Abstract
Denitrification and arginine fermentation are central metabolic processes performed by the opportunistic pathogen Pseudomonas aeruginosa during biofilm formation and infection of lungs of patients with cystic fibrosis. Genome-wide searches for additional components of the anaerobic metabolism identified potential genes for pyruvate-metabolizing NADH-dependent lactate dehydrogenase (ldhA), phosphotransacetylase (pta), and acetate kinase (ackA). While pyruvate fermentation alone does not sustain significant anaerobic growth of P. aeruginosa, it provides the bacterium with the metabolic capacity for long-term survival of up to 18 days. Detected conversion of pyruvate to lactate and acetate is dependent on the presence of intact ldhA and ackA-pta loci, respectively. DNA microarray studies in combination with reporter gene fusion analysis and enzyme activity measurements demonstrated the anr- and ihfA-dependent anaerobic induction of the ackA-pta promoter. Potential Anr and integration host factor binding sites were localized. Pyruvate-dependent anaerobic long-term survival was found to be significantly reduced in anr and ihfA mutants. No obvious ldhA regulation by oxygen tension was observed. Pyruvate fermentation is pH dependent. Nitrate respiration abolished pyruvate fermentation, while arginine fermentation occurs independently of pyruvate utilization.
Pseudomonas aeruginosa is an important opportunistic pathogen causing persistent infections of the lungs of cystic fibrosis patients. Under these conditions, P. aeruginosa forms macrocolonies and generates a microaerobic to anaerobic environment (40). P. aeruginosa is also an important model organism for studying biofilm formation. Biofilms allow bacteria to survive under unfavorable conditions in the habitat (35). It has been shown previously that the deeper layers of biofilms are anaerobic (21, 40, 42, 44).
P. aeruginosa is usually described as a bacterium which favors aerobic growth conditions (29). However, recent data obtained from chemostat experiments suggest that the organism tries to establish a microaerobic milieu for optimal growth (32). Under oxygen-limiting conditions, P. aeruginosa grows in the presence of nitrate or nitrite by using denitrification (11, 46). Under these conditions, nitrate or nitrite replace oxygen as alternative electron acceptors. In the absence of nitrate or nitrite, arginine serves as energy substrate for anaerobic growth (38). P. aeruginosa degrades arginine to ornithine, which is linked to the generation of ATP. For growth under these arginine-fermenting conditions a complex medium is required, because arginine does not serve as carbon source and the ornithine that is produced is excreted into the medium (38). Arginine fermentation was also found to be induced under various stress conditions. It is only partially repressed by the presence of nitrate (24). Without arginine or alternative electron acceptors in the anaerobic growth medium, P. aeruginosa rapidly becomes energy starved and cell numbers decline dramatically from 1 × 109 to 5 × 105 cells per ml within 16 days (6).
The central regulator for the onset of the anaerobic metabolism is Anr (18, 34). This protein is highly homologous to Escherichia coli Fnr. The anr gene encodes the cysteine residues which were found to be essential in E. coli Fnr for the formation of the oxygen-sensitive iron-sulfur cluster. Anr directly induces genes required for arginine fermentation and cyanide biosynthesis (45). Furthermore, Anr induces the gene for a second Fnr-type regulator, named Dnr (4). Dnr, in combination with the two-component regulatory system encoded by the narXL locus, is responsible for the induction of the denitrification pathway (4, 23, 43).
Other strictly aerobic bacteria, such as Bacillus subtilis and Arthrobacter globiformis, possess unexpected fermentation capacities (10, 16, 27). B. subtilis requires either glucose and amino acids or glucose and pyruvate for a mixed acid type of fermentation. However, in contrast to E. coli, a bacterium with highly effective glucose fermentation, B. subtilis and P. aeruginosa lack pyruvate formate lyase (7, 28).
To investigate P. aeruginosa for yet-unknown fermentation pathways, we started a genome-wide search for genes potentially involved in fermentation processes. We identified components of a putative pyruvate fermentation pathway. While this pathway does not support anaerobic growth of P. aeruginosa, it is important for long-term survival under these conditions. An investigation of the genes and physiological reactions involved is described.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are shown in Table 1. Employed E. coli strains were grown in Luria-Bertani (LB) medium (33). Ampicillin was used at a concentration of 100 μg/ml, and gentamicin was used at 10 μg/ml. P. aeruginosa was grown in OS medium supplemented with 2.5 g of yeast extract/liter (38). In indicated experiments 20 mM pyruvate replaced arginine as the energy source. Carbenicillin was used at a concentration of 500 μg/ml, and gentamicin was used at 100 μg/ml. During the procedure for the construction of stable chromosomal gene disruption mutants, sucrose-resistant colonies were obtained by streaking P. aeruginosa merodiploids or cells containing sacB encoding plasmids on LB agar or Pseudomonas Isolation Agar (Gibco, Eggenstein, Germany) supplemented with 5% (wt/vol) sucrose and 200 μg of gentamicin/ml (22).
TABLE 1.
Strains of P. aeruginosa and E. coli and plasmids used
| Bacterial strain or plasmid | Genotype or phenotype | Reference or source |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Wild type | 15 |
| PA6261 | PAO1 Δanr | 43 |
| RM536 | PAO1 dnr::tet Tcr | 3 |
| PAO9104 | PAO1 narL::cat Cmr | 23 |
| CHA-A2 | CHA ihfA::tet Tcr | 13 |
| PAO-MW20 | PAO1 rpoS::aacC1 Gmr | 39 |
| PAO6281 | PAO1 gacA::Ω-Sp/Sm | 30 |
| PAO-ME1 | PAO1 Δpta::aacC1-gfp Gmr | This study |
| PAO-ME3 | PAO1 ΔldhA::aacC1-gfp Gmr | This study |
| E. coli | ||
| DH5α | DH5a supE44 ΔlacU169 (φ80 lacZΔM15) hsdR recA1 endA1 gyrA96 thi-1 relA1 | 20 |
| SM10 | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu Kmr | 12 |
| Plasmids | ||
| pEX18Ap | Apr; oriT+sacB+; gene replacement vector with MCS from pUC18 | 22 |
| pQF50 | Apr; promoterless lacZ gene | 17 |
| pPS858 | Apr, Gmr; source of gentamicin cassette | 22 |
| pMEP1 | Apr; pEX18Ap with a BamHI-HindIII-digested PCR fragment (2,139 bp) of the pta gene | This study |
| pMEP2 | Apr, Gmr; pMEP1 with 288-bp SalI deletion in the pta gene replaced with Gmr-gfp fragment from pPS858 | This study |
| pMEP5 | Apr; pEX18Ap with a BamHI-HindIII-digested PCR fragment (1,865 bp) carrying the 3′ end of gacS, complete ldhA, and the sequence downstream of ldhA | This study |
| pMEP11 | Apr, Gmr; pMEP5 with 727-bp SdaI deletion in the ldhA gene replaced with Gmr-gfp fragment from pPS858 | This study |
| pACKA | pQF50 containing a 291-bp fragment of the putative promoter region of loci ackA-pta | This study |
For anaerobic growth, P. aeruginosa was incubated in completely filled rubber-stoppered bottles shaken at 100 rpm to minimize aggregation of the bacteria. Anaerobic conditions were achieved after 3 min through consumption of residual oxygen by the inoculated bacteria. This was verified by using the redox indicator resazurin at a concentration of 200 μg/liter as well as oxygen electrode measurements in control experiments.
