Significance
The role of FLCN as a tumor suppressor in kidney cancer has been well documented, whereas the functional roles of folliculin (FLCN)-interacting proteins 1 and 2 (FNIP1 and FNIP2) in kidney are unknown. In this study, we demonstrate that double inactivation of Fnip1 and Fnip2 leads to enlarged polycystic kidneys or kidney cancer, which mimics the phenotypes seen in Flcn-deficient kidneys and underscores the significance of Fnip1 and Fnip2 in kidney tumor suppression. Moreover, we found that Fnip1/Fnip2 mRNA ratios differ among organs, which may reflect tissue-specific roles for each Fnip. Our findings define Fnip1 and Fnip2 as critical components of the Flcn complex that are essential for its tumor suppressive function and will aid in the development of novel therapeutics for kidney cancer.
Keywords: folliculin, FNIP1, FNIP2, Birt–Hogg–Dubé syndrome, kidney tumor
Abstract
Folliculin (FLCN)-interacting proteins 1 and 2 (FNIP1, FNIP2) are homologous binding partners of FLCN, a tumor suppressor for kidney cancer. Recent studies have revealed potential functions for Flcn in kidney; however, kidney-specific functions for Fnip1 and Fnip2 are unknown. Here we demonstrate that Fnip1 and Fnip2 play critical roles in kidney tumor suppression in cooperation with Flcn. We observed no detectable phenotype in Fnip2 knockout mice, whereas Fnip1 deficiency produced phenotypes similar to those seen in Flcn-deficient mice in multiple organs, but not in kidneys. We found that absolute Fnip2 mRNA copy number was low relative to Fnip1 in organs that showed phenotypes under Fnip1 deficiency but was comparable to Fnip1 mRNA copy number in mouse kidney. Strikingly, kidney-targeted Fnip1/Fnip2 double inactivation produced enlarged polycystic kidneys, as was previously reported in Flcn-deficient kidneys. Kidney-specific Flcn inactivation did not further augment kidney size or cystic histology of Fnip1/Fnip2 double-deficient kidneys, suggesting pathways dysregulated in Flcn-deficient kidneys and Fnip1/Fnip2 double-deficient kidneys are convergent. Heterozygous Fnip1/homozygous Fnip2 double-knockout mice developed kidney cancer at 24 mo of age, analogous to the heterozygous Flcn knockout mouse model, further supporting the concept that Fnip1 and Fnip2 are essential for the tumor-suppressive function of Flcn and that kidney tumorigenesis in human Birt–Hogg–Dubé syndrome may be triggered by loss of interactions among Flcn, Fnip1, and Fnip2. Our findings uncover important roles for Fnip1 and Fnip2 in kidney tumor suppression and may provide molecular targets for the development of novel therapeutics for kidney cancer.
Germline alteration of the folliculin (FLCN) gene, a novel tumor suppressor, is responsible for Birt–Hogg–Dubé (BHD) syndrome, an inherited kidney cancer syndrome characterized by cutaneous fibrofolliculomas, pulmonary cysts, and an increased risk for the development of kidney cancer (1–4). Genetic studies using Flcn knockout mice have defined important roles for Flcn in metabolism. Kidney-targeted Flcn knockout mice developed enlarged polycystic kidneys with elevated mechanistic target of rapamycin complex 1 (mTORC1) activity (5) and increased mitochondrial biogenesis through up-regulated peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Ppargc1a) (6). Muscle-targeted Flcn knockout mice displayed both red-colored muscle with increased mitochondrial biogenesis caused by elevated Ppargc1, and cardiac hypertrophy with up-regulated mTORC1, which were ameliorated by Ppargc1a inactivation, suggesting that Flcn might serve as a critical link connecting mTORC1 with Ppargc1a-driven mitochondrial biogenesis (5–7). Importantly, mice heterozygous for Flcn inactivation develop renal tumors at 24 mo of age with increased mTORC1/2 activity and Ppargc1a expression, which mimics the human BHD tumor phenotype (6, 8–10), suggesting potential therapeutic targets in metabolic pathways for BHD-associated kidney cancer.
The first FLCN interacting protein FNIP1 was identified through protein–protein interaction studies of the FLCN protein (11). FNIP1 binds to the C terminus of FLCN and to AMP-activated protein kinase (AMPK) (11), a critical molecule for energy sensing, further underscoring a central role for the FLCN/FNIP1 pathway in cellular metabolism. A second folliculin-interacting protein FNIP2 was discovered through bioinformatics searches for sequences similar to FNIP1 (12, 13). Similar to FNIP1, FNIP2 was found to bind to the C terminus of FLCN and to AMPK (12), suggesting a potential functional redundancy with FNIP1. Recent studies with Fnip1 knockout mouse models have demonstrated that Fnip1 is required for B-cell development (14, 15). Interestingly, a Flcn knockout mouse model using the tamoxifen-inducible ER (mutated form of the ligand-binding domain of the estrogen receptor)-Cre system also displayed defects in B-cell development (14), suggesting Fnip1 knockout mice might develop phenotypes similar to those that develop as a consequence of Flcn deficiency.
Furthermore, FLCN has been shown to have a variety of functions that might potentially link AMPK, mTOR, and Ppargc1a with other important pathways. Crystallographic studies have shown that the C terminus of FLCN may be distantly related to Differentially Expressed in Normal Cells and Neoplasia (DENN) domain proteins and may possess guanine nucleotide exchange factor activity toward RAB35 (16). FLCN modulates TFE3 localization (17), which may play an important role in the exit of stem cells from pluripotency (18), and interacts with other signaling pathways including the von Hippel-Lindau–hypoxia inducible factor–vascular endothelial growth factor axis (19–21), the TGF-beta pathway (22, 23), Rho A signaling (24, 25), cell cycle regulation (26, 27), Rag-mediated amino acid sensing (28, 29), and autophagy (30, 31). These findings underscore FLCN as an important molecule, inactivation of which affects multiple pathways.
To clarify the function of FLCN-interacting proteins Fnip1 and Fnip2, we inactivated Fnip1 and/or Fnip2 in mouse kidneys, muscle, and heart and investigated the effect on the mTOR pathway and mitochondrial metabolism. The absolute mRNA copy number of Fnip1 and Fnip2 was measured using droplet digital PCR (ddPCR) technology. To evaluate functional synergy of Fnip1 and Fnip2 with Flcn, we also inactivated Flcn, Fnip1, and Fnip2 simultaneously in mouse kidneys. Finally, we searched for latent tumor development in Fnip1 and Fnip2 knockout mice.
Results
Neither Kidney-Targeted Fnip1 nor Fnip2 Knockout Mice Develop a Kidney Phenotype.
