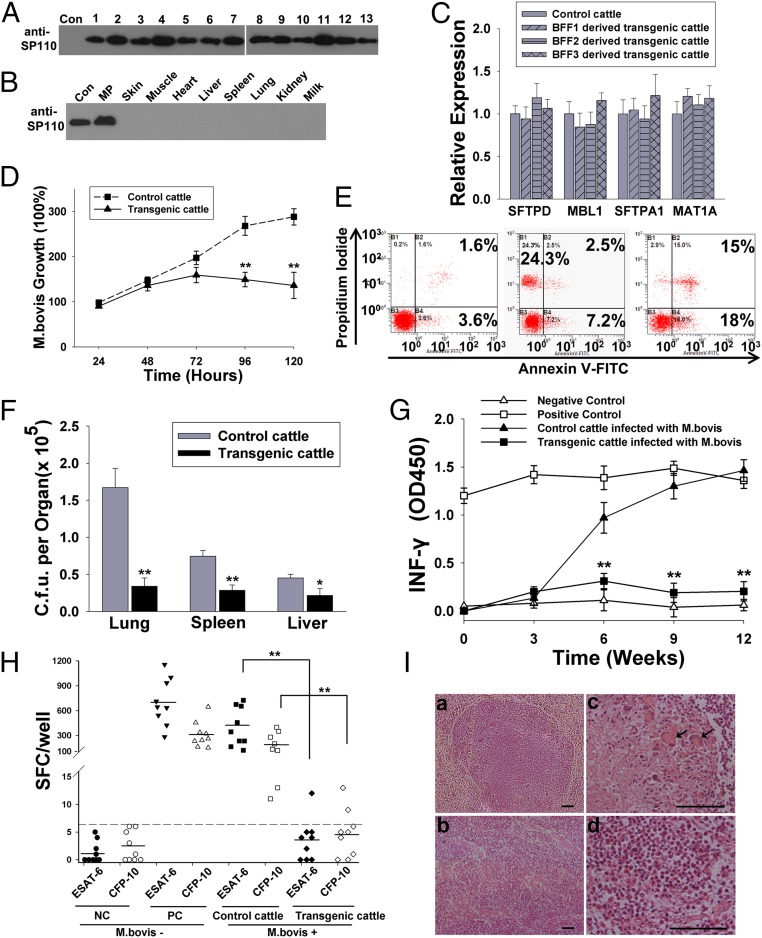
We then determined whether MSR1-controlled SP110 expression is restricted to macrophages only. Macrophages separated from transgenic cattle were used for Western blot. The macrophages from all transgenic cattle expressed SP110 correctly (Fig. 5A). No SP110 protein was detected in the skin, muscle, heart, liver, spleen, lung, kidney, or milk from transgenic cattle (Fig. 5B).
Fig. 5.
Assessment of the ability of transgenic cattle to resist tuberculosis. (A) SP110 was expressed correctly in macrophages isolated from transgenic cattle. Lanes 1–13 represent the 13 live transgenic cattle. Con, control cattle. (B) SP110 was expressed only in macrophages. Organs were obtained from a pool of dead transgenic cattle. Milk was obtained from three live transgenic cattle. Con, control donor cells; MP, macrophages. (C) The addition of SP110 did not affect the expression of nearby endogenous genes. Macrophages were separated from transgenic cattle (n = 9) or control cattle (n = 9). The relative expression levels of SFTPD, MBL1, SFTPA1, and MAT1A were detected by real-time RT-PCR. Each sample was tested individually, but data were analyzed by group. (D) Multiplication of M. bovis in macrophages from control (n = 9) or transgenic (n = 9) cattle in vitro. The macrophages were separated from each animal individually and were mixed by group. M. bovis multiplication was determined by cfu assays. (E) Flow cytometry analysis of the mechanism of cell death of the transgenic cattle macrophages infected with M. bovis. Early apoptotic [annexin V+ propidium iodide (PI)−] late apoptotic (annexin V+ PI+), and necrotic (annexin V− PI+). (Left) Normal macrophages. (Middle) Infected experiment control macrophages. (Right) Infected transgenic macrophages. (F) M. bovis bacterial loads in the organs of the transgenic cattle after endobronchial infection. (G) Amount of IFN-γ produced in experimental control (n = 9) and transgenic (n = 9) cattle that shared a confined airspace with positive control cattle for 12 wk. (H) Concentrations of ESAT-6 and CFP-10 IFN-γ–producing SFCs in PBMCs of control and transgenic cattle. (I) H&E stains show a tubercle in the hilar lymph node of the control cattle (A and C) and normal tissue of transgenic cattle (B and D) 16 wk after infection. Arrows show the Langhans giant cells in the tubercle. (Magnification: 100× in I, a and b; 400× in I, c and d.) (Scale bars: 50 μm.) The transgenic cattle were divided into three groups according to their origin (derived from three different BFFs), and three cattle were picked randomly from each group for the experiments presented in C, D, G, and H. Data are shown as mean ± SD and are derived from at least three independent experiments. NC, negative control; PC, positive control. *P < 0.05; **P < 0.01.
