Significance
α1-Adrenergic receptors are important for the regulation of vascular function and are targeted clinically for blood pressure control. Here, we provide evidence that α1A/B-adrenergic receptors (AR) form heteromeric complexes with chemokine (C-X-C motif) receptor 4 (CXCR4) on the cell surface of vascular smooth muscle cells. We show that disruption of α1A/B-AR:CXCR4 heteromeric complexes inhibits α1-AR–mediated functions in vascular smooth muscle cells and that treatment with CXCR4 agonists enhances the potency of the α1-AR agonist phenylephrine to increase blood pressure. These findings extend the current understanding of the molecular mechanisms regulating α1-AR and provide an example of G protein-coupled receptor heteromerization with important functional implications. Compounds targeting the α1A/B-AR:CXCR4 interaction could provide an alternative pharmacological approach to modulating blood pressure.
Keywords: CXCL12, ubiquitin, AMD3100, phenylephrine, blood pressure
Abstract
Recent evidence suggests that chemokine (C-X-C motif) receptor 4 (CXCR4) contributes to the regulation of blood pressure through interactions with α1-adrenergic receptors (ARs) in vascular smooth muscle. The underlying molecular mechanisms, however, are unknown. Using proximity ligation assays to visualize single-molecule interactions, we detected that α1A/B-ARs associate with CXCR4 on the cell surface of rat and human vascular smooth muscle cells (VSMC). Furthermore, α1A/B-AR could be coimmunoprecipitated with CXCR4 in a HeLa expression system and in human VSMC. A peptide derived from the second transmembrane helix of CXCR4 induced chemical shift changes in the NMR spectrum of CXCR4 in membranes, disturbed the association between α1A/B-AR and CXCR4, and inhibited Ca2+ mobilization, myosin light chain (MLC) 2 phosphorylation, and contraction of VSMC upon α1-AR activation. CXCR4 silencing reduced α1A/B-AR:CXCR4 heteromeric complexes in VSMC and abolished phenylephrine-induced Ca2+ fluxes and MLC2 phosphorylation. Treatment of rats with CXCR4 agonists (CXCL12, ubiquitin) reduced the EC50 of the phenylephrine-induced blood pressure response three- to fourfold. These observations suggest that disruption of the quaternary structure of α1A/B-AR:CXCR4 heteromeric complexes by targeting transmembrane helix 2 of CXCR4 and depletion of the heteromeric receptor complexes by CXCR4 knockdown inhibit α1-AR–mediated function in VSMC and that activation of CXCR4 enhances the potency of α1-AR agonists. Our findings extend the current understanding of the molecular mechanisms regulating α1-AR and provide an example of the importance of G protein-coupled receptor (GPCR) heteromerization for GPCR function. Compounds targeting the α1A/B-AR:CXCR4 interaction could provide an alternative pharmacological approach to modulate blood pressure.
Chemokine (C-X-C motif) receptor 4 (CXCR4) is a G protein-coupled receptor (GPCR) that is essential during development. Animals lacking CXCR4 are not viable and demonstrate defects of the hematopoietic and cardiovascular system (1). After birth, CXCR4 is expressed in many tissues, including the heart and vasculature, and fulfills multiple functions in the immune system, such as regulation of leukocyte trafficking, stem cell mobilization, and homing (2, 3). Moreover, CXCR4 is involved in various disease processes, such as HIV infection, cancer metastasis, and tissue repair (3–5).
In addition to these established functions, recent observations suggest that CXCR4 also contributes to the regulation of hemodynamics and blood pressure. Treatment with the CXCR4 antagonists AMD3100 and AMD3465 reduced blood pressure in experimental models of pulmonary arterial and systemic hypertension (6, 7). We have shown previously that AMD3100 reduces hemodynamic stability and blood pressure during the cardiovascular stress response to traumatic and hemorrhagic shock, whereas selective activation of CXCR4 with the noncognate agonist ubiquitin improves hemodynamic stability and increases systemic blood pressure after traumatic, hemorrhagic, and endotoxic shock (8–13). Because in vivo pharmacological targeting of CXCR4 did not affect myocardial function, these findings suggested that effects of CXCR4 on hemodynamics and blood pressure are mediated via modulation of vascular function (9). Accordingly, we observed that CXCR4 activation enhances and sensitizes vasoconstriction of isolated mesenteric arteries and veins in response to α1-adrenergic receptor (AR) activation with phenylephrine (PE) (9). As these effects were independent of the vascular endothelium, interactions between CXCR4 and α1-AR in vascular smooth muscle likely constitute the physiological basis for these observations (9). The molecular mechanisms underlying interactions between CXCR4 and α1-AR in vascular smooth muscle, however, remain unknown.
Crosstalk between GPCRs is a widely recognized principle that expands the physiological repertoire of GPCR-mediated signaling events and functions (14–19). Receptor crosstalk can be attributed to a variety of molecular mechanisms, including receptor hetero-oligomerization (14–23). The formation of homo- and/or hetero-oligomeric complexes among GPCRs is thought to be important for many aspects of GPCR function (22–24).
CXCR4 has been shown to associate with multiple chemokine receptors in various expression systems (3, 25–28). ARs are also known to be able to form heteromeric receptor complexes (29–35), and recent evidence suggests that AR may also be able to form heteromeric complexes with chemokine receptors (36–38). Thus, we studied whether α1-AR and CXCR4 may interact on the cell surface of vascular smooth muscle cells through the formation of heteromeric receptor complexes.
Here, we provide evidence that heteromeric receptor complexes between α1A-AR and CXCR4 and between α1B-AR and CXCR4 are constitutively expressed in rat and human vascular smooth muscle cells (VSMC). We show that disruption of the quaternary structure of the heteromeric receptor complex by targeting transmembrane helix (TM) 2 of CXCR4 and depletion of heteromeric receptor complexes by CXCR4 knockdown inhibit α1-AR agonist-induced key signaling events and contraction of VSMC. Furthermore, we show that treatment with CXCR4 agonists increases the potency of the α1-AR agonist PE to increase blood pressure in vivo. Our observations suggest that α1-AR function in VSMC is controlled through the formation of heteromeric α1A/B-AR:CXCR4 complexes.
Results and Discussion
α1A/B-AR Associates with CXCR4 on the Cell Surface of Vascular Smooth Muscle Cells.
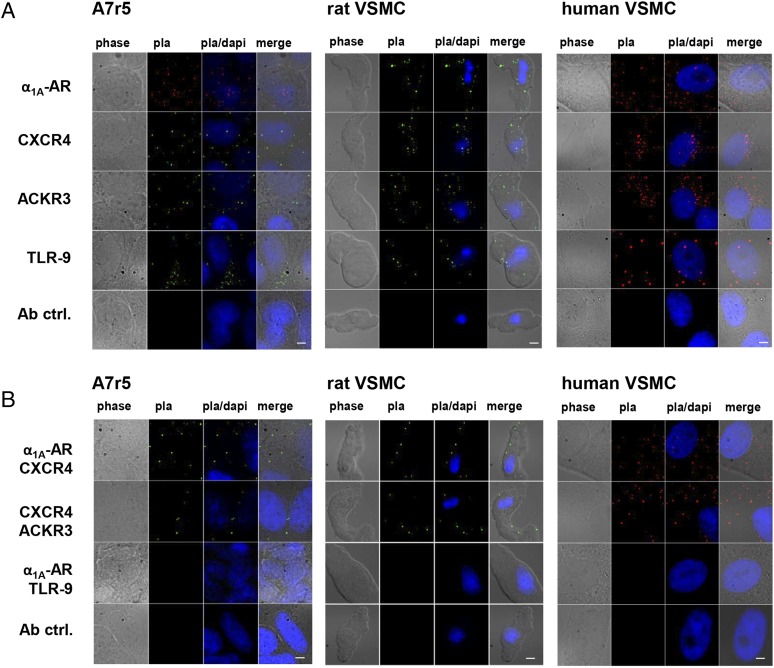
We sought to evaluate whether heteromeric complexes between α1-AR and CXCR4 are expressed on the cell surface of VSMC. Thus, we used proximity ligation assays (PLA) to visualize individual receptors and receptor–receptor interactions (39–41). PLA have been previously used to observe individual proteins and interactions between individual endogenous proteins at a single-molecule resolution (39). We first used PLA to detect CXCR4 and α1A-AR in a format suited for high sensitivity detection of a single protein. As a positive control, we also tested for atypical chemokine receptor (ACKR) 3 (formerly known as RDC1 and CXCR7), which is known to be able to form heteromeric complexes with CXCR4 (3, 28). Toll-like receptor (TLR) 9 was used as a negative control receptor that is unlikely to be associated with α1A-AR. All individual receptors could be visualized by PLA in the rat vascular smooth muscle cell line A7r5, on freshly isolated aortic rat VSMC, and on primary human aortic VSMC (Fig. 1A). When PLA was used to visualize protein–protein interactions at a single-molecule level, we validated the proximity of CXCR4 and ACKR3 in native cells (28) and also detected signals suggesting close proximity of α1A-AR:CXCR4 in A7r5 cells and in rat and human VSMC (Fig. 1B). In contrast, PLA signals for α1A-AR:TLR9 interactions were not detectable (Fig. 1B).