To investigate Fnip1 function in mouse kidney, we crossbred mice carrying loxP-flanked Fnip1 alleles (floxed, f) (14) with cadherin 16 (CDH16)-Cre transgenic mice, which express Cre recombinase driven by the CDH16 promoter, thereby deleting Fnip1 gene sequences specifically in kidney. We observed no significant phenotype in the Fnip1-deficient kidneys except occasional tiny cysts (Fig. 1A). Therefore, we decided to analyze Fnip2 function in kidney by generating a Fnip2 conditional mouse carrying loxP-flanked Fnip2 alleles (floxed, f) (Fig. 1 B–D) and crossbreeding with CDH16-Cre transgenic mice. However, kidney-targeted Fnip2 inactivation also did not cause any phenotype in mouse kidney (Fig. 1E). Indeed, we could not find any phenotype in whole-body Fnip2 knockout mice that affected life span.
Fig. 1.
Neither kidney-targeted Fnip1 nor Fnip2 knockout mice show a kidney phenotype. (A) Conditional Fnip1 knockout mice were crossbred with CDH16-Cre transgenic mice. Inactivation of Fnip1 mRNA was confirmed by real-time PCR. n = 6 each at 3 wk of age. Mean ± SD. Student t test (Left). The size of the Fnip1-deficient kidney was not significantly different from that of the control kidney. n = 11 each at 3 wk of age. Mean ± SD. Student t test (Middle). Representative H&E staining of 3-wk old control and Fnip1-deficient kidneys did not show differences except for infrequent tiny cysts (Right). (B) Fnip2 gene-targeting vector was constructed by recombineering methodology using homologous recombination. A neomycin resistance (Neo r) cassette flanked by Frt (bar) and loxP (triangle) sequences was inserted into intron 11 for positive selection, and the thymidine kinase gene was included for negative selection. A second loxP sequence was inserted into intron 13. Correctly targeted embryonic stem cells were identified by Southern blot analysis and injected into blastocysts to produce chimeras. Backcrossing to C57BL/6 mice produced heterozygous F1 offspring with germline transmission of the Fnip2 floxed (f)-Neo allele. The Neo cassette flanked by Frt sites was excised in vivo by crossing with mice expressing the Flp recombinase transgene under the β-actin promoter. To produce the Fnip2 deleted (d) allele, Fnip2 f/+ mice were crossed with mice expressing the Cre recombinase transgene under the ubiquitous β-actin promoter. Deletion of exon 12 and 13 resulted in a frameshift and premature termination codon in exon 14, which was predicted to cause mRNA degradation by the nonsense-mediated decay mRNA surveillance system. (C) The targeted embryonic stem cells were screened by Southern blotting of BamH1- and EcoRV-digested DNA, using two different external probes located outside the targeting sequence, as shown in B. (D) PCR-based genotyping was performed using DNA extracted from mouse tails for routine monitoring of inheritance in offspring. Locations of PCR primers are indicated by arrows. (E) Kidney-specific inactivation of Fnip2 was achieved by crossing with CDH16-Cre transgenic mice. Inactivation of Fnip2 mRNA was confirmed by real-time PCR. n = 6 each at 3 wk of age. Mean ± SD. Student t test (Left). Size of Fnip2-deficient kidney was not significantly different from that of control kidney. n = 6 each at 3 wk of age. Mean ± SD. Student t test (Middle). Representative H&E staining of 3-wk old control and Fnip2-deficient kidneys did not show any difference in histology (Right).
The Relative Expression Levels of Fnip1 and Fnip2 Differ from Organ to Organ.
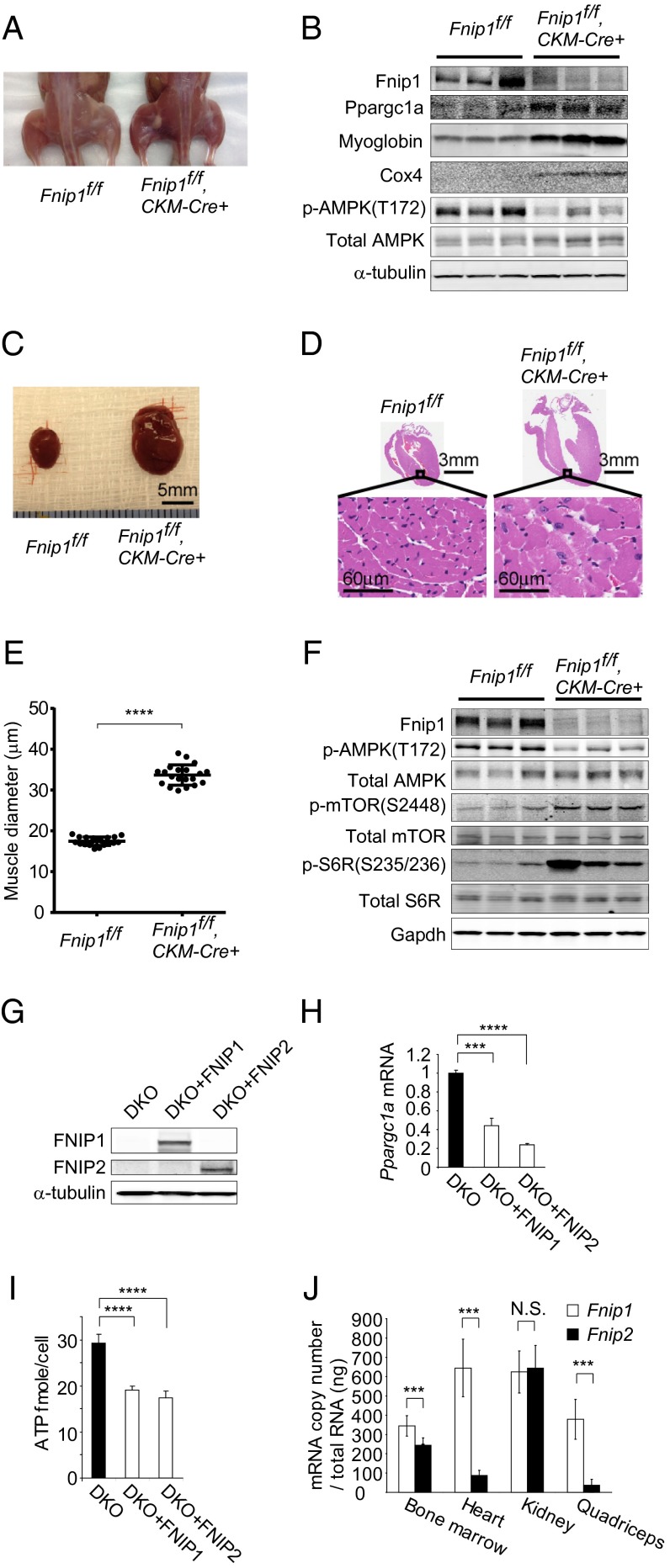
Previously, we reported that Fnip1 knockout mice showed B-cell developmental defects, which were also observed in Flcn knockout mice using the tamoxifen-inducible ER-Cre system (14), suggesting Fnip1 knockout mice might show phenotypes similar to those resulting from Flcn deficiency. In addition to the B-cell phenotype, we found similar Flcn-deficient and Fnip1-deficient phenotypes in skeletal muscle and heart. Muscle-targeted Fnip1 knockout mice showed red-colored muscle with increased mitochondrial biogenesis (myoglobin and cox4 readouts; Fig. 2 A and B), as well as cardiac hypertrophy with elevated mTORC1 activity (Fig. 2 C–F), which we had previously observed in muscle-targeted Flcn knockout mice (6, 32). Sequence similarity between FNIP1 and FNIP2 and the shared interaction of FNIP1 and FNIP2 with FLCN and AMPK (12) implied that FNIP1 and FNIP2 might be functionally redundant. In support of this, we observed that expression of either FNIP1 or FNIP2 in Fnip1/Fnip2 null mouse embryonic fibroblasts (MEFs) suppressed Ppargc1a mRNA and ATP production (Fig. 2 G–I). Because of the potential functional redundancies between Fnip1 and Fnip2, we postulated that Fnip1 and Fnip2 expression might differ from organ to organ and that this variable expression may determine the specific roles for Fnip1 and Fnip2 in those tissues. The recent technology of ddPCR enabled us to measure absolute mRNA copy number. Using this technology, we compared the absolute copy number of Fnip1 and Fnip2 mRNA in wild-type mouse tissues. Interestingly, we observed dominant expression of Fnip1 in heart, skeletal muscle, and bone marrow, the tissues in which we observed phenotypes in Fnip1 knockout mice, whereas there was no significant difference between Fnip1 and Fnip2 mRNA copy number in kidney (Fig. 2J), in agreement with the absence of kidney phenotypes in Fnip1 and Fnip2 knockout mice. These data support the idea that in kidney, the Fnip2 expression level, which is commensurate with that of Fnip1, might maintain Fnip function in Fnip1-deficient kidneys, and therefore, double inactivation of Fnip1 and Fnip2 would be necessary to develop a kidney-specific phenotype.
