Significance
Cerebral ischemia (CI; stroke, brain injury, vascular dementia, neonatal hypoxia, and many other conditions) affects people at all stages of life. Of the many diseases associated with CI, stroke alone causes up to 10% of deaths worldwide and is a leading cause of disability; yet treatment options are extremely limited, so this represents an area of massive unmet clinical need. Inflammation involving the cytokine interleukin-1 is a major contributor to cell death in the ischemic brain. Inflammation can be regulated by large protein complexes called inflammasomes. Here we show that the NLRC4 (NLR family, CARD domain containing 4) and AIM2 (absent in melanoma 2) inflammasomes, but not the NLRP3 (NLR family, pyrin domain containing 3) inflammasome, contribute to inflammation and injury in an ischemic brain and are thus potential therapeutic targets for these devastating diseases.
Keywords: inflammation, inflammasome, cerebral ischemia, brain injury, cell death
Abstract
Inflammation that contributes to acute cerebrovascular disease is driven by the proinflammatory cytokine interleukin-1 and is known to exacerbate resulting injury. The activity of interleukin-1 is regulated by multimolecular protein complexes called inflammasomes. There are multiple potential inflammasomes activated in diverse diseases, yet the nature of the inflammasomes involved in brain injury is currently unknown. Here, using a rodent model of stroke, we show that the NLRC4 (NLR family, CARD domain containing 4) and AIM2 (absent in melanoma 2) inflammasomes contribute to brain injury. We also show that acute ischemic brain injury is regulated by mechanisms that require ASC (apoptosis-associated speck-like protein containing a CARD), a common adaptor protein for several inflammasomes, and that the NLRP3 (NLR family, pyrin domain containing 3) inflammasome is not involved in this process. These discoveries identify the NLRC4 and AIM2 inflammasomes as potential therapeutic targets for stroke and provide new insights into how the inflammatory response is regulated after an acute injury to the brain.
Proinflammatory cytokines of the interleukin-1 (IL-1) family are critical regulators of host responses to infection and orchestrate damaging inflammatory responses that occur during disease (1). One of the main mediators of damaging sterile inflammation is IL-1β, which is implicated in the etiology of many major diseases, including acute cerebrovascular disease (2). Acute cerebrovascular disease presents as a range of conditions, including devastating injuries such as subarachnoid hemorrhage (SAH) and ischemic stroke, which account for up to 10% of mortality worldwide and are the leading cause of morbidity (2). Treatments for acute stroke are limited to thrombolysis for up to 10% of all strokes, antiplatelet agents, and stroke unit care. Thus, treatment of acute cerebrovascular disease remains an area of unmet clinical need. Understanding the mechanisms regulating production of IL-1β during ischemic brain injury may lead to the identification of new therapeutic targets.
IL-1β is produced by many cells, most commonly those of macrophage lineage, as a pro–IL-1β precursor. Pro–IL-1β is expressed in response to pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) that bind to pattern recognition receptors (PRRs) to up-regulate proinflammatory gene expression (3, 4). PAMPs are motifs carried by pathogens, such as bacterial endotoxin (or LPS), and DAMPs are commonly endogenous molecules released by necrosis. Pro–IL-1β is inactive and remains intracellular until a further PAMP or DAMP stimulation activates cytosolic PRRs, often of the nucleotide-binding domain and leucine-rich repeat containing receptor (NLR) family, to form large multiprotein complexes called inflammasomes (5). These complexes consist of the PRR, procaspase-1, and, depending upon the PRR, an adaptor protein called ASC, that interact via CARD and pyrin homology-binding domains (5). When the PRR senses PAMPs or DAMPs, it recruits ASC, which in turn recruits caspase-1, causing its activation. Caspase-1 then processes pro–IL-1β to a mature form that is rapidly secreted from the cell (5). The activation of caspase-1 can also cause cell death (6).