Viable cell counting and live/dead staining.
Viable cell counts were determined by periodic removal of samples from the anaerobic culture with sterile syringes; the serial dilution of cells was obtained in phosphate-buffered saline followed by plating of the dilutions on LB agar. CFU were determined by counting the colonies after incubation of the LB plates at 37°C for 24 h. In addition, a fluorescence microscopic analysis of the samples after staining the cells using the LIVE/DEAD BacLight kit (Molecular Probes, Leiden, The Netherlands) was performed following the instructions of the manufacturer. The kit contained two different dyes: propidium iodide, resulting in red fluorescence from stained chromosomal DNA of dead cells, and SYTO9, resulting in green fluorescence from stained viable cells.
HPLC analysis of fermentation products.
For the measurements of secreted metabolites by long-term surviving P. aeruginosa, aerobically grown bacteria in the early stationary phase were harvested, washed, and resuspended at an optical density at 578 nm (OD578) of 0.3 in OS minimal medium supplemented with 2.5 g of yeast extract/liter at a pH of 7.0. The suspensions were further incubated in rubber-stoppered bottles to achieve anoxic conditions as described above. After addition of the indicated amounts of pyruvate, samples were withdrawn with syringes at various time intervals. Fermentation products were determined by high-performance liquid chromatography (HPLC) analysis from the supernatant of the cultures after removal of the bacteria by centrifugation as previously described (10).
Construction and testing of an ackA-pta-lacZ reporter gene fusion.
The putative promoter region of the ackA-pta loci was fused to lacZ. A 314-bp PCR product covering the region from −310 to +4 upstream of the translational start of the ackA gene was generated with primer Pa-ackA-for (5′-GCATGGTTCGAgAtCTTTTCGATAAAGAAG-3′) and primer Pa-ackA-rev (5′-GCATCACgGaTCCTTGGTCTGCTC-3′). Pa-ackA-for contained a double-base mismatch (lowercase g and t) which introduced a BglII restriction site (underlined) in the resulting PCR fragment into the PCR fragment. Pa-ackA-rev contained a double-base mismatch (lowercase g and a) and introduced a BamHI site (underlined). The PCR fragment was digested by using BglII and BamHI. The resulting 291-bp fragment was fused to lacZ by cloning it into the BglII and BamHI site of pQF50 (17) to generate pACKA. Integrity of the construct was verified by complete DNA sequence determination. Reporter gene fusion assays were performed as previously outlined (31). Obtained activities are given in Miller units (25).
Construction of P. aeruginosa Δpta and ΔldhA mutants.
For construction of the various gene disruption mutants, the previously described sacB-based strategy was employed (22). In the P. aeruginosa Δpta mutant PAO-ME1, 288 bp of the pta gene was deleted and replaced by a gentamicin resistance cassette. For the construction of the required suicide vector pMEP2, a PCR fragment containing 348 bp of the 3′ region and 1,791 bp of the pta gene was cloned into BamHI- and HindIII-digested pEX18Ap vector (22). The primer pair used for amplification of the pta-containing PCR fragment was Pa-pta-cuta-for (5′-CGTCGAAGGCATGGatCCGACCCGTC-3′) and Pa-pta-cutb-rev (5′-GGGTGCGCTGGACaAGcTTCATCCAGC-3′). Pa-pta-cuta-for contained a double-base mismatch (lowercase a and t) which introduced a BamHI site (underlined) into the resulting PCR fragment. Pa-pta-cutb-rev contained a double-base mismatch (lowercase a and c) and introduced a HindIII site (underlined). The resulting vector, pMEP1, was digested with SalI, which removed a 288-bp fragment from the pta gene between two naturally occurring SalI sites. A gentamicin resistance cassette was liberated from plasmid pPS858 by using SalI and was inserted into pMEP1, generating pMEP2. The P. aeruginosa pta mutant was generated with pMEP2 following published strategies (22). The chromosomal disruption of pta was verified by colony PCR as described previously (22). The primer pair used for verification of the constructed mutants was Pa-ptaint-for (5′-GCGACCATGATGCTTGCCCTG-3′) and Pa-ptaint-rev (5′-GCATCATACTGCAAGGGTCCGTC-3′).
In the ΔldhA mutant PAO-ME3, 727 bp of the ldhA gene was deleted and replaced by a gentamicin resistance cassette. For the construction of the suicide vector pMEP11, a PCR fragment containing 550 bp of the 5′ region, the complete ldhA gene (985 bp), and 330 bp of the 3′ region was cloned into the BamHI- and HindIII-digested pEX18Ap vector. The primer pair used for amplification of the PCR fragment was Pa-ldhA-cuta-for (5′-GGTGCCGGgGATCcCGCTCAC-3′) and Pa-ldhA-cutb-rev (5′-CGAAGTCGAGACaAGCtTGGCACTGGT-3′). Pa-ldhA-cuta-for contained a double-base mismatch (lowercase g and c) and introduced a BamHI site (underlined). Pa-ldhA-cutb-rev contained a double-base mismatch (lowercase a and t) and introduced a HindIII site (underlined). The resulting vector, pMEP5, was digested with SdaI, which removed a 727-bp fragment from the ldhA gene at the two natural SdaI sites. A gentamicin resistance cassette liberated from plasmid pPS858 by PstI digestion was inserted into pMEP5, generating pMEP11. The P. aeruginosa ldhA mutant was generated by using pMEP11 following published strategies (22). The primer pair used for verification of the constructed mutants was Pa-ldhAint-for (5′-GGCACGGCTTCGAACTGCAC-3′) and Pa-ldhAint-rev (5′-CGTTGGGGAAGCTCAGCAGG-3′).
Microarray experiments.