Fig. 2.
Fnip1 and Fnip2 expression differs from organ to organ. (A) Muscle-targeted Fnip1 knockout mice show red-colored muscle relative to control muscle (6 wk of age). (B) Western blotting shows increased mitochondrial biogenesis and decreased pAMPK in Fnip1-deficient muscle. α-tubulin served as a loading control. n = 3 each at 6 wk of age. (C) Fnip1-deficient heart is enlarged relative to control heart (8 wk of age). (Scale bar: 5 mm.) (D) Histology of Fnip1-deficient heart shows enlarged cardiac fibers (8 wk of age). (Scale bars: 3 mm and 60 μm.) (E) Muscle diameter of Fnip1-deficient hearts was increased. n = 20 each at 8 wk of age. P < 0.0001, Student t test. (F) Western blotting of Fnip1-deficient hearts showed decreased pAMPK and activated mTORC1 signaling molecules. Gapdh served as a loading control. n = 3 each at 6 wk of age. (G) Western blotting shows the restoration of either FNIP1 or FNIP2 in Fnip1/Fnip2 null MEFs (DKO). α-tubulin served as a loading control. (H) Restoration of either FNIP1 or FNIP2 suppressed Ppargc1a expression in Fnip1/Fnip2 null MEFs (DKO). n = 3 each. Mean ± SD. P < 0.001 for DKO+FNIP1, P < 0.0001 for DKO+FNIP2; Student t test. (I) Restoration of either FNIP1 or FNIP2 suppressed ATP levels in Fnip1/Fnip2 null MEFs (DKO). n = 6 each. Mean ± SD. P < 0.0001. Student t test. (J) ddPCR showed Fnip1 expression was significantly higher than Fnip2 expression in bone marrow, heart, and quadriceps of C57BL/6 mice but was not significantly higher than Fnip2 expression in kidney of C57BL/6 mice. n = 6 each at 6 wk of age. Mean ± SD. P < 0.001 for bone marrow, heart, quadriceps. N.S., not significant. Student t test.
Kidney-Targeted Fnip1/Fnip2 Double-Knockout Mice Develop Enlarged Polycystic Kidneys.
Strikingly, kidney-specific inactivation of both Fnip1 and Fnip2 resulted in enlarged kidneys (Fig. 3A). MRI imaging revealed multiple round-shaped structures in the Fnip1/Fnip2 double-deficient kidneys (Fig. 3B), which were confirmed by H&E staining to be polycystic lesions (Fig. 3C) displaying hyperplastic cells that protruded into the cystic lumen (Fig. 3D). Kidney-specific Fnip1/Fnip2 double-knockout mice showed a significantly increased kidney/body weight ratio (n = 12; percentage kidney/body weight ratio mean = 11.04%; P < 0.001) (Fig. 3E). The survival time of kidney-targeted Fnip1/Fnip2 double-knockout mice was about 3 wk (n = 14; median survival = 20 d; P < 0.0001) (Fig. 3F). The enlarged polycystic kidneys of kidney-targeted Fnip1/Fnip2 double-deficient mice were reminiscent of the kidney phenotype observed in Flcn-deficient kidneys (5), underscoring the phenotypic similarities between these two genotypes.
Fig. 3.
Kidney-targeted Fnip1/Fnip2 double-knockout mice develop enlarged polycystic kidneys. (A) Fnip1 and Fnip2 alleles were deleted specifically in kidney using CDH16-Cre transgenic mice. Double inactivation of Fnip1 and Fnip2 targeted to the kidney resulted in enlarged kidneys relative to the controls (3 wk of age). (B) T2 weighted images (T2WI) of MRI show multiple round-shaped structures in Fnip1/Fnip2 double-knockout kidneys at 3 wk of age (Left). Striations of medulla and renal pelvis are seen (Right). (C) H&E staining shows enlarged polycystic kidneys in 3-wk-old kidney-specific Fnip1/Fnip2 double-knockout mice. (Scale bars: 500 μm.) (D) H&E staining reveals detailed histology of kidneys from 3-wk-old kidney-targeted Fnip1/Fnip2 double-knockout mice displaying hyperplastic cells protruding into the lumen (arrow) within the medulla (Upper). Normal glomeruli (G) and proximal renal tubules (P) were observed in the cortex (Lower). (Scale bars: 50 μm and 20 μm.) (E) Kidney-specific Fnip1/Fnip2 double-knockout mice show an increased kidney/body weight ratio. Homozygous Fnip1/heterozygous Fnip2 double-knockout mice show a slightly increased kidney/body weight ratio as a result of occasionally observed tiny cysts. Mean ± SD. Two-sided Student t test. Three weeks of age. (F) Survival curve of kidney-specific Fnip1/Fnip2 double-knockout mice. Proportion surviving ± SD. Log rank test. n = 14 each at 3 wk of age.
Fnip1/Fnip2 Double-Deficient Kidneys Are Identical to Flcn-Deficient Kidneys.