We inquired whether insertion of the exogenous gene affects the expression of endogenous genes nearby. Real-time RT-PCR analysis detected no significant difference in the relative levels of expression of surfactant, pulmonary-associated protein D (SFTPD), mannose-binding lectin 1 (MBL1), SFTPA1, or MAT1A genes in transgenic and control cattle (Fig. 5C; primers are listed in SI Appendix, Table S4).
In Vitro and in Vivo Challenge and Transmission Experiments.
To estimate the ability of SP110 transgenic cattle to respond to M. bovis infection, macrophages from peripheral blood (SI Appendix, Supplementary Materials and Methods and SI Appendix, Fig. S4) were challenged with M. bovis in vitro. The macrophages from transgenic cattle and control macrophages showed different reactions to M. bovis infection. First, the rate of M. bovis multiplication was lower in the macrophages from SP110 transgenic cattle than in the control macrophages (96 h: 149.3 ± 15.6% vs. 268 ± 19.2%, P = 0.0027; 120 h: 136.4 ± 23.7% vs. 289 ± 17.3%, P = 0.0013) (Fig. 5D). Second, we observed a clear distinction in the mechanism of macrophage cell death after infection. The control cattle macrophages showed characteristic necrosis (24.3%) (Fig. 5E, Center), whereas the transgenic cattle macrophages showed remarkable apoptosis (33.0%) (Fig. 5E, Right).
An in vivo challenge experiment also was performed to confirm the ability of SP110 transgenic cattle to resist M. bovis. Three randomly selected transgenic cattle and three experimental control cattle (derived from the same cells but without the transgene, breed-, sex-, and age-matched with the transgenic cattle) were infected with 5 × 104 cfu of M. bovis by endobronchial instillation. Cattle were killed 16 wk postinfection. The organs susceptible to M. bovis, such as lung, tracheobronchial lymph node, mediastinal lymph node, spleen, and liver tissues, were evaluated for lesions based on a gross pathology scoring system as previously described (33, 34). As shown in Table 2, although only one of three transgenic cattle presented with no histopathological lesions, inserting SP110 into cattle significantly reduced the pathology associated with M. bovis infection by endobronchial instillation (pathology score, 6.5 vs. 32.0) (Table 2). After being examined for gross lesions, the entire organ was homogenized and used for bacterial cfu assay. As shown in Fig. 5F, bacterial loads in the organs of transgenic cattle after infection were reduced significantly compared with those in the control group (spleen, 0.75 ± 0.056 × 105 vs. 0.29 ± 0.062 × 105, P = 0.0005; liver, 0.45 ± 0.030 × 105 vs. 0.21 ± 0.071 × 105, P = 0.0015). The reduction was especially notable in the lung, which is the organ primarily susceptible to virulent M. bovis (1.67 ± 0.26 × 105 vs. 0.33 ± 0.087 × 105, P = 0.000).
Table 2.
Gross pathology of transgenic cattle challenged with M. bovis by endobronchial instillation
| Animal | No. of lobes infected* | Lung score | No. of lymph nodes infected† | Lymph node score | Total pathology score | Mean‡ |
| Transgenic 1 | 2 | 4 | 3 | 4 | 8 | 6.5 |
| Transgenic 2 | 1 | 2 | 2 | 3 | 5 | |
| Transgenic 3 | 0 | 0 | 0 | 0 | 0 | |
| Control 1 | 5 | 21 | 6 | 14 | 35 | 32.0 |
| Control 2 | 4 | 15 | 8 | 18 | 33 | |
| Control 3 | 4 | 14 | 6 | 14 | 28 |
Lung lobes (left apical, left cardiac, left diaphragmatic, right apical, right cardiac, right diaphragmatic, and right accessory lobes) were examined for lesions using a gross pathology scoring system.
Lymph nodes (mandibular, parotid, medial retropharyngeal, mediastinal, tracheobronchial, hepatic, mesenteric, and prescapular lymph nodes) were examined for lesions using a gross pathology scoring system.
Median values per group (n = 3). Only animals with lesions were taken into account.
To estimate further the ability of transgenic cattle to resist tuberculosis, a transmission experiment was performed. Early studies showed that cattle-to-cattle transmission of bovine tuberculosis occurs at a lower rate in animals living in outdoor conditions than in animals sharing a confined airspace (35, 36). Therefore, we performed the transmission experiment in an independent category 3 biosafety accommodation. First, tuberculin skin tests were performed. The comparative changes in skin fold of transgenic cattle were all less than 1 mm (SI Appendix, Table S5), showing that the transgenic cattle being assessed were not infected with M. bovis. It is generally accepted that cellular responses characterized by CD4+ T-cell–derived IFN-γ are helpful in diagnosing people or animals containing MTB (33, 37–40). IFN-γ release assays (IGRAs) were conducted to monitor the IFN-γ release level of control and transgenic cattle that fed with tuberculous cattle. Thus, on stimulation with bovine tuberculin purified protein derivatives (PPD-B), control cattle developed IFN-γ responses within 6 wk of living with tuberculous cattle, and the responses increased steadily throughout the postinfection period. In contrast, the PPD-B–specific IFN-γ responses in transgenic cattle challenged with M. bovis were significantly lower than those in the control cattle challenged with M. bovis (9 wk: transgenic 0.19 ± 0.08 vs. control 1.32 ± 0.12, P = 0.000; 12 wk: transgenic 0.21 ± 0.10 vs. control 1.46 ± 0.15, P = 0.000) (Fig. 5G).