Fig. 1.
α1A-AR and CXCR4 are in close proximity on the cell surface of rat and human vascular smooth muscle cells. (Scale bars: 10 μm.) phase: phase contrast. PLA: PLA fluorescence signal. PLA/DAPI: merged PLA/DAPI signals. Merge: merged phase-contrast/PLA/DAPI signals. (A) Detection of α1A-AR, CXCR4, ACKR3, and TLR-9 on A7r5 cells and on rat and human VSMC by PLA. Ab ctrl.: omission of the primary antibody. Images are representative of three independent experiments. (B) Detection of receptor–receptor associations on A7r5 cells and on rat and human VSMC by PLA. Ab ctrl.: omission of one secondary antibody after incubation with anti–α1A-AR/anti-CXCR4 (rat VSMC) or anti-CXCR4/anti-ACKR3 (A7r5 and human VSMC). Images are representative of three independent experiments.
To define whether all α-ARs associate with CXCR4, we then evaluated human VSMC for interactions between α1-AR and α2-AR subtypes with CXCR4. All α1-AR and α2-AR subtypes were detectable on human VSMC in PLA when used to detect individual receptors (Fig. 2A). Quantification of PLA signals per cell suggested that α1B-ARs were more frequently expressed on human VSMC than all other α-AR subtypes and CXCR4 (Fig. 2C). We then screened human VSMC for possible interactions between CXCR4 and α-AR subtypes and detected PLA signals corresponding to interactions between CXCR4 and α1A-AR and between CXCR4 and α1B-AR (Fig. 2 B and D). Images from PLA signals corresponding to interactions between the receptors at a lower magnification (40×) are shown in Fig. S1. The number of PLA signals per cell corresponding to interactions between all other α-AR subtypes and CXCR4 were not distinguishable from the number of signals obtained in negative control experiments (Fig. 2D). Moreover, 3D reconstruction of the PLA signals from z-stack images confirmed that PLA signals corresponding to α1A-AR:CXCR4 and α1B-AR:CXCR4 are localized on the cell surface of VSMC (Fig. 2E).
Fig. 2.
Screening for proximity of α-AR subtypes with CXCR4 and coimmunoprecipitation analyses of endogenous receptor interactions in human vascular smooth muscle cells. (A) Detection of α-AR subtypes in human VSMC using PLA. Ab ctrl.: omission of the primary antibodies. Phase: phase contrast. PLA: PLA fluorescence signal. PLA/DAPI: merged PLA/DAPI signals. Images are representative of three independent experiments. (Scale bar: 10 μm.) (B) Screening for associations between α-AR subtypes and CXCR4 on human VSMC by PLA. Ab ctrl.: omission of one primary antibody. Phase: phase contrast. PLA: PLA fluorescence signal. PLA/DAPI: merged PLA/DAPI signals. Images are representative of three independent experiments. (Scale bar: 10 μm.) (C and D) Quantification of the number of PLA signals per cell for individual (C) and receptor associations (D), as in A and B, respectively. Eleven randomly selected nonoverlapping vision fields were analyzed for each condition. n = 3. *P < 0.05 vs. ctrl. (E) Three-dimensional representations of α1A-AR:CXCR4 (Top) and α1B-AR:CXCR4 (Bottom) interactions in human VSMC. Deconvolved images were generated from z-stack images (n = 20; thickness: 0.5 μm, bottom to top). Images show merged PLA/DAPI signals. (F) Human VSMC were lysed and CXCR4 was immunoprecipitated (IP) followed by Western blotting (WB) to detect CXCR4 (Top Left), α1A-AR (Bottom Left), α1B-AR (Bottom Right), and α2C-AR (Top Right) in the IP samples. IP control: precipitate after incubation of cell lysates with nonreactive resin. PS: protein standards. The white light images are overlaid at the corresponding position of the standard proteins. Images are representative of n = 3.
To confirm direct physical interactions between α1A/B-AR and CXCR4, we then used a HeLa expression system to perform coimmunoprecipitation analyses of receptor interactions. HeLa cells were cotransfected with FLAG-CXCR4, hemagglutinin (HA)-α1bAR, or HA-ACKR3 (= positive control), followed by immunoprecipitation of cell homogenates with anti-FLAG. HA-α1b-AR and HA-ACKR3 were detected in FLAG-CXCR4 immunoprecipitates, but not in control samples that expressed either receptor alone (Fig. S2). To further consolidate these observations, we next performed coimmunoprecipitation analyses of endogenous receptor interactions in human VSMC. As shown in Fig. 2F, α1A-AR and α1B-AR were detectable in CXCR4 immunoprecipitates, whereas α2C-AR was not detectable. These findings suggest that α1A/B-AR and CXCR4 physically interact in human VSMC and that the observed proximity between the receptors corresponds to direct receptor–receptor interactions.
A Peptide Derived from Transmembrane Domain 2 of CXCR4 Disrupts α1A/B-AR:CXCR4 Complexes and Inhibits α1-AR Function in Vascular Smooth Muscle Cells.
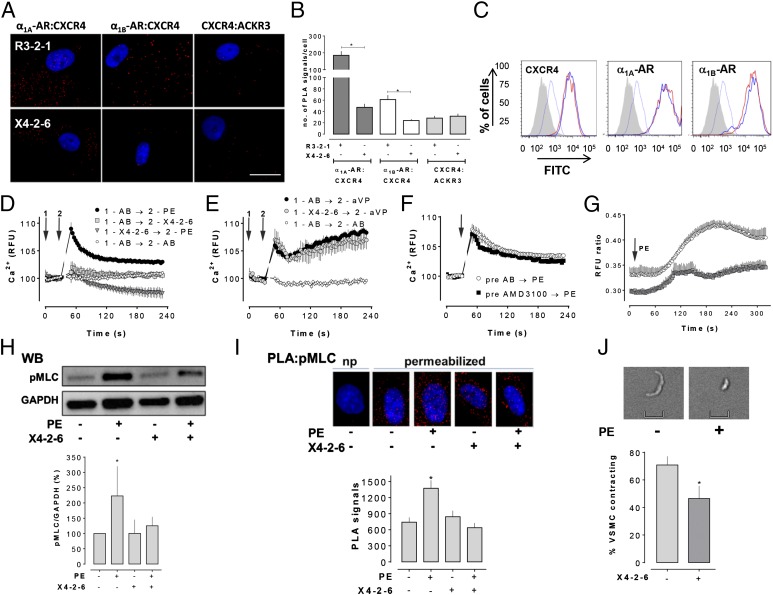
Disruption of transmembrane domains of GPCRs with transmembrane domain-derived peptides can inhibit receptor function and affect receptor dimerization through interference with the correct assembly of the target membrane protein (42, 43). X4-2–6, a peptide derived from TM2 of CXCR4, has previously been shown to inhibit CXCR4 function (43). Therefore, we then evaluated whether X4-2–6 affects the association of CXCR4 with α1A/B-AR and ACKR3 on VSMC by PLA. X4-2–6 reduced the association between α1A-AR and CXCR4 and between α1B-AR and CXCR4 on the cell surface of human VSMC, compared with R3-2–1, a peptide derived from TM2 of chemokine (C-C motif) receptor 3 (Fig. 3 A and B). PLA signals corresponding to CXCR4:ACKR3, however, were not affected by X4-2–6 (Fig. 3 A and B). We observed the same effects of X4-2–6 in PLA experiments with A7r5 cells (Fig. S3 A and B). As assessed by fluorescence-activated cell sorting (FACS) analyses, X4-2–6 did not influence cell-surface expression of the individual receptors, suggesting that X4-2–6 disrupts physical interactions between CXCR4 and α1A/B-AR in the cell membrane without affecting receptor expression levels (Fig. 3C).