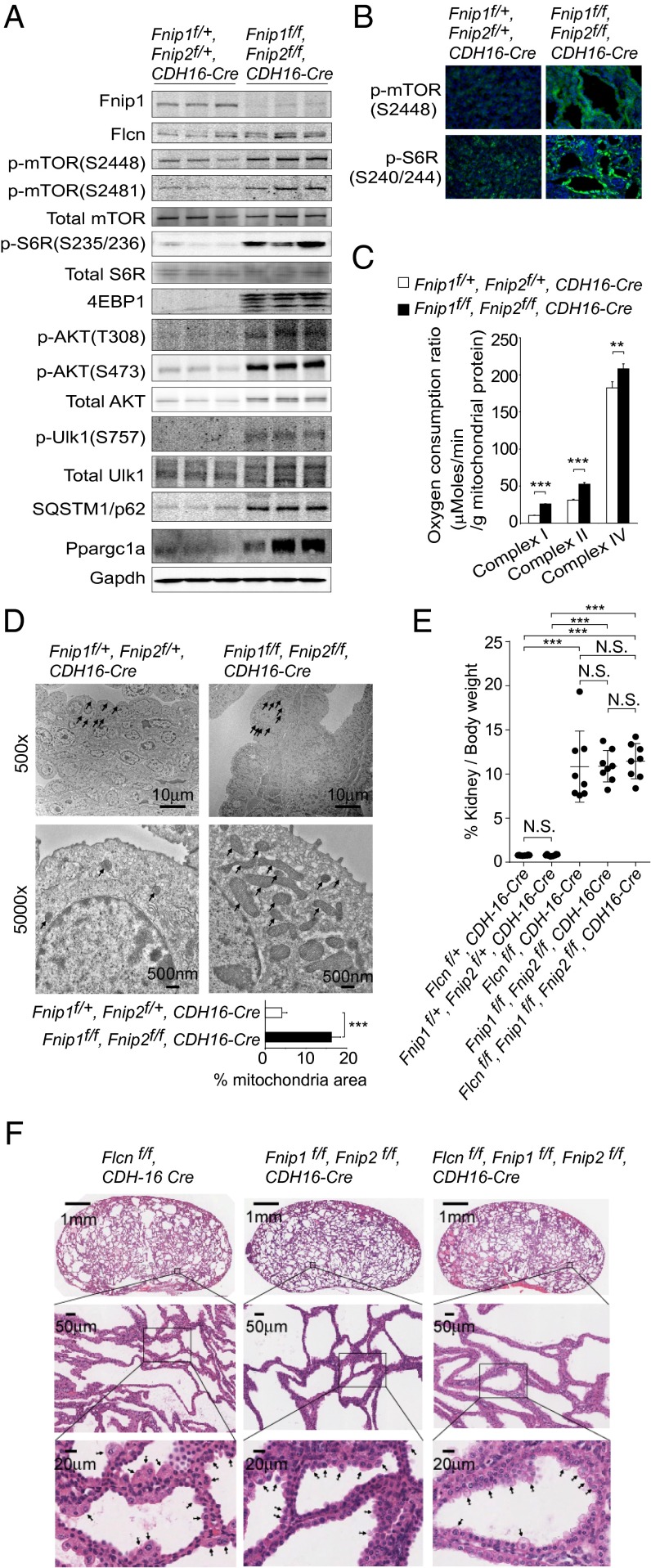
Previously we observed increased mTOR activity (5) and Ppargc1a-driven mitochondrial biogenesis in Flcn-deficient kidneys (6). In fact, Fnip1/Fnip2 double-deficient kidneys showed increased protein expression of Ppargc1a and signaling molecules in the mTOR pathway, including the downstream target of mTORC1, phospho-Ulk1 at Ser757, that suppresses autophagy, which was confirmed by the accumulation of sequestosome-1 (SQSTM1)/p62 (Fig. 4 A and B). Increased respiratory capacity (n = 4 each; P < 0.001) (Fig. 4C) and increased mitochondrial surface area (13 cells each; P < 0.001) (Fig. 4D) were also observed in the Fnip1/Fnip2 double-deficient kidneys. These data further support the concept that double inactivation of Fnip1/Fnip2 in kidney cells mirrors the same phenotype as that associated with kidney-targeted Flcn inactivation. We next asked whether the enlarged polycystic kidney phenotype in kidney-targeted Fnip1/Fnip2 double-knockout mice developed through the same pathway that had produced the identical phenotype in kidney-specific Flcn knockout mice. To answer this question, we crossbred kidney-specific Fnip1/Fnip2 double-knockout mice with kidney-specific Flcn knockout mice to see whether Flcn inactivation might have a synergistic effect on Fnip1/Fnip2 doubly inactivated kidneys. In fact, Flcn inactivation did not enhance the size (n = 8; P = 0.554) (Fig. 4E) or alter the histology (Fig. 4F) of Fnip1/Fnip2 double-deficient kidneys, suggesting that the enlarged polycystic kidney phenotypes of kidney-specific Fnip1/Fnip2 double-knockout mice and kidney-specific Flcn knockout mice developed through the same pathway, possibly triggered by loss of an important functional interaction among Flcn, Fnip1, and Fnip2.
Fig. 4.
Fnip1/Fnip2 double-deficient kidneys are identical to Flcn-deficient kidneys. (A) Western blotting shows increased Ppargc1 and phospho-proteins of the AKT-mTOR pathway in Fnip1/Fnip2 double-deficient kidney. Increased phospho-Ulk1 at Ser757, one of the readouts of mTORC1 activity, correlates with the accumulated SQSTM1/p62, normally degraded by autophagy. Mouse Fnip2 protein is not shown because of technical difficulty developing a unique Fnip2 antibody that does not cross-react with mouse Fnip1. Gapdh served as a loading control. n = 3 at 3 wk of age. (B) Immunofluorescence shows increased staining of p-mTOR (S2448) and pS6R (S240/244) in Fnip1/Fnip2 double-deficient kidney relative to control kidney. Nuclei were stained with DAPI (Blue). Representative of three mice at 3 wk of age. (C) Respiratory capacity of isolated mitochondria is increased in Fnip1/Fnip2 double-deficient kidney relative to control kidney. State 3 respiration of complex I and complex II, and complex IV-dependent respiration, were measured by Seahorse XF96 analyzer. Mean ± SD. n = 4 at 3 wk of age. Student t test (two-sided). (D) Electron microscope images show increased mitochondrial mass in Fnip1/Fnip2 double-deficient kidney compared with control kidney. Arrows indicate mitochondria. (Scale bars: 10 μm and 500 nm.) Percentage of mitochondrial area per cell was quantified for the indicated genotypes. Thirteen cells were evaluated for each genotype. Mean ± SD. Student t test (two-sided). Three weeks of age. (E) The kidney/body weight ratios of 3-wk-old mice with Flcn-deficient, Fnip1/Fnip2 double-deficient, and Flcn/Fnip1/Fnip2 triple-deficient kidneys show no significant differences. Mean ± SD, n = 8 each. Student t test (two-sided). (F) The histologies of kidneys from 3-wk-old Flcn-deficient, Fnip1/Fnip2 double-deficient, and Flcn/Fnip1/Fnip2 triple-deficient mice show no differences. Arrows indicate hyperplastic cells protruding into the cyst lumens that were observed in all of the genotypes. (Scale bars: 1 mm, 50 μm, and 20 μm.)