Further, a more specific assay, namely an MTB-specific enzyme-linked immunospot (ELISPOT) assay, was performed after the transmission experiment to confirm our results. As shown in Fig. 5H, the average number of spot-forming cells (SFC) was significantly lower in transgenic cattle than in control cattle [early secretory antigenic target-6 (ESAT-6): control 401.1 ± 234.1 vs. transgenic 3.56 ± 3.4, P = 0.000; culture filtrate protein-10 (CFP-10): control 182.6 ± 137.7 vs. transgenic 4.78 ± 4.2, P = 0.000]. Moreover, the number of SFC in transgenic cattle was not significantly different from that in negative control cattle (ESAT-6: negative control 1.43 ± 1.37 vs. transgenic 3.56 ± 3.4, P = 0.215; CFP-10: negative control 2.70 ± 2.33 vs. transgenic 4.78 ± 4.2, P = 0.437). After the transmission experiment, all animals in contact with M. bovis were killed for postmortem examination. The lungs and lymph nodes were evaluated for lesions using a gross pathology scoring system adapted from Vordermeier and Waters, et al. (33, 34). As shown in Table 3, six of nine transgenic cattle presented with no visible or histopathological lesions. In addition, a significant reduction in the gross pathology of the lungs and lymph nodes was observed in the transgenic animals (pathology score, 4.7 ± 2.1 vs. 17.8 ± 4.8, P = 0.000) (Table 3). H&E staining also was used to assess the degree of lung pathology present. A representative H&E stain of hilar lymph node is shown in Fig. 5I. The development of large necrotic lung lesions after infection, a characteristic of control cattle, was prevented in transgenic cattle.
Table 3.
Gross pathology of transgenic cattle challenged by transmission experiment
| Animal | No. of lobes infected* | Lung score | No. of lymph nodes infected† | Lymph node score | Total score | Mean ± SD‡ |
| Transgenic group 1 | 1 | 2 | 1 | 1 | 3 | 4.7 ± 2.1 |
| 0 | 0 | 0 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | 0 | ||
| Transgenic group 2 | 3 | 3 | 2 | 4 | 7 | |
| 2 | 2 | 1 | 2 | 4 | ||
| 0 | 0 | 0 | 0 | 0 | ||
| Transgenic group 3 | 0 | 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | 0 | ||
| Control group 1 | 4 | 12 | 6 | 13 | 25 | 17.8 ± 4.8 |
| 4 | 13 | 5 | 10 | 23 | ||
| 3 | 10 | 3 | 8 | 18 | ||
| Control group 2 | 4 | 12 | 4 | 10 | 22 | |
| 3 | 9 | 4 | 9 | 18 | ||
| 2 | 6 | 3 | 6 | 12 | ||
| Control group 3 | 3 | 8 | 4 | 9 | 17 | |
| 3 | 7 | 2 | 5 | 12 | ||
| 2 | 7 | 3 | 6 | 13 |
Lung lobes (left apical, left cardiac, left diaphragmatic, right apical, right cardiac, right diaphragmatic, and right accessory lobes) were examined for lesions using a gross pathology scoring system.
Lymph nodes (mandibular, parotid, medial retropharyngeal, mediastinal, tracheobronchial, hepatic, mesenteric, and prescapular lymph nodes) were examined for lesions using a gross pathology scoring system.
Median values per group (n = 9). Only animals with lesions were taken into account.
The SP110 Transgene Is Heritable and Is Expressed in Offspring Macrophages.
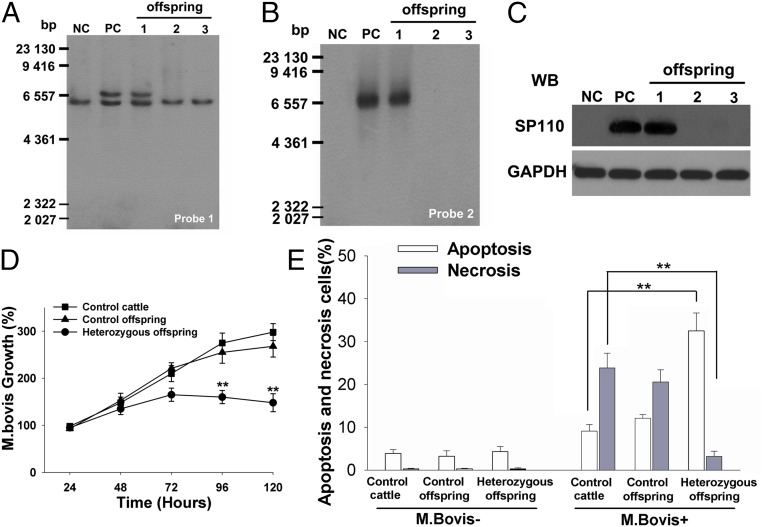
A significant concern in the cultivation of transgenic animals is whether the transgene is maintained in offspring. In this study, we acquired three offspring calves of the transgenic cattle by means of artificial insemination, and one calf was confirmed to be heterozygous for SP110 knockin by Southern blot (Fig. 6 A and B). Western blot was performed to examine whether the expression of SP110 was maintained in the heterozygous offspring. The results indicated that SP110 was expressed in the macrophages of the offspring animal, and there was no significant difference in the level of SP110 expression in the offspring and founder cattle (Fig. 6C). Furthermore, an in vitro challenge experiment was performed to estimate the ability of macrophages from the heterozygous offspring to resist tuberculosis. As shown in Fig. 6D, the rate of M. bovis multiplication was lower in the macrophages from the heterozygous offspring than in the control macrophages (96 h: 160.3 ± 14.6% vs. 275.2 ± 21.7%, P = 0.0047; 120 h: 148.5 ± 18.3% vs. 295 ± 17.4%, P = 0.0020). The distinction in the mechanism of macrophage cell death after infection was observed also. The control macrophages showed necrosis (control 23.8 ± 3.4% vs. heterozygous 3.3 ± 1.5%, P = 0.000), whereas the macrophages from the heterozygous offspring showed characteristic apoptosis (control 9.1 ± 1.5% vs. heterozygous 32.5 ± 4.1%, P = 0.000) (Fig. 6E) after infection with M. bovis. These data indicated that SP110 site-specific knockin for tuberculosis resistance in cattle is heritable.