Microarray analyses of anr-dependent ackA-pta and ldhA transcription were performed. For this purpose, the expression profile of aerobically grown P. aeruginosa PAO1 was compared to that of anaerobically grown PAO1, anr, and dnr mutant strains under aerobic and anaerobic conditions. Strains were inoculated in AB minimal medium (9) supplemented with 50 mM nitrate and 50 mM glucose and grown aerobically to an OD578 of 0.3. Cultures were immediately shifted to anaerobic conditions and were incubated for 2 h. Subsequently, cells were harvested and RNA was extracted by a modified hot phenol method (1). RNA from three independent cultures was pooled for one GeneChip experiment. Two GeneChips for each mutant and growth condition were compared as detailed before (5, 8). Generation of cDNA, labeling, and hybridization of Affymetrix GeneChips was carried out by following protocols provided by the manufacturer (Santa Clara, Calif.). Data analysis was carried out with Affymetrix Microarray Suite 5.0. Affymetrix scaling was used to normalize data from different arrays. A scale factor was derived from the mean signal of all of the probe sets on an array and a user-defined target signal. The signal from each individual probe set is multiplied by this scale factor. The user-defined target signal was set to 100 in our experiments, and all other signals were related to that. Therefore, normalized Affymetrix units represent obtained relative signal intensities under these defined conditions.
Pta assay.
For the preparation of cell extracts from aerobically grown cells, bacteria were grown to exponential phase at an OD578 of 0.3 in LB medium supplemented with 10 mM gluconate and 20 mM pyruvate, harvested, and washed twice with 100 mM Tris-HCl buffer, pH 8.0. Alternatively, wild-type and mutant P. aeruginosa isolates were first cultivated in the same medium as described above under aerobic conditions. After reaching an OD578 of 0.3, cultures were poured into anaerobic flasks and 20 mM pyruvate was added. The bacteria were further incubated under oxygen exclusion for 24 h. The cells were subsequently harvested and washed twice with 100 mM Tris-HCl buffer, pH 8.0. After cell disruption by sonication, the cell homogenate was centrifuged for 10 min at 16,000 × g. To remove residual cell debris and membranes the supernatant was subsequently centrifuged for 2 h at 125,000 × g. The resulting clear supernatant contained 1 to 1.5 mg of protein per ml and was immediately used as a cell extract for enzyme assays. Phosphotransacetylase (Pta) activity was determined spectrophotometrically at 30°C by following the formation of acetyl-coenzyme A (CoA) from acetyl phosphate and CoA resulting in an increase of absorption at 233 nm. The assay mixture (600 μl) contained 90 mM Tris-HCl buffer (pH 8.0), 90 mM potassium chloride, 0.2 mM CoA, 14.5 mM acetyl phosphate, and 5 to 20 μg of cell extract proteins (37). The addition of acetyl phosphate initiated the reaction. An extinction coefficient (ɛ233) of 4.44 mM−1 cm−1 was used in the Pta assay, representing the difference between the extinction coefficients of acetyl-CoA and CoA. One unit of activity was defined as the enzyme activity that converted 1 μmol of acetyl-CoA into product after 60 min at 30°C. Protein concentrations were determined by using the bicinchoninic acid assay (Sigma-Aldrich, Munich, Germany).
RESULTS AND DISCUSSION
Identification of ORFs potentially involved in the anaerobic metabolism of P. aeruginosa.
We searched the complete genome sequence of P. aeruginosa (36) for open reading frames (ORFs) which are potentially involved in anaerobic energy metabolism by using the BLAST 2.0 program (www.ncbi.nlm.nih.gov/BLAST) (2). In addition to the genes involved in the well-established denitrification and arginine fermentation pathways, various other ORFs potentially involved in the anaerobic energy metabolism were identified. The gacS-ldhA operon (PA0926 and PA0927), encoding the sensor kinase GacS of a GacA/GacS component regulatory system and a potential fermentative lactate dehydrogenase (LdhA), were detected. Moreover, the ackA-pta loci (PA0835 and PA0836), potentially encoding acetate kinase and Pta, respectively, and the adhA locus (PA5427), encoding a potential alcohol dehydrogenase (AdhA), were found.
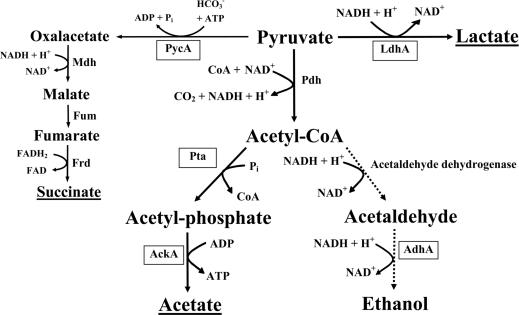
No obvious ORFs encoding proteins homologous to subunits of pyruvate formate lyase, which catalyzes the conversion of pyruvate into acetyl-CoA and formate, or formate hydrogen lyase, which catalyzes the conversion of formate into carbon dioxide and hydrogen, were identified. A model for a putative pyruvate fermentation pathway in P. aeruginosa was deduced from these observations (Fig. 1).
FIG. 1.
Proposed model for P. aeruginosa pyruvate fermentation. The underlined products were detected by HPLC analysis. PycA, pyruvate carboxylase; LdhA, fermentative lactate dehydrogenase; Pdh, pyruvate dehydrogenase; Pta, phosphotransacetylase; AckA, acetate kinase; AdhA, alcohol dehydrogenase. Enzymes of the reductive citric acid cycle: Mdh, malate dehydrogenase; Fum, fumarase; Frd, fumarate reductase.
Pyruvate fermentation provides the basis for anaerobic long-term survival of P. aeruginosa.
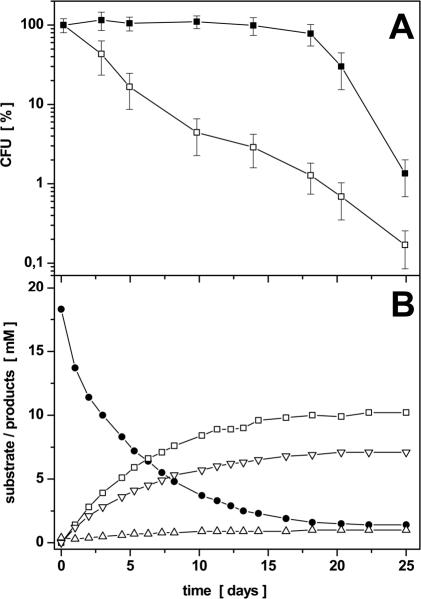
P. aeruginosa is usually described as an aerobic, nonfermenting bacterium (29). This was also proposed for B. subtilis, which was found to perform an anaerobic mixed acid fermentation (10). Initially, we tested whether pyruvate fermentation or the fermentation of glucose or gluconate in the presence of pyruvate sustains anaerobic growth of P. aeruginosa. However, none of these substrates allowed significant growth of P. aeruginosa under anaerobic conditions. We also unsuccessfully tested various minimal and complex media (data not shown). These observations are in agreement with the results of previous investigations, which demonstrated the limitation of anaerobic growth of P. aeruginosa to denitrification and arginine fermentation. Unexpectedly, over a longer period of up to 18 days we observed constant cell numbers for P. aeruginosa when cultured under anaerobic conditions in OS medium containing pyruvate (Fig. 2A). Viable cell counts were obtained by plating cultures at different time points on solid complex medium and direct counting the bacteria in a Thoma counting chamber. We compared the survival of P. aeruginosa in media with and without pyruvate. For that purpose, samples were taken from the parallel cultures at various times and viable cell counts of P. aeruginosa were determined. As shown in Fig. 2A, P. aeruginosa maintains a constant viable cell number over a period of up to 18 days in the presence of pyruvate. In contrast, without pyruvate the culturable cell number of P. aeruginosa decreases logarithmically down to 1% CFU during the same period (Fig. 2A). To rule out the possibility that the observed survival of P. aeruginosa in the presence of pyruvate is caused by a protective effect of pyruvate against peroxides or oxygen stress, the cells were incubated anaerobically in rich medium (LB) containing 50 mM KNO3. The number of the observed CFU for these experiments was comparable to those observed when CFU were determined aerobically.