A number of inflammasome-forming PRRs have been identified, including NLR family, pyrin domain containing 1 (NLRP1); NLRP3; NLRP6; NLRP7; NLRP12; NLR family, CARD domain containing 4 (NLRC4); AIM 2 (absent in melanoma 2); IFI16; and RIG-I (5). Of these inflammasomes identified to date, the best characterized, and most strongly associated with sterile inflammation, is formed by NLRP3 (7). Indeed, there are now several studies suggesting that NLRP3 inflammasomes contribute to ischemic brain injury (8, 9). However, the picture is more complicated. NLRP1 inflammasomes have been implicated in several models of brain injury (6, 10, 11), as have AIM2 inflammasomes, which are suggested to mediate pyroptotic neuronal cell death (12). There is also evidence supporting a role for caspase-1 in brain injury (13), with a selective caspase-1 inhibitor, VRT-018858, a nonpeptide, active metabolite of the prodrug pralnacasan, showing marked protection in a rat model of stroke (14). However, data for the related caspase-1 inhibitor VRT-043198 suggest that it is also an effective inhibitor of caspase-4 (15), a human ortholog of caspase-11. Caspase-11 is also implicated in ischemic brain injury (16, 17), and given that we now also know that the original caspase-1−/− mouse is also deficient in caspase-11 (18), it is clear that caspase-11 could have a role in ischemic brain injury. Our aim here was to elucidate which inflammasomes contribute to ischemic brain injury, using mice in which specific inflammasome components are deleted (−/−).
Results
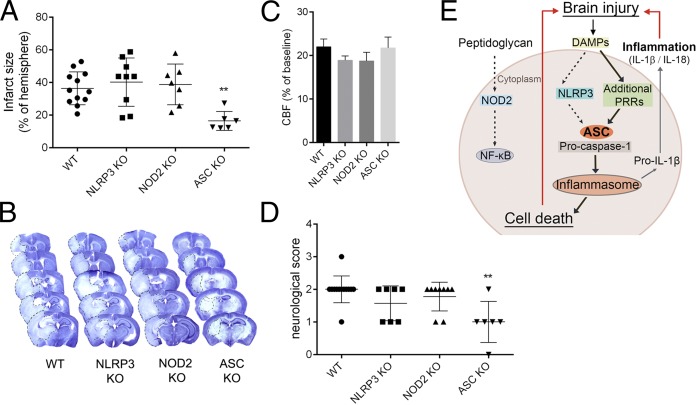
We initially investigated the role of NLRP3 and ASC in ischemic brain injury induced by middle cerebral artery occlusion (MCAo). We discovered that ischemic brain injury was comparable between WT and NLRP3−/− mice, but ASC−/− mice were significantly protected (Fig. 1 A and B). This was not due to altered cerebral perfusion during MCAo (Fig. 1C) or after induction of reperfusion. ASC−/− mice also had improved neurological outcomes following MCAo compared with WT and NLRP3−/−, which were similar (Fig. 1D). We also found that brain injury was not altered by knockout of the noninflammasome-forming PRR NOD2 (19) (Fig. 1 A–D). Thus, after MCAo, ASC is likely to regulate assembly of inflammasomes via activation of PRRs other than NLRP3 (Fig. 1E).
Fig. 1.
ASC-dependent mechanisms contribute to brain injury induced by cerebral ischemia. Infarct volume (A) as measured on cresyl violet-stained brain sections (B) is significantly reduced in ASC−/− mice (**P < 0.01 vs. WT, NLRP3−/− and NOD2−/−). (C) Average cerebral blood flow (CBF) values were unaltered during occlusion of the MCA (A–C; one-way ANOVA followed by Tukey’s post hoc test). (D) Neurological outcome was improved in ASC−/− mice (**P < 0.01, Kruskal–Wallis test followed by Dunn’s multiple comparison). (E) Schematic diagram summarizing the data. The dashed lines highlight dispensable pathways.
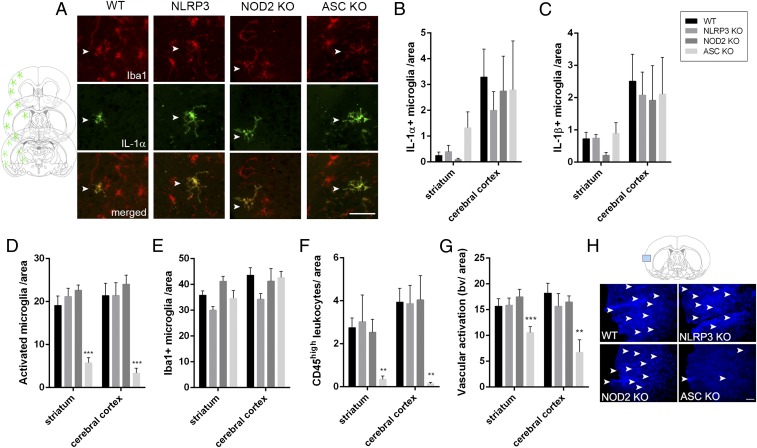
Microglial activation (Iba1+, ramified cells, expressing low levels of CD45), leukocyte recruitment (round or elongated cells, expressing high levels of CD45; Fig. S1), and vascular activation were also reduced in the brains of ASC−/− mice after MCAo (Fig. 2 D–H). However, numbers of microglia expressing IL-1α and IL-1β after MCAo were equivalent in the brains of WT, NLRP3−/−, or ASC−/− mice (Fig. 2 A–C and Fig. S2A). These findings suggest that following MCAo, inflammation and injury progressed independently of the canonical sensor of sterile damage, the NLRP3 inflammasome.