Fig. 6.
The SP110 transgene is heritable and is expressed in offspring macrophages. (A and B) Southern blot analysis of three offspring cattle using probe 1 (A) and probe 2 (B). The results show that one of the offspring is heterozygous for the SP110 knockin. Lanes 1–3 represent the three offspring cattle. NC, negative control; PC, positive control. (C) The expression level of SP110 was detected by Western blots (WB). Lanes 1–3 represent the three offspring cattle. GAPDH serves as a loading control. (D) In vitro multiplication of M. bovis in the macrophages from control cattle, control offspring, or heterozygous offspring. M. bovis multiplication was determined by cfu assay. Control offspring are the two offspring cattle without the SP110 transgene. (E) Apoptosis and necrosis rates of control and offspring macrophages infected with M. bovis were determined by flow cytometry.
Discussion
As of this writing, ZFN, TALEN, and RNA-guided engineered nuclease (RGEN) are the three most widely used and most promising tools for genome modification. However, each nuclease has its own advantages and disadvantages. ZFNs, the first programmable nuclease for genome modification, have been used and improved in academia and industry for the last two decades (41, 42). For example, clinical trials on the ZFNs for CCR5, the most common coreceptor for HIV-1, have been underway for several years, and the therapeutic benefits are very promising (43, 44). However, despite continuous improvements in ZFN technology, a substantial proportion of ZFNs fail, whether they are produced by design or selection (45–47). In contrast, TALENs can be designed to target almost any given DNA sequence and achieve a very high success rate. Because a single mismatch between modules and base pairs can decrease binding significantly, TALENs are generally less toxic and more specific than ZFNs. RGEN derives from an adaptive immune system that is widespread among bacteria and archaea. The unique advantages of RGENs over ZFNs and TALENs are their simplicity and the fact that they are readily multiplexed; however, specificity remains an issue in this system (48–52). TALENs and RGENs possibly may replace ZFNs for routine research based on principles of simplicity, efficiency, and reliability. However, we still need to prove whether these nucleases will have similar or greater utility than ZFNs. More importantly, in addition to the features of these three classes of nucleases, cell type and delivery method also have great effects on the activity and success rate. No reliable rules currently exist to predict nuclease activity before experimental validation.
TALEN technology has been applied successfully to create transgenic animals in most small model animal species (10, 11, 15). Carlson et al. (17) reported successful TALEN-mediated gene knockout in bovine embryos. Huang et al. (53) and Lillico et al. (54) created TALEN-mediated transgenic pigs. Most recently, Proudfoot et al. (21) reported that they have created myostatin gene-knockout sheep and cattle using TALENs. Here we report, for the first time to our knowledge, TALE nickase-mediated gene insertion via homologous recombination to produce transgenic cattle. We introduced a D450A mutation to the FokI of the right hand of wild-type TALEN and established a TALE nickase system, which primarily creates a SSB in the genome. A targeted SSB has the potential to restrict repair to the HDR pathway, thereby eliminating the NHEJ pathway and greatly improving the efficiency of targeting.
The M-S locus was selected for gene targeting for the following reasons. First, macrophages express many surface receptors that facilitate the binding of microorganisms. SFTPA1, one of the surfactant proteins, may modulate the activity of one or more receptors that are responsible for direct binding to M. bovis (55–57); SFTPD, an important paralog of SFTPA1, interacts with compounds, such as bacterial lipopolysaccharides, in the immune response (58). MBL1 recognizes mannose and N-acetylglucosamine in many microorganisms, and it can activate the classical complement pathway (59). Given these facts, we hypothesized that SP110, in conjunction with endogenous genes nearby, may activate anti-M. bovis mechanisms in macrophages (this hypothesis needs to be explored further experimentally). Second, the chromatin encoding these genes is activated in macrophages because of the important functional role of this gene cluster. Therefore, insertion of SP110 in this region could avoid exogenous gene silencing caused by chromatin inactivation.