FIG. 2.
(A) Anaerobic survival of P. aeruginosa without pyruvate (□) and in the presence of 20 mM pyruvate (▪). P. aeruginosa was grown aerobically in OS medium with 20 mM gluconate into the early stationary phase. Bacteria were harvested, washed, resuspended in OS medium at an OD578 of 0.3, and poured into rubber-stoppered bottles. Pyruvate (20 mM) was added where indicated. Viable cell counts were determined by plating the cultures onto LB agar plates. The determined CFU are the means of three independent experiments. Error bars indicate the observed standard deviations; 100% survival represents 5.3 × 108 ± 1.02 × 108 CFU/ml (□) and 5.6 × 107 ± 0.99 × 108 CFU/ml (▪), respectively. (B) Kinetics of pyruvate utilization and product formation under anaerobic survival conditions of P. aeruginosa in the presence of 20 mM pyruvate as the sole carbon and energy source. A representative experiment of a single culture is shown. Observed standard deviations are explained in footnote a of Table 2. The resulting error bars are omitted for the sake of clarity. Pyruvate (•), lactate (□), acetate (▿), and succinate (▵) were detected by using HPLC analysis of the supernatant as described in Materials and Methods.
Previously, viable but nonculturable cells which were not detectable by the plate counting approach were obtained under conditions of oxygen starvation (6). Therefore, we determined the total cell number in a Thoma counting chamber. Additionally, we stained the cells with two different dyes specific for viable cells or dead cells, resulting in green (viable) or red (dead) fluorescence after UV excitation. Using a fluorescence microscope, we observed over a period of 18 days predominantly (more than 95%) green fluorescence and, thus, viable P. aeruginosa cells cultivated in the presence of 20 mM pyruvate under anaerobic conditions. The total cell numbers observed via Thoma counting remained constant over the same period. These observations clearly indicate that P. aeruginosa cells were viable and confirmed the data obtained from the plate counts. In contrast, the total cell number of a P. aeruginosa culture without addition of pyruvate significantly decreased over the same period when analyzed by the Thoma counting chamber. Moreover, the decreased cell numbers of the cultures contained a larger number of dead cells (up to 25%) when analyzed by the differential viable/dead staining method. The latter results are in agreement with previous observations (6). Furthermore, these results clearly demonstrate that pyruvate is essential for long-term survival under anaerobic conditions. Interestingly, pyruvate could not be replaced by other substrates, such as glucose or gluconate, for fermentation. Similarly, the combination of pyruvate with glucose or gluconate did not change the survival rate (data not shown).
P. aeruginosa anaerobically converts pyruvate into lactate, acetate, and succinate.
Because pyruvate was found to be essential for anaerobic survival of P. aeruginosa, we analyzed the growth medium for possible pyruvate fermentation products. HPLC analysis measured increasing amounts of lactate, acetate, and succinate accompanied by a stoichiometric decrease of the pyruvate concentration (Fig. 2B). P. aeruginosa formed under the following anaerobic conditions: 0.56 mol of lactate (9.0 mM), 0.39 mol of acetate (6.3 mM), and 0.04 mol of succinate (0.7 mM) per mol of pyruvate (16.2 mM) (Table 2). No additional compounds, such as ethanol, acetoin, formate, or 2,3-butanediol, were identified during HPLC analysis. The complete stoichiometric conversion of pyruvate to the corresponding fermentation products after 18 days coincided with the beginning of the decrease of viable cell counts (Fig. 2A), underscoring the importance of pyruvate fermentation for the anaerobic survival of P. aeruginosa.
TABLE 2.
Fermentation product formation by wild-type P. aeruginosa and several mutantsa
| P. aeruginosa strains | Pyruvate consumed (mM) | Products formed
|
||||||
|---|---|---|---|---|---|---|---|---|
| Concn (mM)
|
Product/pyruvate (mol/mol)
|
|||||||
| Lactate | Acetate | Succinate | Lactate | Acetate | Succinate | Total | ||
| Wild type | 16.2 | 9.0 | 6.3 | 0.7 | 0.56 | 0.39 | 0.04 | 0.99 |
| ldhA mutant | 1.1 | NDb | 0.6 | 0.5 | NDb | 0.55 | 0.45 | 1.00 |
| pta mutant | 4.8 | 2.2 | 1.5 | 0.4 | 0.46 | 0.31 | 0.08 | 0.85 |
| IHF mutant | 0.4 | NDb | 0.5 | 0.3 | NDb | NCc | NCc | NCc |
| anr mutant | 11.8 | 6.5 | 4.5 | 0.4 | 0.55 | 0.38 | 0.03 | 0.96 |
| dnr mutant | 15.3 | 8.5 | 6.6 | 0.3 | 0.55 | 0.43 | 0.02 | 1.00 |
| narL mutant | 16.3 | 9.1 | 6.4 | 0.6 | 0.56 | 0.39 | 0.04 | 0.99 |
Indicated strains were incubated anaerobically at an OD578 of 0.3 (approximately 6×108 CFU/ml) for 23 days at 37°C in OS medium using 20 mM pyruvate as sole carbon source. The amounts of pyruvate consumed and fermentation products formed were quantified by HPLC analysis of the growth media as described in Materials and Methods. CO2 formed and redox-equivalent turnover, as outlined in Fig. 1, have to be added to balance the fermentation equation. Experiments were repeated three times. The standard deviation of determined concentrations above 9 mM was below 5%, standard deviation of determined concentrations between 1.1 and 9 mM was below 8.5%, and standard deviation of determined concentrations below 1.1 mM was below 22%.
ND, not detected.
NC, not calculated.
In a control experiment we tested the fermentation products acetate (6.5 mM), lactate (9.0 mM), and succinate (0.7 mM), alone and in combination, for their ability to support long-term anaerobic survival in OS medium. However, none of the products or the combination sustained anaerobic survival (data not shown).
These results confirmed our initially proposed model of a mixed acid pyruvate fermentation in P. aeruginosa. The one exception was that we did not observe a significant conversion of acetyl-CoA into ethanol (dashed line in Fig. 1).