Fig. 2.
ASC deficiency reduces inflammation in the brain independently of IL-1 production. Numbers of IL-1α– (A and B) and IL-1β– (C) positive microglia (A, Iba1+, arrowheads) are not significantly altered in the brain by NLRP3, NOD2, or ASC deficiency after cerebral ischemia. Schematic shows the location of IL-1–positive microglia in the ipsilateral hemisphere, which was similar in all animals. Numbers of activated microglia [D, expressing low levels of CD45 (CD45low), Iba1+], total microglial numbers (E), recruitment of leukocytes expressing high levels of CD45 (CD45high) (F), and vascular activation (G, blood vessels with high levels of tomato lectin staining, as shown on H in blue) have been assessed in the ipsilateral striatum and cerebral cortex. n = 6–9, two-way ANOVA followed by Tukey’s post hoc test. **P < 0.01, ***P < 0.001. (Scale bar, A, 50 μm; H, 100 μm.)
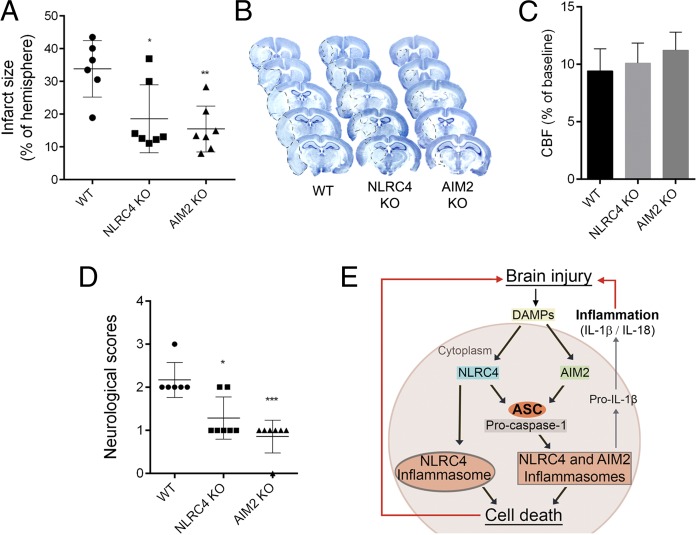
The lack of NLRP3 involvement after MCAo was unexpected and suggests that other inflammasomes must contribute to sterile inflammation in the brain. Available evidence suggests that AIM2 could have a role in sterile inflammatory responses (20), whereas the other inflammasome-forming receptors appear to depend upon a microbial presence. To investigate the involvement of additional PRRs, we induced MCAo in AIM2−/− and NLRC4−/− mice. AIM2 recognizes pathogen and host double-stranded DNA and is composed of a pyrin domain and a DNA-binding HIN domain, and thus AIM2 inflammasomes have an absolute requirement for ASC to recruit and activate caspase-1 (21, 22). NLRC4 has a CARD domain and can directly interact with procaspase-1, although the presence of ASC is known to enhance NLRC4 inflammasome formation (5). AIM2−/− and NLRC4−/− mice had reduced injury and improved behavioral outcomes after MCAo, compared with WT mice (Fig. 3 A, B, and D). As with the ASC−/− experiment above (Fig. 1), the enhanced protection in the AIM2−/− and NLRC4−/− mice was not due to altered cerebral perfusion during MCA occlusion (Fig. 3C). These data suggest that NLRC4 and AIM2 may interact with ASC to mediate brain injury after MCAo (Fig. 3E).
Fig. 3.
AIM2- and NLRC4-dependent mechanisms contribute to brain injury induced by cerebral ischemia. Infarct volume (A) as measured on cresyl violet-stained brain sections (B) was significantly reduced in AIM2−/− and NLRC4−/− mice (*P < 0.05, **P < 0.01). (C) Average CBF values were unaltered during occlusion of the MCA (A–C, one-way ANOVA followed by Tukey’s post hoc test). (D) Neurological outcome was improved in AIM2−/− and NLRC4−/− mice (*P < 0.05, ***P < 0.001), Kruskal–Wallis test followed by Dunn’s multiple comparison). (E) Schematic diagram summarizing the data. n = 6–7.