We created 13 transgenic cattle using M-S locus-targeted heterozygous colonies as donor cells. Heterozygous colonies of cells with SP110 knockin to a single chromosome 28 will retain one normal chromosome, which will be helpful for the survival of transgenic animals. This strategy has been proved in our previously published work (5), mainly because the normal chromosome would counteract the defects or neutralize the side effects introduced by genome modification.
The cleavage efficiency of TALENs at the M-S locus was lower than previously reported (5, 17). This region may be heavily methylated or even inactivated in BFFs because of its function. TALEN cleavage occurs largely during the S phase of the cell cycle when all genomic sequences are exposed for replication. To confirm our hypothesis, the FSCN1-ACTB (F-A) locus was selected. Higher cleavage efficiency was achieved, but relatively lower blastocyst rates, pregnancy rates, and birth rates were observed also (SI Appendix, Supplementary Result, Fig. S5, and Tables S6 and S7). These data suggested that the F-A locus probably is not a safe harbor for the transgene. Although we detected an off-target mutation in one of 19 F-A locus gene-targeted cell clones (SI Appendix, Fig. S6 and Tables S8 and S9), so far there is no convincing evidence that the potential toxicity is associated with off-target effects. The underlying mechanisms should be examined further in future investigations.
Based on in vitro and in vivo challenge and on the transmission experiments, the SP110 transgenic cattle could control the growth and multiplication of M. bovis, activate the apoptotic pathway of cell death instead of necrosis after infection with M. bovis, and efficiently resist the low dose of M. bovis transmitted from tuberculous cattle in nature. In this study, we also acquired three offspring calves of the founder transgenic cattle, and one calf was heterozygous for SP110 knockin. We found that SP110 is expressed in the heterozygous offspring, and an in vitro challenge experiment proved that tuberculosis resistance is maintained in the macrophages from the heterozygous calf (Fig. 6). All these results demonstrate that inserting SP110 into cattle is a highly promising technique for creating resistance to M. bovis infection and that this genome modification for tuberculosis resistance in cattle is heritable.
M. bovis can evade host immune defense by inducing necrosis rather than by inhibiting the apoptosis of macrophages (60). In the present study, SP110 transgenic cattle could activate the apoptotic pathway of cell death instead of necrosis after infection with M. bovis. Therefore, previously undescribed mechanisms of SP110 or SP110-interacting proteins may exist, in which SP110 or SP110-interacting proteins control various aspects of the macrophage life activities, including activation, differentiation, and immune response to pathogens. It would be interesting to investigate further the specific mechanism of SP110 in determining the fate of macrophage. Moreover, other factors associated with SP110 should be examined, and their potential roles of tuberculosis resistance should be determined.
Materials and Methods
Ethics Statement.
This study was carried out in strict accordance with the guidelines for the care and use of animals of Northwest A&F University. All animal experimental procedures were approved by the Animal Care Commission of the College of Veterinary Medicine, Northwest A&F University. Bovine ovaries from slaughtered mature cows were collected from the Tumen abattoir, a local abattoir in Xi’An, China. Six-month-old tuberculosis-free calves were obtained from Keyuan Cloning Co., Ltd. and were kept in the Animal Services Unit in a category 3 biosafety accommodation. Every effort was made to minimize animal pain, suffering, and distress and to reduce the number of animals used.
Surveyor Nuclease Assay.
The capacity of each TALEN for native gene disruption activity at its target locus was determined by Surveyor nuclease (Transgenomic) assay in BFFs. In brief, genomic DNA from TALEN-treated cells was extracted using a Universal Genomic DNA Extraction Kit (TakaRa). The targeted loci were PCR amplified using the following primers: pair 1, F (5′-GAGAAGGAAATGGCAACCCAC-3′) and R (5′-CGGAAATCTTGATTCCAGCTT-3′); pair 2, F (5′-CCTTCCGCCTCTGTAGGTACAGA-3′) and R (5′-GTAGGACACAGTGCCGCAAACCC-3′); pair 3, F (5′-CGAATTCACTTTCACTTTCGT-3′) and R (5′-CAGTTCTTCACTTTCTGCCATA-3′).
PCR products were digested with Surveyor nuclease and analyzed by agarose gel electrophoresis according to the manufacturer's instructions. Quantification was based on relative band intensities using Image J software.
Cell Culture and Transfection.
Primary BFFs were isolated from 1-mo-old fetuses. The tissues were minced, plated on 60-mm Petri dishes (Corning Costar), and cultured with DMEM/F12 (Gibco, Invitrogen) supplemented with 10% (vol/vol) FBS (HyClone) and 10 ng/mL epidermal growth factor. HEK-293FT cells (ATCC) were cultured with DMEM (Invitrogen) supplemented with 10% (vol/vol) FBS. BFFs were harvested using 0.25% trypsin/EDTA solution (Invitrogen). Cells (1 × 107) were resuspended in Opti-MEM (Gibco), mixed, if not otherwise indicated, with 10 µg of linearized donor plasmid and 5 µg of each TALEN-encoding plasmid, and electroporated at 510 V with three pulses of 1-ms duration using the BTX Electro-cell manipulator ECM2001 (BTX Technologies). Electroporated cells were sorted via flow cytometry and plated on 10-cm plates at 1 × 106 cells per plate. Individual colonies were selected and expanded after G418 selection (600 ng/mL) 10–14 d after electroporation.
Nuclear Transfer.