Fig. 3.
X4-2–6 disrupts α1A/B-AR:CXCR4 complexes and inhibits α1-AR function in vascular smooth muscle cells. (A) Human VSMC were incubated with 100 nM of X4-2–6 or R3-2–1 (= control) for 15 min at room temperature. Interactions between α1A/B-AR and CXCR4 and between CXCR4 and ACKR3 were visualized by PLA. Images show merged PLA/DAPI signals and are representative of three independent experiments. (Scale bar: 50 μm.) (B) Quantification of the number of PLA signals per cell for receptor interactions after X4-2–6 or R3-2–1 treatment, as in A. Twenty randomly selected nonoverlapping vision fields were analyzed per experiment; n = 3. *P < 0.05 vs. cells incubated with R3-2–1. (C) FACS analysis of the cell-surface expression of CXCR4 (Left), α1A-AR (Center), and α1B-AR (Right) after vehicle (thick red line) or X4-2–6 (100 μM, thick blue line) treatment. Gray area: unstained cells. Thin blue line: IgG control. (D) Ca2+ fluxes in A7r5 cells upon addition of PE (10 μM). Arrows: Time points when drugs were added. X4-2–6: 100 μM. AB: assay buffer. n = 3–4. (E) Ca2+ fluxes in A7r5 cells upon addition of arginine vasopressin (aVP: 100 pM). Arrows: Time points when drugs were added. X4-2–6: 100 μM. AB: assay buffer. n = 3. (F) Ca2+ fluxes in A7r5 cells upon addition of PE (10 μM). Arrow: Time point when drugs were added. AMD3100: 10 μM. AB: assay buffer. n = 3. (G) Ca2+ fluxes in human VSMC upon addition of PE (1 mM, arrow). Human VSMC were preincubated with or without 100 μM X4–2-6 for 15 min. n = 3. (H, Top) Western blot (WB) analyses of MLC2 phosphorylation (pMLC) after stimulation (45 min at 37 °C) of human VSMC with PE (1 mM). Before PE stimulation, cells were preincubated with (+) or without (−) 100 μM X4-2–6 (15 min at 37 °C). Western blotting with anti-GAPDH was used as a protein loading control. (Bottom) Quantification of the chemiluminescence signals after human VSMC stimulation. Data are expressed as percentage of untreated cells [PE (−), X4-2–6 (−)]. n = 4. *P < 0.05 vs. untreated cells. (I, Top) PLA analyses of pMLC2 phosphorylation after PE stimulation and X4-2–6 treatment, as in E. No PLA signal could be detected in nonpermeabilized cells (np). (Bottom) Quantification of the number of PLA signals per cell after treatment of human VSMC. For each condition and experiment, 11 randomly selected nonoverlapping vision fields were analyzed; n = 3. *P < 0.05 vs. untreated cells. (J) X4-2–6 attenuates rat mesenteric artery smooth muscle cell contraction in response to PE. Freshly isolated cells were treated with or without 100 μM X4-2–6 for 15 min at room temperature and then stimulated with 10 μM PE. (Top) Representative images of cells before (PE −) and after (PE +) exposure to PE. (Scale bar: 10 μm.) (Bottom) Quantification of the number of cells contracting in response to PE. *P < 0.05 X4-2–6 treated (+) vs. untreated (−) cells. n = 3.
To assess whether disruption of the α1A/B-AR:CXCR4 association influences α1-AR–mediated signaling, we tested the effects of X4-2–6 on PE-induced intracellular Ca2+ mobilization and myosin light chain (MLC) 2 phosphorylation (Ser-19) in VSMC. As shown in Fig. 3D, X4-2–6 inhibited PE-induced intracellular Ca2+ mobilization in A7r5 cells. X4-2–6, however, did not affect Ca2+ mobilization in response to arginine vasopressin (Fig. 3E), suggesting specificity of the observed effects for α1-AR. Because pretreatment of A7r5 cells with the selective CXCR4 inhibitor AMD3100 did not affect PE-induced Ca2+ mobilization (Fig. 3F), effects of X4-2–6 cannot be attributed to the inhibition of CXCR4-mediated signaling. As observed in A7r5 cells, X4-2–6 also attenuated Ca2+ mobilization in response to PE in human VSMC (Fig. 3G). Furthermore, X4-2–6 inhibited Ca2+/calmodulin-dependent MLC2 phosphorylation upon PE stimulation of human VSMC, as assessed by Western blotting (Fig. 3H). To confirm results from Western blotting experiments, we then used PLA to visualize and quantify intracellular phosphorylated MLC2 (Fig. 3I). Consistent with the intracellular localization of phospho-MLC2, we detected PLA signals only when VSMC were permeabilized before incubation with anti-phospho MLC2. Quantification of the number of PLA signals per cell showed the same magnitude of PE-induced MLC2 phosphorylation as determined by Western blot analyses and confirmed the inhibition of PE-induced MLC2 phosphorylation by X4-2–6 (Fig. 3H). As these data indicated that X4-2–6 inhibits key signaling events in the pathway mediating α1-AR–induced VSMC contraction, we then tested whether X4-2–6 also influences contraction of freshly isolated rat mesenteric artery smooth muscle cells upon exposure to PE. As shown in Fig. 3J, pretreatment of cells with X4-2–6 reduced the number of cells contracting upon PE stimulation from 71 ± 6% to 46 ± 9%. The observation that the inhibitory effects of X4-2–6 on PE-induced signaling events in human aortic VSMC were more pronounced than effects of X4-2–6 on PE-induced contraction of freshly isolated VSMC from mesenteric arteries could be explained by distinct functional roles of the α1-AR subtypes that have been observed among various vascular beds (44).
Because our observations suggested that X4-2–6 functions as an α1-AR antagonist, we then evaluated whether X4-2–6 can also interact with α1A-AR using NMR spectroscopy. We used reductively methylated membranes prepared from cells overexpressing either α1a-AR or CXCR4 to closely mimic native conditions for receptor folding and interactions with the plasma membrane. A similar approach has recently been used to provide structural insight into ligand regulation of the extracellular surface of the β2-AR (45). The overlaid 13C-1H heteronuclear single quantum coherence (HSQC) spectra of α1a-AR with and without PE are shown in Fig. 4, Upper Left. Addition of 10 μM PE induced chemical shift changes that are indicative of a global structural rearrangement of the receptor induced by ligand binding. No chemical shift changes in the spectrum of α1a-AR were induced by addition of 10 μM X4-2–6 (Fig. 4, Upper Right). To address the possibility that 13CH3 probes on α1a-AR do not report on X4-2–6 binding, we added 10 μM PE to α1a-AR in the presence of 10 μM X4-2–6 (Fig. 4, Lower Left). PE-induced chemical shift changes in α1a-AR were similar to those in the absence of X4-2–6. When X4-2–6 was added to CXCR4, significant chemical shift changes in the spectrum of CXCR4 were detected, indicating peptide-receptor interactions (Fig. 4, Lower Right). Because X4-2–6 also caused loss of proximity between α1A/B-AR and CXCR4 in VSMC, these data suggest that X4-2–6 binding to CXCR4 induces structural rearrangements of the receptor that disrupt the quarternary heteromer interface. Provided that PLA signals for native CXCR4:ACKR3 represent direct receptor interactions, the observation that the proximity between CXCR4 and ACKR3 was not affected by X4-2–6 points toward TM2 of CXCR4 as part of a specific α1A/B-AR:CXCR4 heteromer interface, which does not participate in the formation of the CXCR4:ACKR3 interface.
Fig. 4.
X4-2–6 induces chemical shift changes in the NMR spectrum of CXCR4 in membranes. Superimposition of 1H-13C HSQC NMR spectra of reductively methylated membrane preparations of (Upper Left) α1a-AR (blue) and α1a-AR treated with 10 µM PE (red); (Upper Right) α1a-AR (blue) and α1a-AR treated with 10 µM X4-2–6 (red); (Lower Left) α1a-AR (blue) and α1a-AR treated with 10 µM X4-2–6 and 10 µM PE (red); and (Lower Right) CXCR4 (blue) and CXCR4 treated with 10 µM X4-2–6 (red).