Effect of external pH, nitrate respiration, and arginine fermentation on pyruvate fermentation.
Resting cells of P. aeruginosa with an OD578 of 4 were incubated anaerobically with 50 mM pyruvate at the following external pH values: 5.0 in 100 mM morpholinepropanesulfonic acid (MES) buffer and 6.0, 7.0, and 8.0 in 100 mM phosphate buffer. Cell suspensions of P. aeruginosa in phosphate buffer with pH values of 6.0 and 7.0 were used to perform pyruvate fermentation as described above, which was followed by HPLC analysis. Cell suspensions in MES buffer with a pH of 5.0 neither consumed significant amounts of pyruvate nor formed lactate, acetate, and succinate (data not shown). At a pH of 8.0 in phosphate buffer there was only limited pyruvate consumption and subsequent formation of lactate, acetate, and succinate, indicating a limited pH range of 6 to 7 for pyruvate fermentation (data not shown).
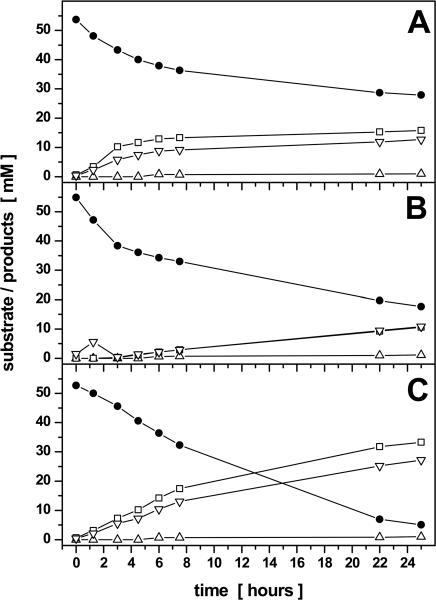
Pyruvate fermentation of P. aeruginosa in the presence of the electron acceptor nitrate was monitored. Nitrate was completely converted into nitrite after 3 h of incubation, which in turn was converted to nitrogen after 6 h (data not shown). Pyruvate was consumed rapidly, but no lactate or succinate was detected by HPLC analysis after 1.5 and 3 h (Fig. 3B). Acetate was only formed transiently at about 1.5 h and was rapidly consumed. Similar observations had previously been made for E. coli and were explained as a glucose overflow metabolism (41). After 3 h of incubation, when all nitrate had been converted into nitrite, pyruvate consumption slowed down and finally resulted in the formation of lactate, acetate, and succinate (compare Fig. 3B to C). These observations clearly indicate the complete metabolization of pyruvate via the Krebs cycle during nitrate respiration analogous to pyruvate metabolization during aerobic growth conditions. Only after full utilization of the primary alternative electron acceptor nitrate does the organism use pyruvate via a fermentative mode of energy generation.
FIG. 3.
Kinetics of pyruvate degradation and product formation by resting cells of P. aeruginosa in 100 mM phosphate buffer (pH 7.4). The cells were grown for 21 h in OS minimal medium with 50 mM nitrate as electron acceptor and 10 mM gluconate-20 mM pyruvate as carbon source under oxygen limitation and then were harvested and washed in phosphate buffer. Finally, the cell suspensions (OD578 between 8 and 9) of bacteria were incubated under anoxic conditions with the addition of 50 mM arginine (A), 50 mM nitrate (B), or without further additions (C). Pyruvate (•), lactate (□), acetate (▿), and succinate (▵) were detected by HPLC analysis of the supernatant as described in Materials and Methods. Graphs represent the results of three independent experiments; standard deviations were below 7% for the determined pyruvate, lactate, and acetate concentrations and below 15% for the already low succinate concentrations.
Moreover, pyruvate was fermented in the presence of arginine, another fermentable substrate. As expected, pyruvate was completely converted into its typical fermentation products from the beginning of incubation (Fig. 3A). After 4.5 h, the pH of the medium had increased to 8.0 because of NH3 generated by arginine fermentation. From that time point pyruvate was only very slowly fermented (Fig. 3A). This was in accordance with the experiment described above, which was performed at a pH of 8. Clearly, both fermentation processes can be maintained in parallel. In theory, the parallel fine-tuned activity of both pathways, the acid-producing pyruvate fermentation and NH3-generating arginine fermentation, would contribute to the stabilization of an appropriate extracellular pH value for long-term survival.
The gacS-ldhA and ackA-pta loci are required for pyruvate fermentation.
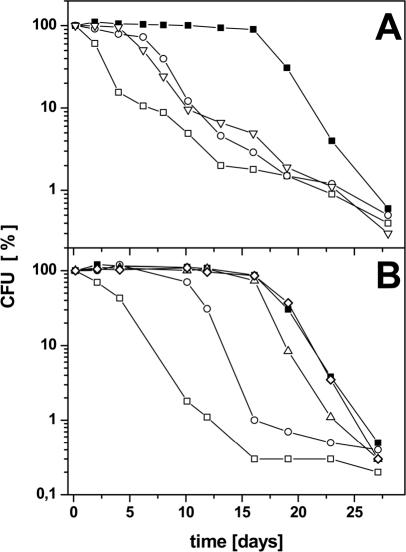
Two loci on the P. aeruginosa chromosome, ldhA, encoding a fermentative lactate dehydrogenase, and ackA-pta, encoding acetate kinase and Pta, were investigated for their potential involvement in the observed fermentative metabolism and its corresponding regulation. The mutants PAO-ME1 (Δpta) and PAO-ME3 (ΔldhA) were constructed and tested for their long-term anaerobic survival capacities and for the production of fermentation products. The viable cell numbers of both mutants decreased significantly upon anaerobic incubation in the presence of pyruvate (Fig. 4A). HPLC analysis of the supernatant of an ldhA knockout mutant culture after 23 days of anaerobic incubation showed neither the consumption of significant amounts of pyruvate nor the formation of lactate, acetate, succinate, or other products (Table 2). However, in the supernatant of the pta knockout mutant a small decrease in the concentration of pyruvate (from 20 to 15.2 mM) as well as small amounts of lactate (2.2 mM), acetate (1.5 mM), and succinate (0.4 mM) were measured. These observations are explained by the activity of other Ptas in P. aeruginosa specific for metabolites, such as propionyl-CoA or butyryl-CoA, which also can accept acetyl-CoA as substrate to a lesser degree. On the other hand, the action of the respiratory pyruvate oxidase (PoxB), which catalyzes the one-step conversion of pyruvate into acetate and carbon dioxide, might account for the small increase of acetate observed in the pta mutant.
FIG. 4.