Ovaries were collected from the local abattoir and transported to the laboratory within 4–6 h in sterile saline at 20 °C. In vitro maturation of oocytes, enucleation, microinjection, and fusion of reconstructed oocytes were carried out in our laboratory according to the previously described methods (5). The reconstructed oocytes were cultured until they developed to blastocyst stage. Three or four fresh day 7 blastocysts produced in vitro were nonsurgically transferred to randomly assigned synchronized recipient heifers on day 7 after estrus. Pregnancy was diagnosed by rectal palpation on day 35 and confirmed by ultrasonography on day 60 after blastocyst transfer.
M. bovis Challenge and Transmission Experiments.
For the challenge experiment, three control and three transgenic calves were infected with 5 × 104 cfu of M. bovis (strain AF 2212/97) by endobronchial instillation as previously described (33, 61). At the end of the experimental period, the calves were killed by i.v. injection of sodium phenobarbitone, and postmortems were performed.
We had set a control group and an experimental group in the transmission experiment. The control group comprised a negative control (a normal animal without the transgene or M. bovis infection) and a positive control (a normal animal without the transgene but infected with M. bovis by endobronchial instillation and diagnosed as tuberculous). The experimental group comprised the control (cloned animals without the transgene) and transgenic animals. Positive controls used for the transmission experiment were produced by endobronchial instillation with 5 × 104 cfu of M. bovis. Skin tests and IFN-γ assays were performed to confirm that cattle were infected with M. bovis. The positive results of bacterial cultures of respiratory fluids proved that infected cattle could transmit M. bovis into the environment. Positive controls were reconfirmed by the presence of tuberculous lesions in the lungs and lymph nodes through postmortems after the transmission experiment. For the transmission experiment, nine positive controls, nine experimental controls, and nine transgenic cattle were fed together in a confined accommodation for 12 wk. Blood samples were collected, and IFN-γ assays were performed to monitor the level of IFN-γ release at the time points indicated in Fig. 5H.
Postmortem and Pathology Scoring System.
Postmortems were performed after the challenge and transmission experiments. Lung lobes (left apical, left cardiac, left diaphragmatic, right apical, right cardiac, right diaphragmatic, and right accessory lobes) were examined externally for the occurrence of lesions, followed by slicing of the lung into 0.5- to 1-cm-thick slices that then were examined individually for lesions. Lymph nodes (mandibular, parotid, medial retropharyngeal, mediastinal, tracheobronchial, hepatic, mesenteric, and prescapular lymph nodes) were sliced into 1- to 2-mm-thick slices that were examined for the presence of visible lesions. Lungs and lymph nodes were evaluated using a semiquantitative gross pathology scoring system adapted from Vordermeier and Waters et al. (33, 34). Lung lobes were scored individually based on the following scoring system: 0 = no visible lesions; 1 = no external gross lesions, but lesions seen upon slicing; 2 = fewer than five gross lesions <10 mm in diameter; 3 = more than five gross lesions <10 mm in diameter; 4 = more than one distinct gross lesion >10 mm in diameter; 5 = gross coalescing lesions. The scores of the individual lobes were added to calculate the lung score. Lymph node pathology was based on the following scoring system: 0 = no necrosis or visible lesions; 1 = small focus (1–2 mm in diameter); 2 = several small foci or a necrotic area at least 5 × 5 mm; 3 = extensive necrosis. The scores of lung lobes and lymph nodes were added to determine the total pathology score per animal. All scoring was performed by the same operator to ensure scoring consistency.
Cfu Assay.
Infection with M. bovis was performed by the State Key Laboratory of Veterinary Etiological Biology (Lanzhou, China). In brief, a bacterial suspension (∼107 bacteria per 106 cells) was added to the medium and incubated at 37 °C and 5% (vol/vol) CO2 for 4 h. Cells then were washed extensively with PBS to remove noningested bacteria. At the time points indicated in the text after infection, bacterial cfu were quantitated by plating on 7H10 agar plates (Difco Laboratories). Quantitative assessment of bacterial burden in organs was performed as previously described (34). In brief, after being examined for gross lesions, the entire organ was homogenized in phenol red nutrient broth. The homogenates then were diluted with PBS, plated on 7H10 agar plates, and incubated for 8 wk at 37 °C.
IGRAs.
IGRAs were performed using a BOVIGAM kit (Prionics AG) according to the manufacturer's instructions. In brief, whole-blood samples were incubated with PPD-B to stimulate the lymphocytes to secrete IFN-γ. The plasma supernatants were harvested after 24 h of incubation, and IFN-γ was estimated using a sandwich enzyme immunoassay. Optical density at 450 nm was determined using a VICTOR ×5 Multilabel Plate Reader (PerkinElmer).
ELISPOT Assay.
MTB-specific ELISPOT assays were performed as previously described (33, 62). In brief, ELISPOT plates (Millipore) were coated overnight at 4 °C with mouse anti-bovine IFN-γ monoclonal antibody (Thermo Scientific). Peripheral blood mononuclear cells (PBMCs) (2 × 105) then were added and cultured at 37 °C for 24 h. The cells were stimulated with ESAT-6 and CFP-10 peptides in separate wells following procedures performed strictly according to the manufacturer's recommendations. The response of stimulated cultures was considered positive when the test well contained at least six more spots than the control well.