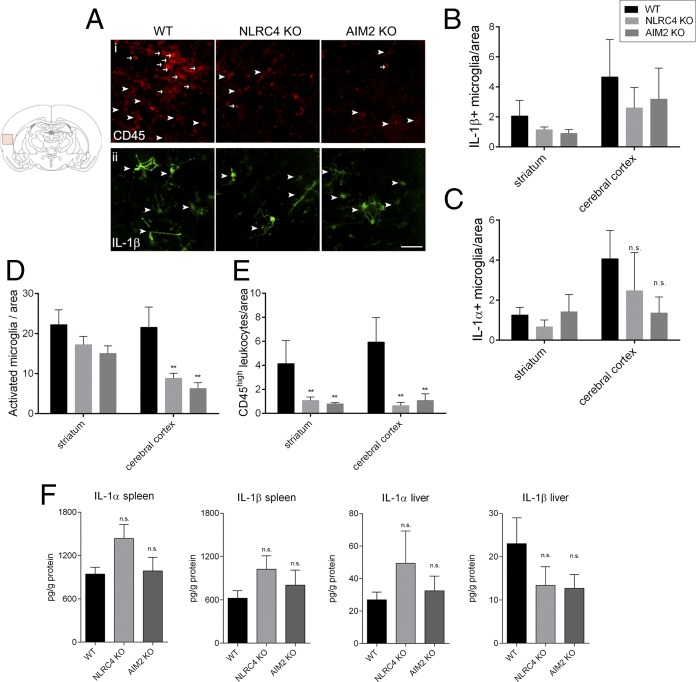
As with the ASC−/− mice above, microglial activation and leukocyte recruitment were reduced in the brains of AIM2−/− and NLRC4−/− mice after MCAo (Fig. 4 D and E). Numbers of microglia expressing IL-1β and IL-1α after MCAo were equivalent in the brains of WT, AIM2−/−, or NLRC4−/− mice (Fig. 4 A–C and Fig. S2B). Furthermore, we did not find any significant difference in IL-1β or IL-1α production (Fig. 4F), as well as CXCL1, TNFα, IL-6, and IL-10 production (Fig. S3) in the liver and the spleen, two major organs that are involved in diverse inflammatory/acute phase responses that contribute to brain injury (23, 24).
Fig. 4.
AIM2 and NLRC4 deficiency reduces inflammation in the brain independently of IL-1 production. Numbers of IL-1β– (A and B) positive microglia (arrowheads), IL-1α–positive microglia (C), activated microglia (A and D, arrowheads), and CD45high leukocytes (A and E, arrows) have been assessed in the ipsilateral hemisphere after MCAo (two-way ANOVA followed by Tukey’s post hoc test). (F) Protein levels of IL-1α and IL-1β were measured with cytometric bead array in liver and spleen homogenates (one-way ANOVA followed by Tukey’s post hoc test, **P < 0.01). n = 6–7. n.s., not significant. (Scale bar, 50 μm.)
Discussion
Here we have shown that ischemic brain injury was reduced in ASC−/−, AIM2−/−, and NLRC4−/− mice and not in mice deficient for the canonical sensor of sterile injury, NLRP3. These data provide the first (to our knowledge) evidence for multiple inflammasomes regulating neuronal injury, identifying AIM2 and NLRC4 as key drivers of sterile inflammatory responses in the brain.
NLRC4 is regarded as a sensor of pathogenic bacteria (25), and thus to see it contributing to ischemic brain injury, a model of sterile inflammation, was an unexpected result. NLRC4 inflammasome activation is, however, also reported in a model of alcohol-induced liver damage (26) and also to regulate tumorigenesis in an inflammation-induced model of colorectal cancer (27). In these conditions it is possible that host microbiota contribute to the inflammatory response. However, our results may also suggest that microbiota do not contribute to brain injury via recognition of peptidoglycan-derived peptides by NOD2. We know that both systemic and central IL-1–dependent inflammation drives injury in experimental cerebral ischemia (28), and thus there is a possibility that NLRC4 drives a systemic inflammatory response following the translocation of gut bacteria/products. However, although there was a characteristic systemic inflammatory response following MCAo, it was identical between the WT and inflammasome knockout strains (Fig. 4). Nevertheless, we cannot rule out the possibility that early actions mediated by circulating leukocytes or inflammatory mediators could contribute to some of the differences observed in the knockout strains.