Although crystal structures of GPCR heteromers are currently not available, crystallographic structures of GPCR homodimers revealed several different interfaces. The main interface of the CXCR4 homodimer is localized at TM5 and TM6, whereas several other GPCR homodimers form interfaces that also include TM2 (46–50). Thus, a TM2 contact site in CXCR4 could permit receptor heteromerization without interfering with the constitutive CXCR4 homodimerization (46). Furthermore, as X4-2–6 did not interfere with α1a-AR and did not affect ligand-induced conformational changes of α1a-AR in membranes, off-target effects of X4-2–6 on α1A/B-AR or PE appear unlikely to account for the inhibitory effects of X4-2–6 on signaling events upon α1-AR activation. Thus, these data led to the hypothesis that α1A/B-AR:CXCR4 heteromers are a prerequisite for α1-AR function in VSMC.
CXCR4 Silencing Inhibits α1-AR Function in Vascular Smooth Muscle Cells.
To test the hypothesis that CXCR4:α1A/B-AR heteromeric complexes are required for α1-AR function, we aimed to reduce CXCR4:α1A/B-AR heteromerization by reducing CXCR4 expression in human VSMC with RNA interference. Fig. 5A shows a typical Western blot with anti-CXCR4 with cell homogenates from human VSMC transfected with nontargeting (NT) or CXCR4-targeted siRNA. As expected, anti-CXCR4 recognized multiple bands in human VSMC transfected with NT siRNA, which likely correspond to proteolytically processed, ubiquitylated, or glycosylated forms of CXCR4 (51–55). The most abundant receptor species after transfection of human VSMC with NT siRNA were detectable at migration positions corresponding to 48–60 kDa. The intensities of these bands were reduced after transfection of the cells with CXCR4 siRNA (Fig. 5A). As estimated by FACS analyses, CXCR4 cell-surface expression was reduced by 69 ± 7% (n = 4) after transfection of human VSMC with CXCR4 siRNA, compared with cells transfected with NT siRNA (Fig. 5B). Cell-surface expressions of α1A/B-AR were not affected by CXCR4 siRNA (Fig. 5B). When CXCR4 expression was quantified by PLA (Fig. 5C), transfection of human VSMC with CXCR4 siRNA resulted in 62 ± 8% reduction of CXCR4 cell-surface expression, compared with cells transfected with NT siRNA.
Fig. 5.
CXCR4 silencing inhibits α1-AR function in human vascular smooth muscle cells. (A) CXCR4 was silenced with siRNA in human VSMC followed by Western blotting of whole-cell lysates with anti-CXCR4 and anti-GAPDH. NT: nontargeting. PS: white light image of prestained protein standards. (B) FACS analysis of CXCR4 (Left), α1A-AR (Center), and α1B-AR (Right) expression on the cell surface of human VSMC after transfection with NT (red line) and CXCR4 (blue line) siRNA. Gray line: IgG control. Gray area: unstained cells. (C–F) Representative PLA images (merged PLA/DAPI) and quantification of PLA signals for CXCR4 (C), α1A-AR:CXCR4 (D), α1B-AR:CXCR4 (E), and ACKR3:CXCR4 (F) after CXCR4 silencing, as in A and B. For each condition and experiment, 11 randomly selected nonoverlapping vision fields were analyzed; n = 3. *P < 0.05 vs. cells transfected with NT siRNA. (Scale bars: 10 μm.) (G) PE (1 mM; arrow)-induced Ca2+ fluxes in human VSMC after CXCR4 silencing, as in A and B. Open circles: cells transfected with NT siRNA. Gray squares: cells transfected with CXCR4 siRNA. For each experiment (n = 3), the fluorescence ratio was calculated from 50 individual cells in each vision field. (H) Western blot (WB) analyses of MLC2 phosphorylation (pMLC) after PE stimulation, as in Fig. 3E, in human VSMC after CXCR4 silencing, as in A and B. Western blotting with anti-GAPDH was used as a protein loading control. (I) Quantification of the chemiluminescence signals after VSMC stimulation as in H. Data are expressed as a percentage of unstimulated cells transfected with NT siRNA. n = 4. *P < 0.05 vs. unstimulated cells transfected with NT siRNA.
Next, we analyzed the expression of α1A/B-AR:CXCR4 and ACKR3:CXCR4 heteromers by PLA. Compared with human VSMC after transfection with NT siRNA, CXCR4 siRNA silencing reduced the PLA signals for α1A-AR:CXCR4 (Fig. 5D), α1B-AR:CXCR4 (Fig. 5E), and ACKR3:CXCR4 (Fig. 5F) heteromers by 90%, 60%, and 59%, respectively. The finding that the degree of reduction of CXCR4 and of the α1B-AR:CXCR4 and ACKR3:CXCR4 heteromers on the cell surface after siRNA silencing was comparable argues for a 1:1 receptor:receptor stoichiometry. Notably, PLA signals for α1A-AR:CXCR4 heteromers were almost completely depleted after CXCR4 silencing. Several explanations may account for this observation, such as alteration of the receptor heteromerization equilibrium in the plasma membrane or PLA signals resulting from the association of CXCR4 with other receptors that form heteromeric complexes with α1A-AR, i.e., during receptor clustering (56).
To assess the functional consequences of CXCR4 silencing on α1-AR function in human VSMC, we then measured PE-induced Ca2+ fluxes and MLC2 phosphorylation. We detected that CXCR4 silencing in human VSMC abolished PE-induced Ca2+ fluxes (Fig. 5G) and MLC2 phosphorylation (Fig. 5 H and I), compared with cells transfected with NT siRNA. Among the α1-AR subtypes, α1A-AR is thought to be most important for mediating vasoconstriction in aortic vascular smooth muscle (44, 57). Thus, the large reduction of α1A-AR:CXCR4 heteromers after CXCR4 silencing in human VSMC (aortic smooth muscle cells) can explain the loss of effect of PE in our experiments, despite only partial reduction of α1B-AR:CXCR4 heteromers.
CXCR4 Agonists Increase the Potency of PE to Increase Blood Pressure.
To evaluate whether CXCR4 influences α1-AR function in vivo, we tested how pharmacological modulation of CXCR4 affects the blood pressure response to PE in rats. Under normal hemodynamic conditions, animals received a single injection of the cognate CXCR4 agonist CXCL12, the noncognate CXCR4 agonist ubiquitin, or the CXCR4 antagonist AMD3100, followed by increasing doses of PE. As a quantifiable marker of the integrated blood pressure response to each dose of PE, we then determined the area under the mean arterial blood pressure (MAP) curve for each dose of PE and generated dose–response curves. As shown in Fig. 6, the EC50 of PE after vehicle treatment was 664 ng/kg (95% confidence interval: 346–1273 ng/kg), and the maximal area under the MAP curve was 396 mmHg × s (95% confidence interval: 335–458 mmHg × s). CXCL12 and ubiquitin pretreatment reduced the EC50 of PE 3.9- and 3.5-fold, respectively. Whereas AMD3100 pretreatment did not affect the EC50 of PE, AMD3100 antagonized the effects of CXCL12 and ubiquitin. None of the CXCR4 ligands influenced the efficacy of PE. These findings suggest that CXCR4 activation enhances the potency of PE in vivo.
Fig. 6.
CXCR4 agonist treatment enhances the potency of PE to increase blood pressure. Rats were treated with vehicle, CXCL12, ubiquitin, or AMD3100 alone or with AMD3100 plus CXCL12 or ubiquitin (750 nmol/kg each), followed by increasing doses of PE at 5-min intervals. For each dose of PE, the area under the MAP curve was calculated, and dose–response curves were generated. LogEC50 vehicle vs. CXCL12: P = 0.0007; vehicle vs. ubiquitin: P = 0.003. CI: confidence interval. There were no differences in MAP at baseline among the individual groups [MAP (mmHg, mean ± SEM): vehicle (n = 5): 89 ± 3; CXCL12 (n = 3): 87 ± 6; ubiquitin (n = 4): 89 ± 3; AMD3100 (n = 4): 97 ± 2; AMD3100/CXCL12 (n = 3): 87 ± 3; AMD3100/ubiquitin (n = 3): 85 ± 2; P > 0.05 among groups].