Statistical Analysis.
Data are presented as the mean ± SD and are derived from at least three independent experiments. Statistical significances were analyzed using the Student's t test. A value of P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Ye Liu, Chengcheng Cui, and Xu Liu for expert technical assistance; Kun Ru and the ViewSolid Biotech Company for assistance in constructing TALEN plasmids; Xiaoning He for assistance in karyotype analysis; and the Keyuan Cloning Company for assistance in the M. bovis challenge and transmission experiments. This work was supported by National Major Project for Production of Transgenic Breeding Grant 2013ZX08007-004 and National High Technology Research and Development Program of China (863 Program) Grant 2011AA100303.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 3854.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421587112/-/DCSupplemental.
References
- 1.Meyer M, de Angelis MH, Wurst W, Kühn R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc Natl Acad Sci USA. 2010;107(34):15022–15026. doi: 10.1073/pnas.1009424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu H, et al. TALEN-mediated gene mutagenesis in rhesus and cynomolgus monkeys. Cell Stem Cell. 2014;14(3):323–328. doi: 10.1016/j.stem.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommer D, et al. Efficient genome engineering by targeted homologous recombination in mouse embryos using transcription activator-like effector nucleases. Nat Commun. 2014;5:3045. doi: 10.1038/ncomms4045. [DOI] [PubMed] [Google Scholar]
- 4.Xue H, et al. A targeted neuroglial reporter line generated by homologous recombination in human embryonic stem cells. Stem Cells. 2009;27(8):1836–1846. doi: 10.1002/stem.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, et al. Zinc-finger nickase-mediated insertion of the lysostaphin gene into the beta-casein locus in cloned cows. Nat Commun. 2013;4:2565. doi: 10.1038/ncomms3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahfouz MM, et al. De novo-engineered transcription activator-like effector (TALE) hybrid nuclease with novel DNA binding specificity creates double-strand breaks. Proc Natl Acad Sci USA. 2011;108(6):2623–2628. doi: 10.1073/pnas.1019533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zu Y, et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat Methods. 2013;10(4):329–331. doi: 10.1038/nmeth.2374. [DOI] [PubMed] [Google Scholar]
- 8.Miller JC, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29(2):143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 9.Bedell VM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491(7422):114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung YH, et al. Knockout mice created by TALEN-mediated gene targeting. Nat Biotechnol. 2013;31(1):23–24. doi: 10.1038/nbt.2477. [DOI] [PubMed] [Google Scholar]
- 11.Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T, Liu B, Spalding MH, Weeks DP, Yang B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol. 2012;30(5):390–392. doi: 10.1038/nbt.2199. [DOI] [PubMed] [Google Scholar]
- 13.Hockemeyer D, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29(8):731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsuyama T, et al. An efficient strategy for TALEN-mediated genome engineering in Drosophila. Nucleic Acids Res. 2013;41(17):e163. doi: 10.1093/nar/gkt638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang P, et al. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol. 2011;29(8):699–700. doi: 10.1038/nbt.1939. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, et al. TALEN-mediated editing of the mouse Y chromosome. Nat Biotechnol. 2013;31(6):530–532. doi: 10.1038/nbt.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson DF, et al. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci USA. 2012;109(43):17382–17387. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan W, et al. Efficient nonmeiotic allele introgression in livestock using custom endonucleases. Proc Natl Acad Sci USA. 2013;110(41):16526–16531. doi: 10.1073/pnas.1310478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan WS, Carlson DF, Walton MW, Fahrenkrug SC, Hackett PB. Precision editing of large animal genomes. Adv Genet. 2012;80:37–97. doi: 10.1016/B978-0-12-404742-6.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghaddassi S, Eyestone W, Bishop CE. TALEN-mediated modification of the bovine genome for large-scale production of human serum albumin. PLoS ONE. 2014;9(2):e89631. doi: 10.1371/journal.pone.0089631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proudfoot C, et al. Genome edited sheep and cattle. Transgenic Res. 2015;24(1):147–153. doi: 10.1007/s11248-014-9832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grange JM. Mycobacterium bovis infection in human beings. Tuberculosis (Edinb) 2001;81(1-2):71–77. doi: 10.1054/tube.2000.0263. [DOI] [PubMed] [Google Scholar]
- 23.Thoen C, Lobue P, de Kantor I. The importance of Mycobacterium bovis as a zoonosis. Vet Microbiol. 2006;112(2-4):339–345. doi: 10.1016/j.vetmic.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 24.Ayele WY, Neill SD, Zinsstag J, Weiss MG, Pavlik I. Bovine tuberculosis: An old disease but a new threat to Africa. Int J Tuberc Lung Dis. 2004;8(8):924–937. [PubMed] [Google Scholar]
- 25.Buddle BM, et al. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect Immun. 2003;71(11):6411–6419. doi: 10.1128/IAI.71.11.6411-6419.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan H, et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434(7034):767–772. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guschin DY, et al. A rapid and general assay for monitoring endogenous gene modification. Methods Mol Biol. 2010;649:247–256. doi: 10.1007/978-1-60761-753-2_15. [DOI] [PubMed] [Google Scholar]