Thus, our data provide the first to our knowledge information that NLRC4 could be a DAMP sensor in brain inflammation. However, sterile inflammatory conditions involving NLRC4 in other tissues are now emerging in the literature. Autoinflammatory conditions caused by activating mutations in NLRC4 (similar to the CAPS syndromes caused by activating mutations in NLRP3) have been described recently (29–31). NLRP3 is regarded as the canonical sensor of sterile injury or stress, and so to consider how NLRC4 may sense DAMPs it is sensible to draw comparisons to NLRP3. Given the diverse structures of DAMPs that activate NLRP3 and the fact that no direct interactions have been observed, a direct interaction between a DAMP and NLRP3 is unlikely (32), and no accessory receptors have been described. Rather, the cellular stress imposed by DAMPs is suggested to give rise to a number of potential mechanisms that lead to posttranslational modification of NLRP3, leading to its activation (32). NLRC4 does not sense bacterial ligands directly but does so through coreceptors called NAIP proteins (33). Given that the NAIP proteins specifically and directly bind and sense bacterial proteins (34), it is unlikely that NAIPs also bind diverse DAMPs. This raises the possibility of specific host protein DAMPs that are homologous with bacterial NAIP ligands, or that certain DAMPs or tissue/cellular stresses activate posttranslational mechanisms, causing activation of NLRC4. Recent work from our group and others has identified ubiquitin posttranslational modification of NLRP3 inflammasome components as a key integrator of diverse DAMP signals (35–38). Interestingly, ubiquitination has also been suggested to regulate the activation of NLRC4 in a model of caspase-8–dependent cell death (39). Phosphorylation of NLRC4 is also known to be pivotal to its activation (40). Thus, cellular/tissue stress may cause posttranslational modification of NLRC4 inflammasome components, resulting in NLRC4 inflammasome activation. A key distinction between NLRP3 and NLRC4 is that NLRP3 requires priming to induce NLRP3 expression before active NLRP3 inflammasomes can be formed (41). This priming step is not required for NLRC4 inflammasome activation (42). It is therefore possible that these pathways operate in the absence of functional NLRP3. NLRC4 has a CARD domain and can directly interact with procaspase-1, although the presence of ASC is known to enhance NLRC4–inflammasome-dependent IL-1β processing (5). Thus, the large protective effect we also observe in the ASC−/− mice could be in part due to an effect on NLRC4–inflammasome-dependent IL-1β release. These data therefore give insights into the regulation of NLRC4 and may trigger new research into NLRC4 as a sensor of sterile injury.
AIM2 recognizes pathogen and host double-stranded DNA and is composed of a pyrin domain and a DNA-binding HIN domain, and thus to recruit caspase-1 into the inflammasome complex requires ASC as a bridging molecule (21, 22). Cultured embryonic rat cortical neurons undergo an AIM2–inflammasome-dependent cell death when challenged with the AIM2 activator poly(dA:dT) (12). AIM2 inflammasomes also drive a protective inflammatory response in the brain, and AIM2−/− mice show reduced survival in response to central infection with Staphylococcus aureus (43). This latter study also shows a disconnect between NLRP3 and ASC because ASC−/− mice are also more susceptible to central S. aureus infection, whereas the survival of NLRP3−/− mice is comparable to WT (43). These observations, together with our data, suggest that AIM2 is an important sensor of infection and injury in the brain in vivo.
We have shown that animals deficient in both IL-1α and IL-1β have markedly reduced infarct size after MCAo (44), and that Anakinra (IL-1Ra) is neuroprotective in this model (e.g., for review see ref. 45). These data, and our observations of protection in the ASC knockout mice, suggest that there is a specific regulation of IL-1 in the brain, and we also have shown previously that both central and peripheral-derived IL-1 contribute to brain injury (28). However, inflammatory caspases may also be involved in neurodegenerative disease in the absence of IL-1 (13), so NLRC4 could also regulate caspase-dependent cell death pathways. AIM2 inflammasomes may also be involved directly in neuronal cell death, and it has been reported that cultured embryonic rat cortical neurons undergo an AIM2–inflammasome-dependent cell death when challenged with the AIM2 activator poly(dA:dT) (12). Thus, inflammasomes may regulate both inflammatory and cell death pathways that modify ischemic brain injury.