The observation that AMD3100 did not affect PE-induced effects in normal animals is consistent with the effects of AMD3100 on PE-induced Ca2+ mobilization in VSMC in the present study and with the previous observation that AMD3100 did not influence PE-induced vasoconstriction in pressure myography experiments (9). As depletion of α1A/B-AR:CXCR4 heteromers by X4-2–6 and CXCR4 knockdown inhibited PE-induced responses in VSMC, our observations of AMD3100 suggest that heteromeric complex formation per se, independent of ligand occupation or the activation status of CXCR4, controls α1-AR function. Similarly, ligand unoccupied ghrelin receptor has been reported to modulate dopamine receptor subtype-2 function via formation of heteromeric ghrelin receptor and dopamine receptor subtype-2 complexes (58). In addition, the finding that CXCR4 agonists enhanced the potency of PE in vivo is in agreement with previous observations from pressure myography experiments with isolated arteries (9). This indicates that ligand activation of CXCR4 further sensitizes α1-AR responses and could be explained through allosteric effects of CXCR4 on α1A/B-AR within the α1A/B-AR:CXCR4 complex when CXCR4 transitions into an activated configuration upon agonist binding.
α1A/B-AR:CXCR4 and CXCR4:ACKR3 heteromers appear to be constitutively expressed in VSMC, and pharmacological activation of CXCR4 and ACKR3 has been reported to result in opposite effects on PE-induced vasoconstriction in isolated arteries (9). This implies that CXCR4 and also ACKR3 function as modulators of α1-AR. Whereas the molecular mechanisms through which ACKR3 influences α1-AR function remain to be determined, the observed effects of ubiquitin in the present study are in agreement with the findings that ubiquitin functions as a noncognate CXCR4 agonist that does not bind to ACKR3 (51, 52, 59–63). The observation that CXCL12, which has a much higher affinity for ACKR3 than for CXCR4 (64), also enhanced the potency of PE in normal animals in vivo in the present study, whereas CXCL12 previously desensitized PE-mediated vasoconstriction of isolated arteries and reduced blood pressure during hemorrhagic shock (9), suggests that effects of CXCL12 depend on the relative functional contribution of CXCR4 and ACKR3 within the specific experimental or (patho)physiological environment (65).
Because CXCR4 antagonists have been reported to reduce blood pressure in experimental models of pulmonary arterial and systemic hypertension and during hemorrhagic shock (6, 7, 9), these findings point toward a pathophysiological role of CXCR4 agonists during blood pressure regulation under disease conditions that are associated with increased catecholamine release, such as shock or hypertension. Interestingly, ubiquitin has been described to be stored along with catecholamines in secretory chromaffin granules in the adrenal gland and to be released into the circulation upon stimulation of chromaffin cells (66), which may reflect a physiological linkage between CXCR4 and adrenergic receptor function.
Conclusively, our data suggest that endogenous α1A/B-AR:CXCR4 heteromers are constitutively expressed on VSMC, that the heteromeric receptor complex is important for α1-AR function, and that ligand activation of CXCR4 further sensitizes α1-AR. Such a regulation of α1-AR function could be explained through allosteric interactions between α1A/B-AR and CXCR4 within the heteromeric receptor complex, which could provide the physiological advantage that α1-AR, and subsequently vascular function, can be selectively regulated to allow fine-tuning of blood pressure in the systemic circulation or in different vascular beds (67, 68). Furthermore, our observations provide an example that signaling events, which have been considered as characteristic intracellular consequences following activation of a homomeric GPCR, reflect a biochemical fingerprint of a GPCR heteromer (69). We believe that our observations extend the current understanding of the molecular mechanisms regulating α1-AR function in VSMC and that compounds targeting the α1A/B-AR:CXCR4 interaction could provide an alternative pharmacological approach to modulate vascular function and blood pressure.
Materials and Methods
Cells and Cell Lines.
A7r5 cells (rat aortic vascular smooth muscle cell line) and human aortic VSMC were obtained from the American Type Culture Collection. HeLa cells were as described (70). Rat aortic and mesenteric artery VSMC were isolated from male Lewis and Sprague–Dawley rats as described elsewhere (71–73). All procedures involving rats were in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Loyola University Chicago. A7r5, rat, and human VSMC were cultured in high-glucose Dulbecco’s Modified’s Eagle Medium, 10 mg/mL sodium pyruvate, 2 mM l-glutamine, 10% (vol/vol) FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Proteins, Peptides, and Reagents.
PE, arginine vasopressin, and AMD3100 were from Sigma-Aldrich. CXCL12 was as described (9, 74) and also obtained from Protein Foundry. Ubiquitin was purchased from R&D Systems. X4-2–6, a peptide derived from the second transmembrane helix of CXCR4, was prepared as described (43); R3-2–1 (LLFLVTLPFWIHYVRGHNWVFGHDDD-PEG27-NH2), a peptide derived from the second transmembrane helix of chemokine receptor CCR3, was designed and produced similarly to the X4-2–6 peptide and used as a control. Solid-phase synthesis on a 433A Applied Biosystems Peptide Synthesizer using Fmoc amino acid derivatives was used for the production of the peptides. After cleavage with 87.5% (vol/vol) trifluoroacetic acid containing 5% (vol/vol) water, 5% (vol/vol) thioanisol, and 2.5% (vol/vol) triisopropylsilane, the peptides were purified by reverse phase HPLC using an Atlantis C3 column (Agilent Technologies). The peptide structure and purity was confirmed by ion-spray mass-spectrometry combined with HPLC.
Reductive Methylation of Membrane Preparations.
ChemiSCREEN membrane preparations of α1aAR and CXCR4 were purchased from EMD Millipore. Reductive methylation of the membrane preparations was performed as described previously (75). In brief, 20 µL of 1 M borane–ammonia complex and 4 µL of 13C formaldehyde were added to 1 mL of membrane preparation. This mixture was incubated with stirring for 2 h at 4 °C. The addition of borane–ammonia and formaldehyde was repeated, and the mixture was incubated with stirring for an additional 2 h. Ten microliters of 1 M borane–ammonia complex were then added and the mixture was incubated at 4 °C overnight with stirring. The reaction was then stopped by adding 110 µL of 2 M Tris⋅HCl (pH 7.6). Thereafter, the membrane preparations were dialyzed in PBS and used for NMR experiments.
Heteronuclear Single Quantum Coherence NMR.
1H-13C HSQC NMR experiments were carried out on a 900-MHz Bruker Avance Spectrometer equipped with a cryogenic probe. Data were processed and analyzed using the NMRPipe software.
Coimmunoprecipitation Analyses of Receptor Interactions.
HeLa cells were transiently transfected with DNA encoding FLAG-CXCR4 or empty expression vector pcDNA and pcDNA, HA-ACKR3, or HA-ARα1b, using TransIT-LT1 transfection reagent, similar to previously published protocols (70). Twenty-four hours later, cells were collected in 1.0 mL immunoprecipitation buffer [20 mM Na2PO4, pH 6.5, 150 mM NaCl, 1% (vol/vol) Triton-X 100, 10 µg/mL leupeptin, 10 µg/mL aprotinin, and 10 µg/mL pepstatin A] and incubated at 4 °C for 30 min. Cells were sonicated and centrifuged, and 500 µg of the clarified lysates were incubated with an anti-FLAG polyclonal antibody (Sigma) to immunoprecipitate FLAG-CXCR4 followed by immunoblotting to detect bound HA-ACKR3 or HA-α1b-AR, similar to previously published protocols (76).
Coimmunoprecipitation experiments with human VSMC were performed using the Thermo Scientific Pierce coimmunoprecipitation kit according to the manufacturer’s protocol. Anti–α1A/B-AR and anti–α2C-AR antibodies were purchased from Abcam. Forty micrograms of anti-CXCR4 was incubated with 50 µL Amino LinK Plus coupling resin for 2 h. Three hundred micrograms of cell lysates was precleared with 25 µL of the control agarose resin slurry (30 min at 4 °C). Immobilized anti-CXCR4 resin and nonreactive resin (= control) were incubated with precleared lysate overnight at 4 °C. After incubation, the resins were washed three times with 200 µL IP lysis/wash buffer, and protein was eluted using 50 μL of elution buffer. Samples were analyzed by Western blotting.
CXCR4 Gene Silencing by RNA Interference.
CXCR4 siRNA gene silencing was performed as described previously (51). In brief, VSMC cells were grown in 1 mL Accell siRNA delivery media per well (Thermo Scientific Dharmacon) in 12-well plates (Nunc). Commercially available Accell CXCR4 siRNA was reconstituted with 1× siRNA buffer to a stock concentration of 100 µM. Cells were then transfected with 10 nmol CXCR4 siRNA and incubated for 72 h at 37 °C, 5% (vol/vol) CO2. Accell NT siRNA pool was used as negative control. After 72 h, cells were assayed for receptor cell-surface expression and used for signaling experiments.