Fnip1/Fnip2 Double-Knockout Mice Develop Kidney Cancer.
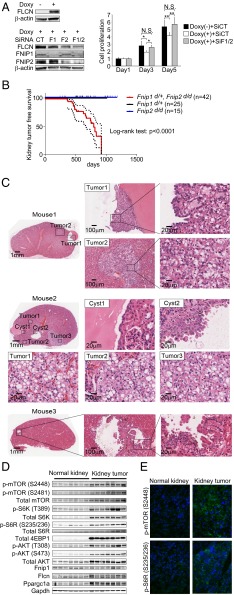
Our previous finding that heterozygous Flcn knockout mice developed kidney tumors by 24 mo of age after the loss of the remaining Flcn allele, thereby mimicking human BHD renal tumorigenesis, strongly supports a tumor-suppressive role for FLCN in kidney and underscores FLCN as a classical tumor suppressor (8, 9). Because Fnip1/Fnip2 double deficiency in kidney resulted in the identical enlarged polycystic kidney phenotype seen under Flcn deficiency, and knockdown of FNIP1/FNIP2 was permissive for proliferative cell growth in a FLCN-restored human BHD-associated kidney cancer cell line (Fig. 5A), we postulated that double inactivation of Fnip1 and Fnip2 might lead to tumor development in the kidney. Double homozygous inactivation of Fnip1 and Fnip2 in whole body resulted in embryonic lethality (Table 1), further supporting the essential nature of the Fnips and the similarity between Flcn and Fnip1/Fnip2 double-deficient phenotypes. Homozygous Fnip1 knockout mice did not survive long enough to observe latent tumorigenesis (median survival = 292 d) because of severe immunodeficiency resulting from B-cell developmental defects (14). Therefore, we decided to achieve double inactivation of Fnip1 and Fnip2 by heterozygous knockdown of Fnip1 together with homozygous knockdown of Fnip2. Strikingly, these mice developed kidney cancer without developing any particular extrarenal phenotype, whereas neither heterozygous Fnip1 knockout mice nor homozygous Fnip2 knockout mice displayed a kidney tumor phenotype (n = 42; median kidney tumor-free survival = 796 d; P < 0.0001) (Fig. 5B). Kidney cancer in human BHD syndrome presents as multiple tumors with a variety of histologies including hybrid oncocytic tumors, chromophobe renal cell carcinoma, oncocytoma, papillary renal cell carcinoma, and clear cell renal cell carcinoma (4). Indeed, we observed multiple kidney tumor lesions with a variety of histologies in heterozygous Fnip1/homozygous Fnip2 double-knockout mice, most of which were hybrid oncocytic tumors (Fig. 5C). We observed increased protein expression of mTORC1/2 pathway members and Ppargc1a in these tumors by Western blot analysis (Fig. 5D) and immunostaining (Fig. 5E), further supporting the idea that these tumors might develop through the same pathway as tumors that develop in the heterozygous Flcn knockout mouse model.
Fig. 5.
Fnip1/Fnip2 double-knockout mice develop kidney cancer. (A) Double knockdown of FNIP1 and FNIP2 in human kidney cancer cell line caused increased cell proliferation. UOK257 FLCN-null kidney cancer cell line was reconstituted with wild-type FLCN in a doxycycline-inducible manner (Upper Left). FNIP1 and FNIP2 expression was knocked down with siRNA (Lower Left). Cell proliferation was evaluated using MTT assay at days 1, 3, and 5 (Right). CT, Control siRNA; F1, FNIP1 siRNA; F2, FNIP2 siRNA; F1/2, FNIP1 siRNA + FNIP2 siRNA. Mean ± SD. Student t-test (two-sided). n = 5 each. N.S., no significance. (B) Kidney tumor-free survival demonstrates that heterozygous Fnip1/homozygous Fnip2 double-knockout mice developed renal tumors at a median age of 796 d. Neither heterozygous Fnip1 knockout mice nor homozygous Fnip2 knockout mice developed kidney cancer. Kidney tumor-free survival ± SD. Log rank test. n = 25, 15, and 42 for Fnip1d/+, Fnip2d/d, and Fnip1d/+/Fnip2d/d, respectively. (C) H&E staining shows kidney tumor development in heterozygous Fnip1/homozygous Fnip2 double-knockout mice. Tumor developed from cyst wall (Tumor1 of Mouse1, 692 d old) or within the kidney without prominent infiltration (Tumor2 of Mouse1). Cells lining the cyst walls (Cyst1 of Mouse2) were occasionally piled up (Cyst2 of Mouse2, 670 d old). The most frequent histology was the hybrid oncocytic tumor (Tumor1–Tumor3 of Mouse2). Papillary projections from the cyst wall were occasionally observed (Mouse3, 699 d old). (Scale bars: 1 mm, 100 μm, and 20 μm.) (D) Western blotting shows increased Ppargc1a and phospho-proteins of the AKT-mTOR pathway in kidney tumors from Fnip1/Fnip2 double-knockout mice. Gapdh served as a loading control. n = 7 each, 628–699 d old. (E) Immunostaining of kidney tumors from Fnip1/Fnip2 double-knockout mice demonstrate increased phospho-mTOR and pS6R (downstream readout of mTOR) compared with adjacent normal kidney.
Table 1.
Neonates from matings of Fnip1d/+ Fnip2d/d males and females
| Genotype | Neonates | Percentage neonates with genotype |
| Fnip1wt/wt Fnip2d/d | 47 | 36 |
| Fnip1d/+ Fnip2d/d | 85 | 64 |
| Fnip1d/d Fnip2d/d | 0 | 0 |
| Total | 132 | 100 |
Fnip1d/+ Fnip2d/d males and Fnip1d/+ Fnip2d/d females were mated, and 132 neonates were obtained from 53 litters. No Fnip1d/d Fnip2d/d neonates were observed.
Discussion
Here we report kidney tumor-suppressive roles for Fnip1 and Fnip2 in cooperation with Flcn. Fnip1/Fnip2 double inactivation targeted to mouse kidney resulted in an enlarged multicystic kidney phenotype shortly after birth, which was identical to the phenotype observed in Flcn-deficient kidneys (5). The ratio of absolute Fnip1 to Fnip2 mRNA copy number was high in the organs that demonstrated a phenotype after Fnip1 inactivation, whereas in kidney where no Fnip1-deficient or Fnip2-deficient phenotype was observed, the absolute Fnip2 mRNA copy number was comparable to that of Fnip1. Therefore, these data suggest that the ratio of absolute Fnip1 to Fnip2 mRNA copy number may determine the function of each Fnip in a particular organ. Moreover, whole-body heterozygous Fnip1/homozygous Fnip2 double-knockout mice developed kidney tumors at 24 mo of age, implying that loss of interaction between Flcn, Fnip1, and Fnip2 may trigger kidney cancer development.