We report that microglial cell activation, leukocyte recruitment, and vascular activation are reduced in the brains of ASC−/−, AIM2−/−, and NLRC4−/− mice after MCAo (Figs. 2 and 4). It is important to note that these inflammatory changes could occur as a consequence, in addition to being a cause, of the reduced damage. Changes in inflammatory markers are likely to result from a reduction in IL-1 processing and secretion but also result from reduced injury. We have reported previously that systemic injection of IL-1β increases leukocyte recruitment into the brain after MCAo and that induced neutropenia in this model is neuroprotective, suggesting that an IL-1β–dependent recruitment of leukocytes exacerbates ischemic brain injury (46). We have also reported that DAMPs can induce inflammasome-independent chemokine production from cultures of mixed glia (astrocytes and microglia) in the absence of IL-1, and that in the brains of IL-1αβ double-knockout mice there is chemokine production and microglial cell activation (47). Thus, the extent of microglial cell activation and leukocyte recruitment is likely to be dependent upon both IL-1–dependent inflammation and IL-1–independent cell death. In these studies we chose to study brain injury and inflammation 24 h post MCAo. In this model the extent of injury is almost completely developed at 24 h postinjury. This time point is most suitable for proof of concept studies, when both injury and neurological assessment have to be performed. Also, we have reported sustained protective effects of Anakinra up to 48 h post MCAo (48) and 7 d post MCAo, using a caspase-1 inhibitor (14). Thus, we are confident that the protective effects observed in ASC−/−, AIM2−/−, and NLRC4−/− mice are consistent with our previous and other published work.
In the introduction we describe studies suggesting that NLRP3 inflammasomes contribute to ischemic brain injury (8, 9), but that also NLRP1 inflammasomes are implicated in several models of brain injury (6, 10, 11), as are AIM2 inflammasomes (12). Here, using mice deficient in specific inflammasome components, we have shown that ischemic brain injury is profoundly influenced by multiple inflammasomes and, importantly, here was independent of the canonical sensor of sterile inflammation, the NLRP3 inflammasome. We report here an involvement of NLRC4 and AIM2 inflammasomes, but it is possible that others known [e.g., NLRP1 (8-10)] and other yet to be fully characterized inflammasomes are also involved.
In rodent models of Alzheimer’s disease the memory deficit and inflammation have been reported to depend upon the NLRP3 inflammasome (49), and aggregated Aβ is a DAMP known to activate NLRP3 (50). However, NLRP1 has also been implicated in a rodent model of Alzheimer’s disease (51). An explanation for this difference may be the respective cellular location of the inflammasomes. NLRP3 expressed by microglia drives inflammation, and NLRP1 expressed by neurons drives pyroptotic neuronal cell death (51). Thus, activation of multiple inflammasomes in our model may reflect different cellular compartments and possibly cell death in addition to inflammatory mechanisms. The types of inflammasome activated in the brain may also depend upon the nature of the injury. For example, in a mouse model of intracerebral hemorrhage the NLRP3 inflammasome is reported to drive brain edema and behavioral deficits (52). It was reported recently that heme, a breakdown product of blood, activates the NLRP3 inflammasome (53). Thus, in models of hemorrhagic stroke there is likely to be a significant NLRP3-driven component, which was not the case in our study on cerebral ischemia. Thus, the types of inflammasome activated reflect their cellular location, the functional output (e.g., cell death and/or inflammation), and the nature of the injury (e.g., hemorrhagic versus ischemic stroke). Following our discoveries reported here we can now investigate these mechanisms fully.
Inflammation in the brain affects the outcome of neurodegenerative disease and so is an attractive therapeutic target. However, the molecular and cellular mechanisms regulating brain inflammation remain poorly defined. We have discovered that brain inflammation after cerebral ischemia is regulated by ASC, AIM2, and NLRC4 inflammasomes. This insight now gives us the opportunity to fully elucidate inflammatory regulatory networks in the brain and to establish therapeutic targets. The complexity of inflammasome responses in the brain where multiple inflammasomes are activated in the various neurodegenerative conditions studied suggests that perhaps the most promising therapeutic antiinflammatory targets for the treatment of neurodegenerative disease will be ASC and inflammatory caspases.
Materials and Methods
Animals.
Experiments were carried out in 12–16-wk-old male mice (n = 71), all on C57BL/6 background (WT, NLRP3−/−, NOD2−/−, ASC−/−, NLRC4−/−, and AIM2−/−), breeding at the animal facility of the University of Manchester. Animals were allowed free access to food and water and maintained under temperature-, humidity-, and light-controlled conditions. All animal procedures adhered to the UK Animals (Scientific Procedures) Act (1986), and experiments were performed in accordance with STAIR and ARRIVE guidelines.
Focal Cerebral Ischemia Induced by Middle Cerebral Artery Occlusion.