Proximity Ligation Assays.
Duolink proximity ligation assays were performed as described previously (40, 41). In brief, VSMC and A7r5 cells were grown and fixed on eight-well tissue culture slides. For the visualization of individual receptors, slides were blocked with 3% (wt/vol) BSA in PBS and incubated with rabbit anti-CXCR4 (ab2074, Abcam) (1:400), rabbit anti-ACKR3 (LS-B1815, LSBio) (1:400), rabbit anti-α1AAR (ab137123, Abcam) (1:400), rabbit anti-α1BAR (ab169523, Abcam) (1:400), rabbit anti-α1DAR (ab84402, Abcam) (1:400), rabbit anti-α2AAR (SAB4500548, Sigma) (1:400), mouse anti-α2BAR (ab21768, Abcam) (1:400), mouse anti-α2CAR (ab167433, Abcam) (1:400), or rabbit anti-TLR9 (ab62577, Abcam) (1:400) at 37°C for 2 h in a humidifying chamber. Slides were then washed and incubated (1 h at 37°C) with secondary anti-rabbit antibodies conjugated with plus and minus Duolink II PLA probes (1:5). Slides were washed again and then incubated with ligation-ligase solution (30 min at 37°C) followed by incubation with amplification-polymerase solution (2 h at 37°C). Slides were then mounted with minimal volume of Duolink II mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) for 15–30 min, and PLA signals [Duolink In Situ Detection Reagents Green (λexcitation/emission 495/527 nm) or Red (λexcitation/emission 598/634 nm)] were identified as fluorescent spots under a fluorescence microscope [Carl Zeiss Axiovert 200M with EC Plan-Neofluor objective lenses (40× and 100×/1.30 oil) equipped with Axio CamMRc5 (Carl Zeiss) and AxioVision Rel. 4.8.2 (Carl Zeiss) acquisition software] at room temperature. For the visualization of receptor interactions in VSMC, slides were blocked as described above and incubated with rabbit anti-ACKR3 (LS-B1815, LSBio)/mouse anti-CXCR4 (M04, clone 2G9, Abnova) (1:400), mouse anti-CXCR4 (M04, clone 2G9, Abnova)/rabbit anti-α1AAR (ab137123, Abcam) (1:400), mouse anti-CXCR4 (M04, clone 2G9, Abnova)/rabbit anti-α1BAR (ab169523, Abcam) (1:400), mouse anti-CXCR4 (M04, clone 2G9, Abnova)/rabbit anti-α1DAR (ab84402, Abcam) (1:400), mouse anti-CXCR4 (M04, clone 2G9, Abnova)/anti-α2AAR (SAB4500548) (1:400), rabbit anti-CXCR4 (ab2074, Abcam) (1:400)/mouse anti-α2BAR (ab21768) (1:400), rabbit anti-CXCR4 (ab2074, Abcam) (1:400)/mouse anti-α2CAR (ab140702) (1:400), rabbit anti-ACKR3 (LS-B1815, LSBio)/goat anti-α1AAR (Sc-1477, Santa Cruz) (1:400), or mouse anti-TLR9 (ab12121, Abcam)/rabbit anti-α1AAR (ab137123, Abcam) (1:400). The same antibody combinations were used for A7r5 cells, except that ACKR3:α1AAR interactions were visualized with mouse anti-ACKR3 (LS-C64035, LSBio)/rabbit anti-α1AAR (ab137123, Abcam) (1:400). For the visualization of phosphorylation of MLC2, slides were treated as described, except that cells were permeabilized in 0.5% Triton-X before blocking. Slides were incubated with mouse anti-phospho (S19) MLC2 (Cell Signaling). Slides were then washed and incubated with secondary anti-rabbit/mouse antibodies conjugated with plus and secondary anti-mouse/goat antibodies conjugated with minus Duolink II PLA probes (1:5). All following steps were as described above for the visualization of individual receptors. As antibody controls, slides were incubated without primary antibodies or without one of the secondary antibodies conjugated with minus Duolink II PLA probes. PLA signals were quantified using the Duolink Image Tool software (Sigma-Aldrich). Images were imported in merged.tiff formats containing both signal and nuclei channels. Merged images were visually verified for analytical quality. Comparisons and statistical analyses were performed only when PLA assays were performed on the same day in parallel experiments and fluorescence microscopy was performed with the identical settings. For each experiment and condition, 20–24 randomly selected nonoverlapping vision fields were analyzed, and the mean from all experiments was calculated.
Deconvolution 3D Imaging.
Z-stack images were collected (from bottom to top) using identical acquisition parameters with a DeltaVision wide-field fluorescent microscope (Applied Precision, GE) equipped with a digital camera (CoolSNAP HQ; Photometrics), using a 1.4-numerical aperture 100× objective lens. Excitation light was generated using the Insight SSI solid-state illumination module (Applied Precision, GE), and images were deconvolved with the SoftWoRx deconvolution software (Applied Precision, GE). Following deconvolution, images were quantified by Imaris (Bitplane) software using the Surfaces feature function, generating surfaces around red puncta, as described (77). Three-dimensional views of images were generated using Surpass mode of Imaris software (78).
Calcium Assays.
Intracellular Ca2+ in A7r5 cells was measured using the Fluo-4 NW Calcium Assay Kit (Molecular Probes), as described (51, 52, 79). Intracellular Ca2+ in human VSMC was measured using the ratiometric Ca2+ indicator dye Fura-2. VSMC were grown on 15-mm round coverslips for 3 d until 80–100% confluence. Coverslips were washed twice with modified Krebs solution (135 mM NaCl, 5.9 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 11.5 mM glucose, 11.6 mM Hepes, pH 7.3) and then incubated in the same solution with 5 µM Fura-2-AM, 0.1% BSA, and 0.02% Pluronic F127 detergent for 60 min at room temperature in the dark. Cells were next washed twice and incubated in the dark in control medium for 0.5–2 h. All experiments were performed at room temperature in the presence of continuous perfusion of bath solution (1× Hanks’ balanced salt solution, 20 mM Hepes) at a rate of 2 mL/min. Cell images were acquired using C Imaging System (Compix Inc.) with an Olympus 1× 71 inverted epifluorescence microscope (10× fluorescence objective) and Simple PCI software (Vers.5.3.1.). Fura-2 fluorescence (340 and 380 nm excitation, 510 nm emission) was measured every 2 s for 1–2 min before application of PE, followed by 5 min after PE application. Background fluorescence was determined at the end of each experiment by quenching Fura-2 fluorescence with 2 µM ionomycin and 6 mM MnCl2 for 2 min and subtracted from individual wavelength measurements before calculating 340:380 nm fluorescence ratios. For Ca2+ flux analyses, 50 individual cells in each field were continuously monitored, and the corrected 340/380 fluorescence ratio was calculated for each cell and averaged among all cells in the vision field.
FACS Analyses.
FACS analyses of receptor cell-surface expression were performed as described previously (51, 79). Cells were labeled with polyclonal rabbit anti-CXCR4 (ab2074, Abcam), polyclonal rabbit anti-α1AAR (ab137123, Abcam), and polyclonal rabbit anti-α1BAR (ab169523, Abcam) in combination with anti-rabbit FITC-conjugated goat IgG (ab6717, Abcam). Rabbit IgG (GWB-3274CD, R&D Systems) in combination with FITC-conjugated anti-rabbit goat IgG (ab6717, Abcam) was used as a negative control. The geometric fluorescence intensities of at least 3 × 104 cells were recorded and analyzed using the FlowJo software (Tree Star).
Western Blots.
Western blotting with mouse monoclonal anti–phospho-MLC2 (S19) (Cell Signaling), rabbit polyclonal anti-CXCR4 (ab2074, Abcam), rabbit polyclonal anti-α1AAR (ab137123, Abcam), rabbit polyclonal anti-α1BAR (ab169523, Abcam), and mouse anti-α2C AR (ab167433, Abcam) in combination with anti-rabbit or mouse IgG horseradish peroxidase-linked whole antibody (GE Healthcare) was performed as described (52, 79). Rabbit anti-GAPDH (Cell Signaling) in combination with anti-rabbit IgG horseradish peroxidase-linked whole antibody (GE Healthcare) was used as a protein loading control.
Vascular Smooth Muscle Cell Contraction Assay.