According to protein sequence, FNIP1 and FNIP2 show 49% identity and 74% similarity (12), suggesting possible functional redundancy. Double homozygous inactivation of Fnip1 and Fnip2 specifically in mouse kidney resulted in an enlarged polycystic kidney phenotype. However, expression of one allele of either Fnip1 or Fnip2 in kidney-targeted Fnip1/Fnip2 knockout mice was sufficient to rescue this phenotype, suggesting Fnip1 and Fnip2 may have functional redundancy. Because worms and flies have only one Fnip protein (12), it is possible that a second Fnip with overlapping functions evolved from the primary Fnip to ensure redundancy and conserve its critical role in regulating kidney cell proliferation through its interaction with Flcn.
In contrast, the differences between FNIP1 and FNIP2 amino acid sequences imply potentially distinct functions for each FNIP. Therefore, the nonoverlapped functions of the FNIPs, as well as additional overlapping functions, will need to be elucidated in future experiments.
Crystallographic studies revealed that the C terminus of FLCN shows distant homology to DENN domain proteins, a family of GDP–GTP exchange factors that activate Rab GTPases involved in membrane trafficking in eukaryotes (16). Notably, a subsequent bioinformatics study reported that FNIP1 and FNIP2 also have novel DENN modules (33), raising the possibility that complex assembly of FLCN-FNIP1 or FLCN-FNIP2 might form a noncanonical DENN module critical for GDP–GTP exchange that would suffer functional arrest if any of the components were absent. Disruption of a noncanonical DENN module, whose conformation may be controlled by Flcn/Fnip1 or Flcn/Fnip2 interactions and may be essential for regulation of proper kidney cell proliferation rates, might result in development of the polycystic kidney phenotype in our kidney-targeted in vivo models.
After FLCN was identified as a two-hit tumor suppressor gene for BHD-associated chromophobe, hybrid oncocytic, and clear cell kidney cancers, a search for FLCN mutations in a broad spectrum of sporadic kidney tumors was conducted, but genetic analyses of these samples only rarely identified mutations in the FLCN gene (34, 35). Somatic FNIP1 or FNIP2 mutations have been detected in several cancers by whole-exome sequencing as part of The Cancer Genome Atlas (TCGA) project. Sequencing efforts by TCGA project detected infrequent FNIP1/FNIP2 mutations in urologic cancers, including clear cell renal carcinomas (7/424; 1.7%) and bladder cancer (7/130; 5.4%), and in all but one of the urologic cancer samples, the tumors had a single mutation of either FNIP1 or FNIP2 (36). In comparison, uterine corpus endometrioid cancer displayed the highest percentage of tumors with FNIP1 and FNIP2 alterations (17/240; 7.1%), of which 5 (2.1%) had mutations in both genes (37). Infrequent somatic mutations of FLCN, FNIP1, and FNIP2 genes in sporadic kidney tumors indicate that genetic alteration of these genes is not the direct cause of sporadic kidney tumorigenesis, but rather, that the status of the FLCN/FNIP1 or FLCN/FNIP2 interactions might be critical for sporadic kidney tumor suppression. Protein–protein interaction studies of sporadic kidney cancers would be important to elucidate the functional status of the FLCN/FNIP1/FNIP2 complex in sporadic kidney cancer, especially in sporadic chromophobe renal cell carcinoma and oncocytoma, which are the most frequent histologic subtypes observed in human BHD syndrome (Fig. S1).
The findings of this study, which characterize the phenotype of Fnip1, Fnip2, and Fnip1/Fnip2 knockout mouse models in multiple organs, further our understanding of the Flcn tumor suppressor pathway and underscore important roles for Flcn/Fnip1/Fnip2 interactions in inhibiting kidney cancer development. These data may lead to the development of novel diagnostics and therapeutics for kidney cancer that target the FLCN-FNIP pathway.
Materials and Methods
Animals.
Mice carrying Flcn alleles and Fnip1 alleles flanked by loxP sites (floxed, f) and mice carrying a Fnip1 deleted (d) allele were generated as previously described (5, 14). Mice carrying Fnip2 alleles flanked by loxP sites (floxed, f) and mice carrying a Fnip2 deleted (d) allele were generated using the same strategy (5, 14). Briefly, the Fnip2 targeting vector was generated by inserting a neomycin resistance cassette flanked by Frt and loxP sequences into intron 11 of Fnip2 and inserting a second loxP sequence into intron 13. Deletion of exon 12 and exon 13 in the Fnip2d/+ mice resulted in a reading frameshift and premature termination codon in exon 14. CDH16-Cre transgenic mice, which express Cre recombinase under the cadherin 16 (CDH16) promoter specifically in adult renal tubules and developing genitourinary tract (38), were crossed with mice carrying floxed (f) alleles of Flcn, Fnip1, and Fnip2 to inactivate those genes. Because we did not observe any phenotypic difference between Fnip1f/f, Fnip2 f/f, CDH16-Cre and Fnip1f/d, Fnip2f/d, CDH16-Cre mice, we used these two genotypes interchangeably throughout these studies. Muscle-targeted inactivation of Fnip1 was done by crossing Fnip1f/f mice and CKM-Cre transgenic mice (Jackson Laboratories). Mice were housed in National Cancer Institute animal facilities and killed by CO2 asphyxiation for analyses according to the National Cancer Institute–Frederick Animal Care and Use Committee guidelines. Animal care procedures followed the National Cancer Institute Animal Care and Use Committee guidelines.
Southern Blot Analysis of Embryonic Stem Cells and PCR Genotyping of Fnip2 Knockout Mice.
KOD Hot DNA polymerase (Novagen) was used for generating probes and routine PCR genotyping. A 5′ external probe for Southern blot analysis of targeted embryonic stem cells was generated by PCR with the following primers: forward, 5′-GAACCAAGCGGGGAGAATCGAA-3′; reverse, 5′-GCAAAGGCTGAACCCGTTACCA-3′. A 3′ external probe was also generated by PCR with the following primers: forward, 5′-TCAGGCCAAACCATAGCCTCA-3′; reverse, 5′-TCAGACTCAGCTGGGGAACGAA-3′. Genomic DNA was isolated from tail samples of mice using DirectPCR Reagent (Viagen Biotech, Inc.) according to manufacturer’s protocols. Nonradioactive Southern blotting was performed with DIG OMNI System for PCR probes (Roche Molecular Biochemicals) according to the manufacturer’s protocol. PCR genotyping was performed with three primer sets to amplify wild-type (146-bp PCR product), floxed (238-bp PCR product), and deleted (457-bp PCR product) Fnip2 alleles: P1, 5′-ATGCTAGTGAGGAGGAGCCATTG-3′, P2, 5′-AGGACAGAGAAAGCACGTGCTAG-3′ and P3, 5′-AGTGTGCCACTTCCTTCTGGTCG-3′. We confirmed that the CDH16-Cre transgenes had no detectable effect on mouse phenotypes.
ddPCR and Real-Time PCR.