(A) Anaerobic survival of P. aeruginosa wild type (▪), ldhA mutant (○), and pta mutant (▿) in the presence of 20 mM pyruvate. CFU of wild-type P. aeruginosa without pyruvate served as a control (□). P. aeruginosa wild type and mutants were grown aerobically in OS minimal medium using 20 mM gluconate as sole carbon source. At an OD578 of 0.3, cells and media were transferred to rubber-stoppered bottles and 20 mM pyruvate was added. Survival under anaerobic conditions without alternative electron acceptors was determined with viable cell counts on agar plates and was confirmed with fluorescence microscopy using the LIVE/DEAD BacLight kit as outlined in Materials and Methods. Graphs represent the results of at least three independent experiments. Standard deviations were found comparable to those shown for the experiments in Fig. 2A and were omitted for the sake of clarity. (B) Anaerobic survival of P. aeruginosa wild type (▪), anr mutant (○), dnr mutant (▵), narL mutant (◊), and IHF mutant (□) in the presence of 20 mM pyruvate. P. aeruginosa wild type and mutants were grown aerobically in OS minimal medium with 20 mM gluconate as sole carbon source. At an OD578 of 0.3 cells were transferred to rubber-stoppered bottles and 20 mM pyruvate was added. Survival under anaerobic conditions without end electron acceptors was determined with viable cell counts on agar plates as outlined in Materials and Methods. Graphs represent the results of at least three independent experiments. Standard deviations were found comparable to those shown for the experiments in Fig. 2A and were omitted for the sake of clarity.
These results obtained from the ΔldhA and Δpta mutants confirm that the investigated ORFs are coding for the enzymes essential for pyruvate fermentation as proposed in our model (Fig. 1). Moreover, the almost complete failure of both systems, one NAD+ regenerating, the other ATP producing, in single chromosomal mutants indicates their close cooperation during long-term survival and pyruvate formation.
Expression of the ackA-pta locus is regulated by oxygen tension via Anr and integration host factor (IHF).
To investigate the oxygen tension-dependent expression of the ldhA and ackA-pta loci, DNA microarray experiments were performed. Whole cellular RNA preparations were screened for the presence of ldhA-, ackA-, and pta-specific mRNA formation. RNA preparations from aerobically and anaerobically grown P. aeruginosa were compared. No obvious changes in ldhA expression in response to changes in oxygen tension were observed (Table 3). In agreement with these findings, there were no obvious differences in NADH-dependent lactate dehydrogenase activity in the cell extracts that were prepared from aerobically and anaerobically grown P. aeruginosa.
TABLE 3.
Expression of ackA, pta, and ldhA in wild-type P. aeruginosa and various mutants depending on oxygen tensiona
| Strain | Analyzed parameter and condition
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
ldhA mRNA
|
ackA mRNA
|
pta mRNA
|
ackA-lacZ (Miller units)
|
Pta activity (U/mg)d
|
||||||
| Aerobic | Anaerobice | Aerobic | Anaerobic | Aerobic | Anaerobic | Aerobic | Anaerobic | Aerobic | Anaerobic | |
| Wild type | 3.6 ± 0.14b | 2.8 ± 1.3b | 25.6 ± 1.2b | 142.8 ± 32b | 13.4 ± 3.0b | 90.8 ± 33b | 95 ± 7 | 355 ± 38 | 4.2 ± 0.5 | 24 ± 1.3 |
| Δanr | NDc | 2.9 ± 1.5b | ND | 50.3 ± 4.1b | ND | 25.1 ± 6.1b | 90 ± 5 | 110 ± 13 | ||
| dnr::tet | ND | 3.3 ± 1.5b | ND | 152 ± 0b | ND | 140 ± 18b | 104 ± 15 | 201 ± 14 | ||
| ifhA::tet | 45 ± 6 | 90 ± 6 | ||||||||
| narL::cat | 102 ± 7 | 290 ± 14 | ||||||||
| ldhA::aacC1 | 4.3 ± 0.7 | 26 ± 1.8 | ||||||||
| pta::aacC1 | 0.4 ± 0.2 | 0.5 ± 0.2 | ||||||||
The results were obtained from microarray experiments and from a ackA-lacZ fusion. The Pta activity in P. aeruginosa wild type and the Δpta and ΔldhA mutants were determined as described in Materials and Methods.
Observed signal strength is given in normalized Affymetrix units as defined in Materials and Methods (5, 8). The standard deviation represents the detected differences between the independently performed GeneChip experiments.
ND, not determined. Because Anr and Dnr activity is known to be limited to anaerobic conditions, analysis of aerobically grown cells was not performed.
One unit of activity is the amount of enzyme that converts 1 μmol of acetyl-CoA to product per 60 min at 30°C.
Anaerobic conditions for the microarrays, the reporter gene fusions, and enzyme activity measurements differed depending on the nature of determined parameters. Due to the short half-life of the mRNAs, the RNA for the microarray analysis was prepared 2 h after the shift to anaerobic conditions. Reporter gene fusion expression was determined after 48 h of anaerobic incubation of the culture, while Pta activity was measured after 24 h of fermentative conditions. Employed media, test principles, and given units are defined in Materials and Methods.
However, the expression of the ackA-pta operon was increased sixfold under anaerobic conditions. Due to the short half-life of mRNA, we failed to follow the transcriptional profile for a longer time period. For that purpose, the complete promoter region of the P. aeruginosa ackA-pta locus was fused to the lacZ reporter gene. After 2 days under pyruvate fermentation conditions, the induction of this reporter gene fusion was 3.7-fold that under aerobic conditions. The observed induction factor was a little lower than the observed sixfold induction by the DNA microarray approach. In order to confirm the anaerobic induction of the ackA-pta loci at the enzyme level, Pta enzyme assays were performed. Pta activity in cell extracts increased about sixfold under anaerobic condition compared to that under aerobic growth conditions (Table 3). Interestingly, in the ΔldhA mutant full anaerobic induction of Pta formation was observed. Nevertheless, in the absence of lactate dehydrogenase (ΔldhA) no obvious pyruvate utilization and long-term survival was observed, indicating the close interplay between both enzymatic systems. As expected, no significant pta activity was found in the pta mutant.
In order to identify the regulatory loci involved in the oxygen-dependent control of ackA-pta expression, DNA microarray and ackA-lacZ reporter gene fusion experiments with various regulatory mutants were performed. Both strategies revealed the essential role of the anr and ihfA loci for anaerobic ackA-pta induction (Table 3). The anr gene encodes an Fnr type of regulatory protein which most likely contains a 4Fe-4S cluster responsible for oxygen tension perception (18, 19, 34). The ihfA locus encodes one subunit of the DNA-bending IHF (13, 14). The β-galactosidase activity of the ackA-lacZ reporter gene fusion was measured in the anr, dnr, narL, and ihfA mutant strains after 2 days of incubation under fermentative conditions. Expression of the ackA-lacZ reporter gene fusion in wild-type P. aeruginosa increases 3.7-fold under fermentative conditions. There was no increase in β-galactosidase activity of the anr mutant, whereas β-galactosidase activity of the ihfA mutant was even a little lower under fermentative conditions compared to that of the anr mutant (Table 3). The narL mutant strains revealed a 2.9-fold increase of reporter gene expression under fermentative conditions comparable to wild-type conditions. The dnr mutant led to an only slightly decreased ackA-lacZ expression. In agreement with this, both mutant strains revealed efficient anaerobic long-term survival (Fig. 4B).