Freshly isolated VSMC from mesenteric arteries were dispensed onto a glass coverslip base of the recording chamber and allowed to adhere for at least 15 min at room temperature. All experiments were performed with continuous perfusion of the bath solution containing 140 mM NaCl, 5.36 mM KCl, 1.2 mM MgCl2, 2 mM CaCl2, 10 mM Hepes, and 10 mM d-glucose (pH 7.3), 298 mOsm/L. Images were acquired using a C Imaging System (Compix Inc.) with an Olympus 1 × 71 inverted epifluorescence microscope (10× objective, phase contrast) and Simple PCI software (Vers.5.3.1.) every 2 s for 1 min before the application of 10 µM PE and for an additional 3 min after PE application. The total number of the cells and the number of the cells that contracted in response to PE were counted in each field. For each experiment, cells from a single animal were tested in triplicate or quadruplicate.
In Vivo Testing of PE Responsiveness.
Male Sprague–Dawley rats (300–325 g body weight, Harlan) were anesthetized with 2.5% (vol/vol) isoflurane, and a central venous catheter and an arterial line were placed in the femoral vessels. After stable baseline conditions were achieved, animals received an i.v. bolus injection of the CXCR4 ligands (AMD3100, CXCL12, or ubiquitin; 750 nmol/kg in 0.5 mL of normal saline). Five minutes later, i.v. bolus injections of increasing doses of PE (5 ng/kg–40 μg/kg) in 0.5 mL of normal saline were administered at 5-min intervals. In blocking experiments, AMD3100 was administered 5 min before CXCL12 or ubiquitin treatment. MAP was recorded at 10-s intervals for the duration of the experimental period. At the end of the experimental period, animals were euthanized (isoflurane inhalation, bilateral pneumothorax). For each dose of PE, the area under the MAP curve was calculated with the GraphPad-Prism 6 software, and dose–response curves were generated.
Data Analyses.
Data are expressed as mean ± SEM from n independent experiments that were performed on different days. Data were analyzed using the GraphPad-Prism 6 software. Unpaired Student’s t test or one-way analyses of variance with Bonferroni’s multiple comparison post hoc test for multiple comparisons were used, as appropriate. Dose–response curves were generated using nonlinear regression analyses. Best-fit values were compared with the extra sum-of-squares F test. A two-tailed P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Adriano Marchese for performing co-immunoprecipitation experiments in HeLa cells; Dr. Edward M. Campbell for help with the three-dimensional imaging analyses; and Heather M. LaPorte for technical help. This research was made possible, in part, by a grant that was awarded and administered by the US Army Medical Research and Materiel Command and the Telemedicine and Advanced Technology Research Center under Contract W81XWH1020122. This research was also supported by the National Institute of General Medical Sciences (Awards R01GM107495 and T32GM008750); by the National Cancer Institute (Award R01CA135341); by the National Heart, Lung, and Blood Institute (Award R21HL118588); by the National Institute of Allergy and Infectious Diseases (Award R01AI058072) of the National Institutes of Health; and by the American Heart Association (Award 13GRNT17230072).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.R.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417564112/-/DCSupplemental.
References
- 1.Tachibana K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 2.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135(3):561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachelerie F, et al. International Union of Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66(1):1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16(11):2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 5.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: Their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10(3):179–185. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- 6.Chu PY, et al. CXCR4 antagonism attenuates the cardiorenal consequences of mineralocorticoid excess. Circ Heart Fail. 2011;4(5):651–658. doi: 10.1161/CIRCHEARTFAILURE.110.960831. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, Hales CA. Effect of chemokine receptor CXCR4 on hypoxia-induced pulmonary hypertension and vascular remodeling in rats. Respir Res. 2011;12:21. doi: 10.1186/1465-9921-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach HH, IV, et al. Initial assessment of the role of CXC chemokine receptor 4 after polytrauma. Mol Med. 2012;18:1056–1066. doi: 10.2119/molmed.2011.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach HH, IV, et al. Chemokine (C-X-C motif) receptor 4 and atypical chemokine receptor 3 regulate vascular α₁-adrenergic receptor function. Mol Med. 2014;20:435–447. doi: 10.2119/molmed.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earle SA, Proctor KG, Patel MB, Majetschak M. Ubiquitin reduces fluid shifts after traumatic brain injury. Surgery. 2005;138(3):431–438. doi: 10.1016/j.surg.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Majetschak M, et al. Effects of exogenous ubiquitin in lethal endotoxemia. Surgery. 2004;135(5):536–543. doi: 10.1016/j.surg.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Majetschak M, Cohn SM, Obertacke U, Proctor KG. Therapeutic potential of exogenous ubiquitin during resuscitation from severe trauma. J Trauma. 2004;56(5):991–999; discussion 999–1000. doi: 10.1097/01.ta.0000127770.29009.5a. [DOI] [PubMed] [Google Scholar]
- 13.Baker TA, et al. Effects of exogenous ubiquitin in a polytrauma model with blunt chest trauma. Crit Care Med. 2012;40(8):2376–2384. doi: 10.1097/CCM.0b013e3182514ed9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 15.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768(4):952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchese A, Chen C, Kim YM, Benovic JL. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci. 2003;28(7):369–376. doi: 10.1016/S0968-0004(03)00134-8. [DOI] [PubMed] [Google Scholar]
- 17.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415(6868):206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 18.Diviani D, et al. Effect of different G protein-coupled receptor kinases on phosphorylation and desensitization of the alpha1B-adrenergic receptor. J Biol Chem. 1996;271(9):5049–5058. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- 19.Collins S, et al. Mechanisms involved in adrenergic receptor desensitization. Biochem Soc Trans. 1990;18(4):541–544. doi: 10.1042/bst0180541. [DOI] [PubMed] [Google Scholar]
- 20.McGraw DW, et al. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest. 2006;116(5):1400–1409. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzimiri N. Receptor crosstalk. Implications for cardiovascular function, disease and therapy. Eur J Biochem. 2002;269(19):4713–4730. doi: 10.1046/j.1432-1033.2002.03181.x. [DOI] [PubMed] [Google Scholar]
- 22.Rozenfeld R, Devi LA. Receptor heteromerization and drug discovery. Trends Pharmacol Sci. 2010;31(3):124–130. doi: 10.1016/j.tips.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferré S, et al. G protein-coupled receptor oligomerization revisited: Functional and pharmacological perspectives. Pharmacol Rev. 2014;66(2):413–434. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milligan G, Canals M, Pediani JD, Ellis J, Lopez-Gimenez JF. 2006. The role of GPCR dimerisation/oligomerisation in receptor signalling. Ernst Schering Found Symp Proc (2):145–161. [DOI] [PubMed]
- 25.Rodríguez-Frade JM, et al. Blocking HIV-1 infection via CCR5 and CXCR4 receptors by acting in trans on the CCR2 chemokine receptor. EMBO J. 2004;23(1):66–76. doi: 10.1038/sj.emboj.7600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts AO, et al. Identification and profiling of CXCR3-CXCR4 chemokine receptor heteromer complexes. Br J Pharmacol. 2013;168(7):1662–1674. doi: 10.1111/bph.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contento RL, et al. CXCR4-CCR5: A couple modulating T cell functions. Proc Natl Acad Sci USA. 2008;105(29):10101–10106. doi: 10.1073/pnas.0804286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113(24):6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 29.Stanasila L, Perez JB, Vogel H, Cotecchia S. Oligomerization of the alpha 1a- and alpha 1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J Biol Chem. 2003;278(41):40239–40251. doi: 10.1074/jbc.M306085200. [DOI] [PubMed] [Google Scholar]
- 30.Small KM, et al. Alpha2A- and alpha2C-adrenergic receptors form homo- and heterodimers: The heterodimeric state impairs agonist-promoted GRK phosphorylation and beta-arrestin recruitment. Biochemistry. 2006;45(15):4760–4767. doi: 10.1021/bi052074z. [DOI] [PubMed] [Google Scholar]
- 31.Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: A functional role for receptor-receptor interaction in vivo. Circulation. 2003;108(13):1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie C, et al. Beta 1/beta 2-adrenergic receptor heterodimerization regulates beta 2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem. 2002;277(38):35402–35410. doi: 10.1074/jbc.M204163200. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, et al. Heterodimerization of alpha 2A- and beta 1-adrenergic receptors. J Biol Chem. 2003;278(12):10770–10777. doi: 10.1074/jbc.M207968200. [DOI] [PubMed] [Google Scholar]