- 28.Sanders KL, Catto LE, Bellamy SR, Halford SE. Targeting individual subunits of the FokI restriction endonuclease to specific DNA strands. Nucleic Acids Res. 2009;37(7):2105–2115. doi: 10.1093/nar/gkp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim E, et al. Precision genome engineering with programmable DNA-nicking enzymes. Genome Res. 2012;22(7):1327–1333. doi: 10.1101/gr.138792.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y, et al. TALE nickase mediates high efficient targeted transgene integration at the human multi-copy ribosomal DNA locus. Biochem Biophys Res Commun. 2014;446(1):261–266. doi: 10.1016/j.bbrc.2014.02.099. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez CL, et al. Engineered zinc finger nickases induce homology-directed repair with reduced mutagenic effects. Nucleic Acids Res. 2012;40(12):5560–5568. doi: 10.1093/nar/gks179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, et al. Targeted gene addition to a predetermined site in the human genome using a ZFN-based nicking enzyme. Genome Res. 2012;22(7):1316–1326. doi: 10.1101/gr.122879.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vordermeier HM, et al. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun. 2002;70(6):3026–3032. doi: 10.1128/IAI.70.6.3026-3032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waters WR, et al. Efficacy and immunogenicity of Mycobacterium bovis DeltaRD1 against aerosol M. bovis infection in neonatal calves. Vaccine. 2009;27(8):1201–1209. doi: 10.1016/j.vaccine.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollock JM, Rodgers JD, Welsh MD, McNair J. Pathogenesis of bovine tuberculosis: The role of experimental models of infection. Vet Microbiol. 2006;112(2-4):141–150. doi: 10.1016/j.vetmic.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 36.Morris RS, Pfeiffer DU, Jackson R. The epidemiology of Mycobacterium bovis infections. Vet Microbiol. 1994;40(1-2):153–177. doi: 10.1016/0378-1135(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 37.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 38.Flynn JL, Ernst JD. Immune responses in tuberculosis. Curr Opin Immunol. 2000;12(4):432–436. doi: 10.1016/s0952-7915(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 39.Delogu G, Li A, Repique C, Collins F, Morris SL. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect Immun. 2002;70(1):292–302. doi: 10.1128/IAI.70.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15(5):321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 42.Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188(4):773–782. doi: 10.1534/genetics.111.131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez EE, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26(7):808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carroll D. Genome engineering with targetable nucleases. Annu Rev Biochem. 2014;83:409–439. doi: 10.1146/annurev-biochem-060713-035418. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez CL, et al. Unexpected failure rates for modular assembly of engineered zinc fingers. Nat Methods. 2008;5(5):374–375. doi: 10.1038/nmeth0508-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JS, Lee HJ, Carroll D. Genome editing with modularly assembled zinc-finger nucleases. Nat Methods. 2010;7(2):91–, author reply 91–92. doi: 10.1038/nmeth0210-91b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sander JD, et al. ZiFiT (Zinc Finger Targeter): An updated zinc finger engineering tool. Nucleic Acids Res. 2010;38(Web Server issue):W462–468. doi: 10.1093/nar/gkq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mali P, et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013;31(9):833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu Y, et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31(9):822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cradick TJ, Fine EJ, Antico CJ, Bao G. CRISPR/Cas9 systems targeting β-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res. 2013;41(20):9584–9592. doi: 10.1093/nar/gkt714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pattanayak V, et al. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol. 2013;31(9):839–843. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu PD, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31(9):827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J, et al. RAG1/2 knockout pigs with severe combined immunodeficiency. J Immunol. 2014;193(3):1496–1503. doi: 10.4049/jimmunol.1400915. [DOI] [PubMed] [Google Scholar]
- 54.Lillico SG, et al. Live pigs produced from genome edited zygotes. Sci Rep. 2013;3:2847. doi: 10.1038/srep02847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66(4):1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasula R, et al. Surfactant protein A (SP-A) mediates attachment of Mycobacterium tuberculosis to murine alveolar macrophages. Am J Respir Cell Mol Biol. 1997;17(2):209–217. doi: 10.1165/ajrcmb.17.2.2469. [DOI] [PubMed] [Google Scholar]
- 57.Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155(11):5343–5351. [PubMed] [Google Scholar]
- 58.Leth-Larsen R, et al. A common polymorphism in the SFTPD gene influences assembly, function, and concentration of surfactant protein D. J Immunol. 2005;174(3):1532–1538. doi: 10.4049/jimmunol.174.3.1532. [DOI] [PubMed] [Google Scholar]
- 59.Garred P, Honoré C, Ma YJ, Munthe-Fog L, Hummelshøj T. MBL2, FCN1, FCN2 and FCN3-The genes behind the initiation of the lectin pathway of complement. Mol Immunol. 2009;46(14):2737–2744. doi: 10.1016/j.molimm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: Is death an exit strategy? Nat Rev Microbiol. 2010;8(9):668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vordermeier HM, et al. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin Diagn Lab Immunol. 1999;6(5):675–682. doi: 10.1128/cdli.6.5.675-682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Losi M, et al. European Tuberculosis Network TBNET Use of a T-cell interferon-gamma release assay for the diagnosis of tuberculous pleurisy. Eur Respir J. 2007;30(6):1173–1179. doi: 10.1183/09031936.00067307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.