mRNA was extracted from bone marrow, heart, kidney, and quadriceps of C57BL/6, using TRIzol (Invitrogen), and 500 ng mRNA was transcribed into cDNA, using SuperScript III reverse transcriptase (Invitrogen) with 10 μL scale. A microliter of cDNA was used to make droplets using the QX100 ddPCR system (Bio-Rad), and droplets were amplified and analyzed with the Taqman assays for Fnip1 (Mm00620486_m1) labeled with FAM and for Fnip2 (Mm01220192_m1) labeled with VIC. Real-time PCR was done as previously described (6). Primer sequences are as follows: mouse Fnip1-forward, 5′-cacagttagtaatgggctgcttgg-3′; mouse Fnip1-reverse, 5′-ctgcaaagaaagaggcactcctg-3′; mouse Fnip2-forward, 5′-tttgctgccttactgactgcggtg-3′; mouse Fnip2-reverse, 5′-cgagaaggctttaatgggagggtg-3′; mouse Ppargc1a-forward, 5′-atgaccctcctcacaccaaacccacag-3′; mouse Ppargc1a-reverse, 5′-cttgagcatgttgcgactgcggttgtg-3′; mouse β-actin forward, 5′-gacatggagaagatctggca-3′; and mouse β-actin reverse, 5′-ggtctcaaacatgatctgggt-3′.
MRI Imaging.
T2-weighted images were obtained using a fast spin echo sequence (rapid acquisition of relaxation enhancement) with an echo time of 13 ms and a repetition time of 2,500 ms by a 7 T MRI scanner controlled with ParaVision 5.0 (Bruker BioSpin MRI GmbH).
Western Blotting, Immunofluorescence Staining, and Antibodies.
Frozen kidney samples were homogenized in RIPA buffer (20 mM Tris⋅HCl at pH 7.5, 150 mM NaCl, 1 mM EDTA, 1.0% Triton X-100, 0.5% deoxycholate, 0.1% SDS) supplemented with Complete protease inhibitor mixture and PhosStop phosphatase inhibitor mixture (Roche). For immunoblotting, 20 μg protein was loaded in each well. Immunofluorescence staining of kidney samples was done as previously reported (5, 9). Antibodies used for Western blotting included phospho-mTOR (Ser2448), phospho-mTOR (Ser2481), total mTOR, total AKT, phospho-AKT (Ser473), phospho-AKT (Thr308), phospho-S6K (Thr389), total S6K, 4EBP1, phospho-S6R(Ser235/236), total S6R, phospho-Ulk1(Ser757), total Ulk1, SQSTM1/p62, phospho-AMPK (Thr172), total AMPK, FLCN, GAPDH, β-actin (all from Cell Signaling), FNIP1 (11), FNIP2 (12) at 1:1,000 dilution, and PPARGC1A (H300), myoglobin (Santa Cruz Biotechnology) at 1:200 dilution. Phospho-mTOR (Ser2448), pshopho-S6R (Ser235/236), and COX4 antibodies (Cell Signaling) were used for immunofluorescence at 1:100 dilution. Antibody–protein complexes were detected using Odyssey imager (LI-COR biotechnology). Because of high sequence similarity between mouse Fnip1 and Fnip2, developing an antibody that detects mouse Fnip2 but does not cross-react with mouse Fnip1 was technically difficult and unsuccessful.
Electron Microscopy.
Mouse kidney samples were immediately immersed in 4% (vol/vol) formaldehyde/2% (vol/vol) glutaraldehyde (Electron Microscopy Sciences)/PBS. Small blocks were then cut, osmicated, and dehydrated before embedding. The blocks were sectioned and observed in a Hitachi H7600 (Tokyo, Japan) transmission electron microscope equipped with an XR41B CCD camera (Advanced Microscopy Techniques Corporation). Percentage mitochondrial area was analyzed with Image J (National Institutes of Health). The ratio of mitochondrial area/cell area was measured in 13 cells and represented as means and 95% confidence intervals.
Respiratory Capacity of Isolated Mitochondria from Kidney Tissue.
For respiratory capacity measurements of isolated mitochondria from kidney, an XF96 V3 PET plate (Seahorse Bioscience) was coated overnight with 1:15,000 polyethylenimine solution/assay buffer (137 mM KCl, 2 mM KH2PO4, 2.5 mM MgCl2, 20 mM Hepes, 0.5 mM EGTA, 0.2% fatty acid-free BSA). Mitochondria isolated from kidney using the standard Nagarse method was attached to the plate bottom at 936 g for 10 min. The plate was warmed at 37 °C for 10 min and transferred to the Seahorse XF96 analyzer. State III respiration (maximum ADP-stimulated oxygen consumption ratio under sufficient substrate for mitochondrial complex) of complex I was measured with 16 μg mitochondria immediately after the addition of 5 mM glutamate, 5 mM malate, and 0.5 mM adenosine diphosphate. State III respiration of complex II was measured with 8 μg mitochondria immediately after the addition of 5 mM succinate, 0.28 μM rotenone, and 0.5 mM adenosine diphosphate. Complex IV-dependent respiration was measured with 2 μg mitochondria immediately after the addition of 0.5 mM tetramethyl-phenylenediamine and 3 mM ascorbic acid.
Cell Lines.
Fnip1/Fnip2 null MEFs (DKO) were generated by deleting all alleles of Fnip1 and Fnip2 in MEFs extracted from Fnip1f/f, Fnip2 f/f mice, using adenoviral Cre recombinase (17). These MEFs were restored with either wild-type FNIP1 (DKO+FNIP1) or wild-type FNIP2 (DKO+FNIP2), using Tet3G system (Clontech) (32). To eliminate a possible artifact from doxycycline, 0.5 ng/μL doxycycline was added to all lines. Doxycycline-inducible wild-type FLCN-expressing UOK257 (6) was transfected with Silencer Select Predesigned SiRNA (Ambion) for FNIP1: 5′- GGCAUAUAAUCGAAUAGUUtt-3′ and FNIP2: 5′-CCACAACUGAUGAUUAGUAtt-3′, using Lipofectamine RNAi MAX (Invitrogen). Cell proliferation was analyzed using TetraColorOne (Seikagakukogyo) according to manufacturer’s protocol.
ATP Measurement.
ATP was measured using ATPlite 1step (Perkin-Elmer) according manufacture’s protocol.
Statistical Analysis.
Experimental data are summarized as the mean values with SD. Statistical analyses were performed using a two-tailed Student t test (SPSS Statistics version 20), and differences were considered to be statistically significant at a value of P < 0.05. Survival curves were obtained using GraphPad Prism version 6.01.
Animal Care.
National Cancer Institute–Frederick is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the National Research Council’s “Guide for Care and Use of Laboratory Animals.”
Supplementary Material
Acknowledgments
We thank Dr. Peter Igarashi for CDH16-Cre transgenic mice, Dr. Daniel Crooks for helpful discussions, and Louise Cromwell for excellent technical support with the mouse studies. M.L. was supported by an annual outbound fellowship from Fondazione Italiana Ricerca sul Cancro, “Fellowship For Abroad 2011.” This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project has been funded in part with federal funds from the Frederick National Laboratory for Cancer Research, NIH, under Contract HHSN261200800001E.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419502112/-/DCSupplemental.