- 34.Prinster SC, Holmqvist TG, Hall RA. Alpha2C-adrenergic receptors exhibit enhanced surface expression and signaling upon association with beta2-adrenergic receptors. J Pharmacol Exp Ther. 2006;318(3):974–981. doi: 10.1124/jpet.106.106526. [DOI] [PubMed] [Google Scholar]
- 35.Chandrasekera PC, Wan TC, Gizewski ET, Auchampach JA, Lasley RD. Adenosine A1 receptors heterodimerize with β1- and β2-adrenergic receptors creating novel receptor complexes with altered G protein coupling and signaling. Cell Signal. 2013;25(4):736–742. doi: 10.1016/j.cellsig.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mustafa S, et al. Identification and profiling of novel α1A-adrenoceptor-CXC chemokine receptor 2 heteromer. J Biol Chem. 2012;287(16):12952–12965. doi: 10.1074/jbc.M111.322834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaRocca TJ, et al. β2-Adrenergic receptor signaling in the cardiac myocyte is modulated by interactions with CXCR4. J Cardiovasc Pharmacol. 2010;56(5):548–559. doi: 10.1097/FJC.0b013e3181f713fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J Exp Med. 2014;211(13):2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Söderberg O, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3(12):995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 40.Brueggemann LI, et al. Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J Biol Chem. 2014;289(4):2099–2111. doi: 10.1074/jbc.M113.527820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tripathi A, Davis JD, Staren DM, Volkman BF, Majetschak M. CXC chemokine receptor 4 signaling upon co-activation with stromal cell-derived factor-1α and ubiquitin. Cytokine. 2014;65(2):121–125. doi: 10.1016/j.cyto.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hebert TE, et al. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271(27):16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- 43.Tarasov SG, et al. Structural plasticity of a transmembrane peptide allows self-assembly into biologically active nanoparticles. Proc Natl Acad Sci USA. 2011;108(24):9798–9803. doi: 10.1073/pnas.1014598108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satoh M, Enomoto K, Takayanagi I, Koike K. Analysis of alpha1-adrenoceptor subtypes in rabbit aorta and arteries: Regional difference and co-existence. Eur J Pharmacol. 1999;374(2):229–240. doi: 10.1016/s0014-2999(99)00340-4. [DOI] [PubMed] [Google Scholar]
- 45.Bokoch MP, et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature. 2010;463(7277):108–112. doi: 10.1038/nature08650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu B, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330(6007):1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manglik A, et al. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature. 2012;485(7398):321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H, et al. Structure of the human κ-opioid receptor in complex with JDTic. Nature. 2012;485(7398):327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salom D, et al. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci USA. 2006;103(44):16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J, Chen S, Zhang JJ, Huang XY. Crystal structure of oligomeric β1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat Struct Mol Biol. 2013;20(4):419–425. doi: 10.1038/nsmb.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem. 2010;285(20):15566–15576. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saini V, et al. The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1α function through distinct receptor interactions. J Biol Chem. 2011;286(38):33466–33477. doi: 10.1074/jbc.M111.233742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soto AG, Trejo J. N-linked glycosylation of protease-activated receptor-1 second extracellular loop: A critical determinant for ligand-induced receptor activation and internalization. J Biol Chem. 2010;285(24):18781–18793. doi: 10.1074/jbc.M110.111088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lapham CK, et al. CXCR4 heterogeneity in primary cells: Possible role of ubiquitination. J Leukoc Biol. 2002;72(6):1206–1214. [PubMed] [Google Scholar]
- 55.Carlisle AJ, Lyttle CA, Carlisle RY, Maris JM. CXCR4 expression heterogeneity in neuroblastoma cells due to ligand-independent regulation. Mol Cancer. 2009;8:126. doi: 10.1186/1476-4598-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grochmal A, Ferrero E, Milanesi L, Tomas S. Modulation of in-membrane receptor clustering upon binding of multivalent ligands. J Am Chem Soc. 2013;135(27):10172–10177. doi: 10.1021/ja404428u. [DOI] [PubMed] [Google Scholar]
- 57.Gnus J, et al. In vitro study on the effects of some selected agonists and antagonists of alpha(1)-adrenergic receptors on the contractility of the aneurysmally-changed aortic smooth muscle in humans. J Physiol Pharmacol. 2012;63(1):29–34. [PubMed] [Google Scholar]
- 58.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73(2):317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Clin Cancer Res. 2013;19(17):4706–4716. doi: 10.1158/1078-0432.CCR-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muppidi A, et al. Targeted delivery of ubiquitin-conjugated BH3 peptide-based Mcl-1 inhibitors into cancer cells. Bioconjug Chem. 2014;25(2):424–432. doi: 10.1021/bc4005574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steagall RJ, et al. Extracellular ubiquitin increases expression of angiogenic molecules and stimulates angiogenesis in cardiac microvascular endothelial cells. Microcirculation. 2014;21(4):324–332. doi: 10.1111/micc.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan C, Lu X, Chen W, Chen S. Serum ubiquitin via CXC chemokine receptor 4 triggered cyclooxygenase-1 ubiquitination possibly involved in the pathogenesis of aspirin resistance. Clin Hemorheol Microcirc. 2014 doi: 10.3233/CH-141900. [DOI] [PubMed] [Google Scholar]
- 63.Cao Y, Li C, Zhang Q, Wang Y, Xia R. Extracellular ubiquitin enhances the suppressive effects of regulatory T cells on effector T cell responses. Clin Lab. 2014;60(12):1983–1991. doi: 10.7754/clin.lab.2014.140314. [DOI] [PubMed] [Google Scholar]
- 64.Balabanian K, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 65.Boudot A, et al. Differential estrogen-regulation of CXCL12 chemokine receptors, CXCR4 and CXCR7, contributes to the growth effect of estrogens in breast cancer cells. PLoS ONE. 2011;6(6):e20898. doi: 10.1371/journal.pone.0020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kieffer AE, et al. The N- and C-terminal fragments of ubiquitin are important for the antimicrobial activities. FASEB J. 2003;17(6):776–778. doi: 10.1096/fj.02-0699fje. [DOI] [PubMed] [Google Scholar]
- 67.Goupil E, et al. 2015. Angiotensin II type I and prostaglandin F2alpha receptors cooperatively modulate signaling in vascular smooth muscle cells. J Biol Chem 290(5):3137–3148.
- 68.Milligan G, Smith NJ. Allosteric modulation of heterodimeric G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28(12):615–620. doi: 10.1016/j.tips.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Ferré S, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5(3):131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malik R, Soh UJ, Trejo J, Marchese A. Novel roles for the E3 ubiquitin ligase atrophin-interacting protein 4 and signal transduction adaptor molecule 1 in G protein-coupled receptor signaling. J Biol Chem. 2012;287(12):9013–9027. doi: 10.1074/jbc.M111.336792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brueggemann LI, Mackie AR, Mani BK, Cribbs LL, Byron KL. Differential effects of selective cyclooxygenase-2 inhibitors on vascular smooth muscle ion channels may account for differences in cardiovascular risk profiles. Mol Pharmacol. 2009;76(5):1053–1061. doi: 10.1124/mol.109.057844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henderson KK, Byron KL. Vasopressin-induced vasoconstriction: Two concentration-dependent signaling pathways. J Appl Physiol (1985) 2007;102(4):1402–1409. doi: 10.1152/japplphysiol.00825.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackie AR, et al. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther. 2008;325(2):475–483. doi: 10.1124/jpet.107.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veldkamp CT, Seibert C, Peterson FC, Sakmar TP, Volkman BF. Recognition of a CXCR4 sulfotyrosine by the chemokine stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) J Mol Biol. 2006;359(5):1400–1409. doi: 10.1016/j.jmb.2006.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abraham SJ, Kobayashi T, Solaro RJ, Gaponenko V. Differences in lysine pKa values may be used to improve NMR signal dispersion in reductively methylated proteins. J Biomol NMR. 2009;43(4):239–246. doi: 10.1007/s10858-009-9306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malik R, Marchese A. Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4. Mol Biol Cell. 2010;21(14):2529–2541. doi: 10.1091/mbc.E10-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Connor C, et al. p62/sequestosome-1 associates with and sustains the expression of retroviral restriction factor TRIM5alpha. J Virol. 2010;84(12):5997–6006. doi: 10.1128/JVI.02412-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eiraku M, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 79.Tripathi A, et al. Modulation of the CXC chemokine receptor 4 agonist activity of ubiquitin through C-terminal protein modification. Biochemistry. 2013;52(24):4184–4192. doi: 10.1021/bi400254f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.