The putative promoter region in front of the ackA-pta locus was scanned for potential binding sites of known regulatory proteins of P. aeruginosa by using the weight matrix search program of the PRODORIC database (26). The search revealed the presence of the Anr-Dnr binding motif TTGATTTTCATCAG (88% identity to the Anr consensus) and the IHF binding motif CAACACCTGCGCCAC (89% identity to the IHF consensus), which are localized 182 to 195 bp and 122 to 136 bp upstream of the translational start codon of ackA, respectively.
The anr and ihfA genes are required for overall pyruvate fermentation.
P. aeruginosa anr, dnr, ihfA, and narL deletion mutants were grown aerobically to an OD578 of 0.3 followed by addition of pyruvate and cultivation under anaerobic conditions for 4 weeks as described for wild-type P. aeruginosa. Total and viable cell numbers with three different approaches were determined, and HPLC analyses from the supernatant of all mutants were performed. The viable cell numbers of the anr mutant had already decreased after 10 days (Fig. 4B), and HPLC analysis showed no further conversion from pyruvate to lactate, acetate, and succinate after that time (data not shown). In agreement with the anr-dependent ackA-pta expression, this oxygen regulator is required for a prolonged survival of P. aeruginosa under anaerobic conditions using the pyruvate fermentation pathway in the absence of external electron acceptors. In contrast, the dnr mutant maintained constant viable cell numbers under the anaerobic conditions tested, comparable to those of P. aeruginosa wild type (Fig. 4B). Additionally, the HPLC analysis of the dnr mutant culture supernatants showed no difference in the kinetics of pyruvate consumption and product formation in comparison to the wild type (Table 2).
The viable cell number of a P. aeruginosa ihfA knockout mutant decreased logarithmically from the start of anaerobic cultivation down to 0.3% survivors after 16 days (Fig. 4B). HPLC analysis detected neither pyruvate utilization nor product formation (Table 2). The ihfA gene encodes one subunit of the IHF, a DNA bending protein, which is essential for pyruvate formation. To exclude the possibility that the tested mutant P. aeruginosa strains did not survive due to reduced cellular concentrations of protective catalases or peroxidases, cells were plated on LB agar containing 20 mM pyruvate. After anaerobic incubation the plates were removed and further incubated aerobically for 24 h. The observed numbers of CFU remained unchanged compared to those of solely aerobically grown cells.
No differences in the viable counts or the HPLC profiles were observed for a knockout mutant in the narL gene in comparison to those of the wild type (data not shown). We did not detect a significant participation of the stationary sigma factor RpoS nor the response regulator GacA on anaerobic survival. Survival of the mutant strains was comparable to that of the wild type. Obviously, pyruvate fermentation is not a typical stress condition which activates RpoS or GacA, even when P. aeruginosa is not growing. RpoS and GacA are certainly essential for the anaerobic starvation that follows after pyruvate has been consumed.
Conclusions.
(i) Under anaerobic growth conditions, in the absence of alternative electron acceptors P. aeruginosa utilizes the conversion of pyruvate into acetate and lactate for long-term survival. This process does not contribute to anaerobic cell growth. (ii) Long-term survival is sustained by NAD+-regenerating lactate dehydrogenase, a Pta, and an ATP-generating acetate kinase. These enzymes are encoded by ldhA and the ackA-pta operon. The ackA-pta operon is induced under oxygen-limiting conditions by the oxygen regulator Anr and DNA-bending IHF, whereas the ldhA gene expression is independent of oxygen tension. (iii) The observed pyruvate fermentation may contribute to the survival of P. aeruginosa in biofilms and during host infection, when oxygen-limiting conditions dominate and nitrate concentration is limited.
Acknowledgments
We thank Barbara Schulz for critically reading the manuscript and Dieter Haas (Université de Lausanne, Switzerland) for providing the P. aeruginosa gacA and anr mutants. We are indebted to Hiruide Arai (Riken Institute, Tokyo, Japan) for the P. aeruginosa dnr mutant and E. P. Greenberg (University of Iowa) for the rpoS mutant strain.
The investigation was funded by the Deutsche Forschungsgemeinschaft, the German Research Centre for Biotechnology, and the Fonds der Chemischen Industrie to D.J.
REFERENCES
- 1.Aiba, H., S. Adhya, and B. de Crombrugghe. 1981. Evidence for two gal promoters in intact Escherichia coli. J. Biol. Chem. 256:11905-11910. [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai, H., Y. Igarashi, and T. Kodama. 1995. Expression of the nir and nor genes for denitrification of Pseudomonas aeruginosa requires a novel CRP/FNR-related transcriptional regulator, DNR, in addition to ANR. FEBS Lett. 371:73-76. [DOI] [PubMed] [Google Scholar]
- 4.Arai, H., T. Kodama, and Y. Igarashi. 1997. Cascade regulation of the two CRP/FNR-related transcriptional regulators (ANR and DNR) and the denitrification enzymes in Pseudomonas aeruginosa. Mol. Microbiol. 25:1141-1148. [DOI] [PubMed] [Google Scholar]
- 5.Bakay, M., Y. W. Chen, R. Borup, P. Zhao, K. Nagaraju, and E. P. Hoffman. 2002. Sources of variability and effect of experimental approach on expression profiling data interpretation. BMC Bioinformatics 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binnerup, S. J., and J. Sorensen. 1993. Long-term oxidant deficiency in Pseudomonas aeruginosa PAO303 results in cells which are non-culturable under aerobic conditions. FEMS Microbiol. Ecol. 13:79-84. [Google Scholar]
- 7.Bock, A., and G. Sawers. 1996. Fermentation, p. 262-282. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella, cellular and molecular biology, 2nd ed., vol. I. ASM Press, Washington, D.C.