References
- 1.Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113(12):1674–1677. [PubMed] [Google Scholar]
- 2.Zbar B, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11(4):393–400. [PubMed] [Google Scholar]
- 3.Nickerson ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2(2):157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 4.Vocke CD, et al. High frequency of somatic frameshift BHD gene mutations in Birt-Hogg-Dubé-associated renal tumors. J Natl Cancer Inst. 2005;97(12):931–935. doi: 10.1093/jnci/dji154. [DOI] [PubMed] [Google Scholar]
- 5.Baba M, et al. Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer Inst. 2008;100(2):140–154. doi: 10.1093/jnci/djm288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasumi H, et al. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN. J Natl Cancer Inst. 2012;104(22):1750–1764. doi: 10.1093/jnci/djs418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, et al. Deficiency of FLCN in mouse kidney led to development of polycystic kidneys and renal neoplasia. PLoS ONE. 2008;3(10):e3581. doi: 10.1371/journal.pone.0003581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman TR, et al. The role of the Birt-Hogg-Dubé protein in mTOR activation and renal tumorigenesis. Oncogene. 2009;28(13):1594–1604. doi: 10.1038/onc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasumi Y, et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc Natl Acad Sci USA. 2009;106(44):18722–18727. doi: 10.1073/pnas.0908853106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klomp JA, et al. Birt-Hogg-Dubé renal tumors are genetically distinct from other renal neoplasias and are associated with up-regulation of mitochondrial gene expression. BMC Med Genomics. 2010;3:59. doi: 10.1186/1755-8794-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baba M, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci USA. 2006;103(42):15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasumi H, et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415(1-2):60–67. doi: 10.1016/j.gene.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takagi Y, et al. Interaction of folliculin (Birt-Hogg-Dubé gene product) with a novel Fnip1-like (FnipL/Fnip2) protein. Oncogene. 2008;27(40):5339–5347. doi: 10.1038/onc.2008.261. [DOI] [PubMed] [Google Scholar]
- 14.Baba M, et al. The folliculin-FNIP1 pathway deleted in human Birt-Hogg-Dubé syndrome is required for murine B-cell development. Blood. 2012;120(6):1254–1261. doi: 10.1182/blood-2012-02-410407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park H, et al. Disruption of Fnip1 reveals a metabolic checkpoint controlling B lymphocyte development. Immunity. 2012;36(5):769–781. doi: 10.1016/j.immuni.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nookala RK, et al. Crystal structure of folliculin reveals a hidDENN function in genetically inherited renal cancer. Open Biol. 2012;2(8):120071. doi: 10.1098/rsob.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong SB, et al. Inactivation of the FLCN tumor suppressor gene induces TFE3 transcriptional activity by increasing its nuclear localization. PLoS ONE. 2010;5(12):e15793. doi: 10.1371/journal.pone.0015793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betschinger J, et al. Exit from pluripotency is gated by intracellular redistribution of the bHLH transcription factor Tfe3. Cell. 2013;153(2):335–347. doi: 10.1016/j.cell.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preston RS, et al. Absence of the Birt-Hogg-Dubé gene product is associated with increased hypoxia-inducible factor transcriptional activity and a loss of metabolic flexibility. Oncogene. 2011;30(10):1159–1173. doi: 10.1038/onc.2010.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastola P, et al. Folliculin contributes to VHL tumor suppressing activity in renal cancer through regulation of autophagy. PLoS ONE. 2013;8(7):e70030. doi: 10.1371/journal.pone.0070030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan M, et al. The tumor suppressor folliculin regulates AMPK-dependent metabolic transformation. J Clin Invest. 2014;124(6):2640–2650. doi: 10.1172/JCI71749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cash TP, Gruber JJ, Hartman TR, Henske EP, Simon MC. Loss of the Birt-Hogg-Dubé tumor suppressor results in apoptotic resistance due to aberrant TGFβ-mediated transcription. Oncogene. 2011;30(22):2534–2546. doi: 10.1038/onc.2010.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong SB, et al. Tumor suppressor FLCN inhibits tumorigenesis of a FLCN-null renal cancer cell line and regulates expression of key molecules in TGF-beta signaling. Mol Cancer. 2010;9:160. doi: 10.1186/1476-4598-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medvetz DA, et al. Folliculin, the product of the Birt-Hogg-Dube tumor suppressor gene, interacts with the adherens junction protein p0071 to regulate cell-cell adhesion. PLoS ONE. 2012;7(11):e47842. doi: 10.1371/journal.pone.0047842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahorski MS, et al. Folliculin interacts with p0071 (plakophilin-4) and deficiency is associated with disordered RhoA signalling, epithelial polarization and cytokinesis. Hum Mol Genet. 2012;21(24):5268–5279. doi: 10.1093/hmg/dds378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai A, Kobayashi T, Hino O. Folliculin regulates cyclin D1 expression through cis-acting elements in the 3′ untranslated region of cyclin D1 mRNA. Int J Oncol. 2013;42(5):1597–1604. doi: 10.3892/ijo.2013.1862. [DOI] [PubMed] [Google Scholar]
- 27.Laviolette LA, et al. Human folliculin delays cell cycle progression through late S and G2/M-phases: Effect of phosphorylation and tumor associated mutations. PLoS ONE. 2013;8(7):e66775. doi: 10.1371/journal.pone.0066775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petit CS, Roczniak-Ferguson A, Ferguson SM. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J Cell Biol. 2013;202(7):1107–1122. doi: 10.1083/jcb.201307084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsun ZY, et al. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell. 2013;52(4):495–505. doi: 10.1016/j.molcel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Possik E, et al. Folliculin regulates ampk-dependent autophagy and metabolic stress survival. PLoS Genet. 2014;10(4):e1004273. doi: 10.1371/journal.pgen.1004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunlop EA, et al. FLCN, a novel autophagy component, interacts with GABARAP and is regulated by ULK1 phosphorylation. Autophagy. 2014;10(10):1749–1760. doi: 10.4161/auto.29640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasumi Y, et al. Folliculin (Flcn) inactivation leads to murine cardiac hypertrophy through mTORC1 deregulation. Hum Mol Genet. 2014;23(21):5706–5719. doi: 10.1093/hmg/ddu286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, Iyer LM, He F, Aravind L. Discovery of Novel DENN Proteins: Implications for the Evolution of Eukaryotic Intracellular Membrane Structures and Human Disease. Front Genet. 2012;3:283. doi: 10.3389/fgene.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.da Silva NF, et al. Analysis of the Birt-Hogg-Dubé (BHD) tumour suppressor gene in sporadic renal cell carcinoma and colorectal cancer. J Med Genet. 2003;40(11):820–824. doi: 10.1136/jmg.40.11.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoo SK, et al. Inactivation of BHD in sporadic renal tumors. Cancer Res. 2003;63(15):4583–4587. [PubMed] [Google Scholar]
- 36.Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499(7456):43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandoth C, et al. Cancer Genome Atlas Research Network Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002;13(7):1837–1846. doi: 10.1097/01.asn.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.