MCAo was performed, using the intraluminal filament technique, as described earlier (54). Animals were anesthetized with isoflurane, and a silicone-coated monofilament (210-μm-tip diameter, Doccol) was introduced to the left external carotid artery and advanced along the internal carotid artery to occlude the MCA for 45 min, followed by 24 h reperfusion. Occlusion was confirmed by a laser Doppler (Moor Instruments) measurement of blood flow, and animals showing less than 80% signal drop compared with baseline or absence of ischemia in the striatum were excluded pre hoc. During surgery, core temperature was maintained at 37 ± 0.5 °C. Three mice were excluded pre hoc from further analysis, due to improper occlusion of the MCA (n = 1) or surgical artifacts (n = 2).
Tissue Processing.
Under terminal anesthesia, animals were perfused transcardially with saline, followed by paraformaldehyde [PFA; 4% (mass/vol) in PBS, Sigma]. Brains were postfixed in 4% PFA at 4 °C for 24 h, and cryoprotected in sucrose/PBS. Twenty-micrometer-thick coronal brain sections were cut on a sledge microtome (Bright series 8000; Bright Instruments). For cytokine measurement, saline-perfused spleen and liver samples were homogenized and processed as described earlier (55).
Measurement of Infarct Size and Neurological Outcome.
The size of ischemic damage was measured as described previously (54) on cresyl violet-stained brain sections, corrected for edema and expressed as the percentage of the hemisphere. There were no differences in brain size between groups. Neurological status in mice was assessed according to a neurological grading score of increasing severity of deficit (56).
Cytokine Measurement.
Sample processing and protein determination were performed as described previously (55). Saline-perfused liver and spleen homogenates were measured for TNFα, CXCL1, IL-6, IL-1β, IL-1α, and IL-10, using CBA Flex Sets (BD Biosciences) according to the manufacturers’ protocol, on a BD FACSVerse flow cytometer (BD Biosciences). Values are expressed as pg/g protein, and protein concentration was determined using Pierce BCA assay kit.
Immunofluorescence.
Immunostaining was performed as described earlier (10). In brief, appropriate mixtures of rat anti-mouse CD45 1:200 (Serotec), goat anti-mouse IL-1α, goat anti-mouse IL-1β 1:100 (R&D Systems), and rabbit anti-Iba1 1:1,000 (Wako Chemicals) antibodies were used followed by the appropriate fluorochrome-conjugated Alexa594 or Alexa488 donkey antisera (1:500, Invitrogen). Biotinylated tomato lectin (10 μg/mL, Sigma.) was visualized with streptavidin–Alexa 350 conjugate (Invitrogen). Activated (CD45low, Iba1+) and total number of microglia, CD45high leukocytes, activated blood vessels (based on high levels of tomato lectin staining), and IL-1α or IL-1β–positive microglia (Iba1+) were counted in the striatum and cerebral cortex on 3–3 serial sections rostrocaudally (two random 20× fields per section).
Quantitative Analysis and Statistics.
Animals were randomized for the experiments, and surgeries were performed without the operator being aware of the genetic condition of the animals. All quantitative analysis was performed under blinded conditions. Group sizes were determined by power calculation based on results from our previous MCAo studies (5% confidence level, 80% power, and an estimated 20–40% SD). Data were analyzed with one-way or two-way ANOVA followed by Tukey's post hoc comparison (PrismGraph 6.0). Neurological scores were analyzed with nonparametric Kruskal–Wallis test followed by Dunn’s multiple comparison test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors thank Mr. László Barna, the Nikon Microscopy Center at the Institute of Experimental Medicine, Nikon Austria GmbH, and Auro-Science Consulting, Ltd., for kindly providing microscopy support and the Flow Cytometry Core Facility at the Institute of Experimental Medicine. The authors are grateful to Dr. Vishva Dixit (Genentech) for providing the NLRP3−/−, NLRC4−/−, and AIM2−/− mice. D.B. was funded by a Wellcome Trust Fellowship. We are grateful for funding provided by the Medical Research Council and the European Union's Seventh Framework Programme (FP7/2008-2013) under Grant Agreements 201024 and 202213 [European Stroke Network (to N.R. and A.D.). A.D. is supported by OTKA (Hungarian Scientific Research Fund) K 109743, TÁMOP-4.2.4.A/2-11/1-2012-0001, and the Hungarian Brain Research Program KTIA_13_NAP-A-I/2.
Footnotes
Conflict of interest statement: N.R. is a nonexecutive director of AstraZeneca, although the company had no involvement with this work. None of the other authors have any conflicts to declare.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419090112/-/DCSupplemental.