- 8.Chen, Y. W., P. Zhao, R. Borup, and E. P. Hoffman. 2000. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J. Cell Biol. 151:1321-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, D. J., and O. Maaløe. 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23:99-112. [Google Scholar]
- 10.Cruz Ramos, H., T. Hoffmann, M. Marino, H. Nedjari, E. Presecan-Siedel, O. Dreesen, P. Glaser, and D. Jahn. 2000. Fermentative metabolism of Bacillus subtilis: physiology and regulation of gene expression. J. Bacteriol. 182:3072-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, K. J., D. Lloyd, and L. Boddy. 1989. The effect of oxygen on denitrification in Paracoccus denitrificans and Pseudomonas aeruginosa. J. Gen. Microbiol. 135:2445-2451. [DOI] [PubMed] [Google Scholar]
- 12.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405 [DOI] [PubMed] [Google Scholar]
- 13.Delic-Attree, I., B. Toussaint, A. Froger, J. C. Willison, and P. M. Vignais. 1996. Isolation of an IHF-deficient mutant of a Pseudomonas aeruginosa mucoid isolate and evaluation of the role of IHF in algD gene expression. Microbiology 142:2785-2793. [DOI] [PubMed] [Google Scholar]
- 14.Delic-Attree, I., B. Toussaint, and P. M. Vignais. 1995. Cloning and sequence analyses of the genes coding for the integration host factor (IHF) and HU proteins of Pseudomonas aeruginosa. Gene 154:61-64. [DOI] [PubMed] [Google Scholar]
- 15.Dunn, N. W., and B. W. Holloway. 1971. Pleiotrophy of p-fluorophenylalanine-resistant and antibiotic hypersensitive mutants of Pseudomonas aeruginosa. Genet. Res. 18:185-197. [DOI] [PubMed] [Google Scholar]
- 16.Eschbach, M., H. Mobitz, A. Rompf, and D. Jahn. 2003. Members of the genus Arthrobacter grow anaerobically using nitrate ammonification and fermentative processes: anaerobic adaptation of aerobic bacteria abundant in soil. FEMS Microbiol. Lett. 223:227-230. [DOI] [PubMed] [Google Scholar]
- 17.Farinha, M. A., and A. M. Kropinski. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J. Bacteriol. 172:3496-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galimand, M., M. Gamper, A. Zimmermann, and D. Haas. 1991. Positive FNR-like control of anaerobic arginine degradation and nitrate respiration in Pseudomonas aeruginosa. J. Bacteriol. 173:1598-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green, J., C. Scott, and J. R. Guest. 2001. Functional versatility in the CRP-FNR superfamily of transcription factors: FNR and FLP. Adv. Microb. Physiol. 44:1-34. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 21.Hassett, D. J., J. Cuppoletti, B. Trapnell, S. V. Lymar, J. J. Rowe, S. Sun Yoon, G. M. Hilliard, K. Parvatiyar, M. C. Kamani, D. J. Wozniak, S. H. Hwang, T. R. McDermott, and U. A. Ochsner. 2002. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug. Deliv. Rev. 54:1425-1443. [DOI] [PubMed] [Google Scholar]
- 22.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 23.Krieger, R., A. Rompf, M. Schobert, and D. Jahn. 2002. The Pseudomonas aeruginosa hemA promoter is regulated by Anr, Dnr, NarL and integration host factor. Mol. Genet. Genomics 267:409-417. [DOI] [PubMed] [Google Scholar]
- 24.Mercenier, A., J. P. Simon, C. Vander Wauven, D. Haas, and V. Stalon. 1980. Regulation of enzyme synthesis in the arginine deiminase pathway of Pseudomonas aeruginosa. J. Bacteriol. 144:159-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, J. M. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria, p. 72-74. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Munch, R., K. Hiller, H. Barg, D. Heldt, S. Linz, E. Wingender, and D. Jahn. 2003. PRODORIC: prokaryotic database of gene regulation. Nucleic Acids Res. 31:266-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano, M. M., Y. P. Dailly, P. Zuber, and D. P. Clark. 1997. Characterization of anaerobic fermentative growth of Bacillus subtilis: identification of fermentation end products and genes required for growth. J. Bacteriol. 179:6749-6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano, M. M., and P. Zuber. 1998. Anaerobic growth of a “strict aerobe” (Bacillus subtilis). Annu. Rev. Microbiol. 52:165-190. [DOI] [PubMed] [Google Scholar]
- 29.Palleroni, N. J. 1984. Family I. Pseudomonaceae, p. 141-219. In N. R. Krieg and J. G. Hold (ed.), Bergey's manual of systematic bacteriology. The Williams and Wilkins Co., Baltimore, Md.
- 30.Reimmann, C., M. Beyeler, A. Latifi, H. Winteler, M. Foglino, A. Lazdunski, and D. Haas. 1997. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309-319. [DOI] [PubMed] [Google Scholar]
- 31.Rompf, A., C. Hungerer, T. Hoffmann, M. Lindenmeyer, U. Romling, U. Gross, M. O. Doss, H. Arai, Y. Igarashi, and D. Jahn. 1998. Regulation of Pseudomonas aeruginosa hemF and hemN by the dual action of the redox response regulators Anr and Dnr. Mol. Microbiol. 29:985-997. [DOI] [PubMed] [Google Scholar]
- 32.Sabra, W., E. J. Kim, and A. P. Zeng. 2002. Physiological responses of Pseudomonas aeruginosa PAO1 to oxidative stress in controlled microaerobic and aerobic cultures. Microbiology 148:3195-3202. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.Sawers, R. G. 1991. Identification and molecular characterization of a transcriptional regulator from Pseudomonas aeruginosa PAO1 exhibiting structural and functional similarity to the FNR protein of Escherichia coli. Mol. Microbiol. 5:1469-1481. [DOI] [PubMed] [Google Scholar]
- 35.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 36.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 37.Thompson, D. K., and J. S. Chen. 1990. Purification and properties of an acetoacetyl coenzyme A-reacting phosphotransbutyrylase from Clostridium beijerinckii (“Clostridium butylicum”) NRRL B593. Appl. Environ. Microbiol. 56:607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vander Wauven, C., A. Pierard, M. Kley-Raymann, and D. Haas. 1984. Pseudomonas aeruginosa mutants affected in anaerobic growth on arginine: evidence for a four-gene cluster encoding the arginine deiminase pathway. J. Bacteriol. 160:928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whiteley, M., M. R. Parsek, and E. P. Greenberg. 2000. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 182:4356-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Doring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu, B., M. Jahic, G. Blomsten, and S. O. Enfors. 1999. Glucose overflow metabolism and mixed-acid fermentation in aerobic large-scale fed-batch processes with Escherichia coli. Appl. Microbiol. Biotechnol. 51:564-571. [DOI] [PubMed] [Google Scholar]
- 42.Xu, K. D., P. S. Stewart, F. Xia, C. T. Huang, and G. A. McFeters. 1998. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 64:4035-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye, R. W., D. Haas, J. O. Ka, V. Krishnapillai, A. Zimmermann, C. Baird, and J. M. Tiedje. 1995. Anaerobic activation of the entire denitrification pathway in Pseudomonas aeruginosa requires Anr, an analog of Fnr. J. Bacteriol. 177:3606-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell. 3:593-603. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann, A., C. Reimmann, M. Galimand, and D. Haas. 1991. Anaerobic growth and cyanide synthesis of Pseudomonas aeruginosa depend on anr, a regulatory gene homologous with fnr of Escherichia coli. Mol. Microbiol. 5:1483-1490. [DOI] [PubMed] [Google Scholar]
- 46.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]