References
- 1.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galea J, Brough D. The role of inflammation and interleukin-1 in acute cerebrovascular disease. J Inflamm Res. 2013;6:121–128. doi: 10.2147/JIR.S35629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Rivero Vaccari JP, et al. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cerebral Blood Flow Metabolism. 2009;29(7):1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassel SL, Sutterwala FS. Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur J Immunol. 2010;40(3):607–611. doi: 10.1002/eji.200940207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang F, et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cerebral Blood Flow Metabolism. 2014;34(4):660–667. doi: 10.1038/jcbfm.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fann DY, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4:e790. doi: 10.1038/cddis.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abulafia DP, et al. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cerebral Blood Flow Metabolism. 2009;29(3):534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- 11.de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28(13):3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamczak SE, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cerebral Blood Flow Metabolism. 2014;34(4):621–629. doi: 10.1038/jcbfm.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denes A, Lopez-Castejon G, Brough D. Caspase-1: Is IL-1 just the tip of the ICEberg? Cell Death Dis. 2012;3:e338. doi: 10.1038/cddis.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross J, Brough D, Gibson RM, Loddick SA, Rothwell NJ. A selective, non-peptide caspase-1 inhibitor, VRT-018858, markedly reduces brain damage induced by transient ischemia in the rat. Neuropharmacology. 2007;53(5):638–642. doi: 10.1016/j.neuropharm.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Wannamaker W, et al. (S)-1-((S)-2-[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18. J Pharmacol Exp Ther. 2007;321(2):509–516. doi: 10.1124/jpet.106.111344. [DOI] [PubMed] [Google Scholar]
- 16.Kang SJ, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149(3):613–622. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata M, et al. Caspases determine the vulnerability of oligodendrocytes in the ischemic brain. J Clin Invest. 2000;106(5):643–653. doi: 10.1172/JCI10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 19.Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23(21):7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dombrowski Y, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3(82):82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010;24(5):708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13(4):325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeSantis DA, et al. Alcohol-induced liver injury is modulated by Nlrp3 and Nlrc4 inflammasomes in mice. Mediators Inflamm. 2013;2013:751374. doi: 10.1155/2013/751374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA. 2010;107(50):21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denes A, et al. Central and haematopoietic interleukin-1 both contribute to ischaemic brain injury in mice. Dis Model Mech. 2013;6(4):1043–1048. doi: 10.1242/dmm.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romberg N, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46(10):1135–1139. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canna SW, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46(10):1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J Exp Med. 2014;211(12):2385–2396. doi: 10.1084/jem.20141091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Nardo D, De Nardo CM, Latz E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am J Pathol. 2014;184(1):42–54. doi: 10.1016/j.ajpath.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39(3):432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477(7366):592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodgers MA, et al. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J Exp Med. 2014;211(7):1333–1347. doi: 10.1084/jem.20132486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Castejon G, et al. Deubiquitinases regulate the activity of caspase-1 and interleukin-1β secretion via assembly of the inflammasome. J Biol Chem. 2013;288(4):2721–2733. doi: 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juliana C, et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287(43):36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49(2):331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Kumar Y, Radha V, Swarup G. Interaction with Sug1 enables Ipaf ubiquitination leading to caspase 8 activation and cell death. Biochem J. 2010;427(1):91–104. doi: 10.1042/BJ20091349. [DOI] [PubMed] [Google Scholar]
- 40.Qu Y, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490(7421):539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 41.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7(6):576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 43.Hanamsagar R, Aldrich A, Kielian T. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J Neurochem. 2014;129(4):704–711. doi: 10.1111/jnc.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boutin H, et al. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21(15):5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brough D, Tyrrell PJ, Allan SM. Regulation of interleukin-1 in acute brain injury. Trends Pharmacol Sci. 2011;32(10):617–622. doi: 10.1016/j.tips.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 46.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27(16):4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savage CD, Lopez-Castejon G, Denes A, Brough D. NLRP3-inflammasome activating DAMPs stimulate an inflammatory response in glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol. 2012;3:288. doi: 10.3389/fimmu.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol. 2003;140(3):471–476. doi: 10.1038/sj.bjp.0705462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heneka MT, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan MS, et al. Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 2014;5:e1382. doi: 10.1038/cddis.2014.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Q, et al. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75(2):209–219. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dutra FF, et al. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci USA. 2014;111(39):E4110–E4118. doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denes A, Humphreys N, Lane TE, Grencis R, Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci. 2010;30(30):10086–10095. doi: 10.1523/JNEUROSCI.1227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chapman KZ, et al. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cerebral Blood Flow Metabolism. 2009;29(11):1764–1768. doi: 10.1038/jcbfm.2009.113. [DOI] [PubMed] [Google Scholar]
- 56.Bederson JB, et al. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.