Significance
We show that by organizing immunomodulatory nucleic acids into spherical nucleic acid (SNA) form, significant increases in activity are observed. Treatment of mice with cancer using immunostimulatory SNAs and nonalcoholic steatohepatitis (NASH) using immunoregulatory SNAs leads to improved disease outcomes vs. their unstructured counterparts. These improvements derive from several key SNA properties, including rapid cellular uptake, endosomal delivery, and multivalent binding. Overall, this work underscores the importance of the spatial orientation and presentation of oligonucleotides in the design of novel immunomodulators.
Keywords: nanotechnology, vaccines, TLRs, immune regulation, oligonucleotides
Abstract
Immunomodulatory nucleic acids have extraordinary promise for treating disease, yet clinical progress has been limited by a lack of tools to safely increase activity in patients. Immunomodulatory nucleic acids act by agonizing or antagonizing endosomal toll-like receptors (TLR3, TLR7/8, and TLR9), proteins involved in innate immune signaling. Immunomodulatory spherical nucleic acids (SNAs) that stimulate (immunostimulatory, IS-SNA) or regulate (immunoregulatory, IR-SNA) immunity by engaging TLRs have been designed, synthesized, and characterized. Compared with free oligonucleotides, IS-SNAs exhibit up to 80-fold increases in potency, 700-fold higher antibody titers, 400-fold higher cellular responses to a model antigen, and improved treatment of mice with lymphomas. IR-SNAs exhibit up to eightfold increases in potency and 30% greater reduction in fibrosis score in mice with nonalcoholic steatohepatitis (NASH). Given the clinical potential of SNAs due to their potency, defined chemical nature, and good tolerability, SNAs are attractive new modalities for developing immunotherapies.
Modulating immunity in a targeted and specific manner is an attractive approach for treating disease. Promising early results have been observed in preclinical and clinical studies, particularly in infectious disease (1, 2), cancer (3–5), allergy (6–8), and autoimmune disease applications (9, 10). Several classes of agents are currently being evaluated for their ability to stimulate or repress immunity, but few are as versatile as nucleic acids. Nucleic acids can stimulate immunity by binding endosomal toll-like receptors (TLR3, TLR7/8, and TLR9), a finding that has been used to develop immunotherapies (11, 12). Nucleic acids can also antagonize endosomal TLRs to regulate aberrant immunity and treat autoimmune disorders such as psoriasis (13) and systemic lupus erythematosus (14). Despite these advances, immunomodulatory nucleic acids have not yet reached their full potential in the clinic. A key challenge pertains to how therapeutic biological activity in humans can be safely increased.
Nanomaterials have shown potential for increasing immunomodulatory nucleic acid activity. Efforts have primarily focused on constructs for immunostimulation (IS) and include complexing oligonucleotides with albumin (15), gold nanoparticles with two-component external coronas consisting of IS-oligonucleotides together with protein antigens (16), gold nanoparticle–IS-oligonucleotide conjugates (17), IS-oligonucleotides conjugated to polymeric nanoparticles (18), and IS-oligonucleotides in complex with lipids (19). Although these studies have uncovered the promise of nanomaterials for immunomodulation, it is still unclear what the dominant structural factors are. This information is important not only to increase activity, but also to simplify and enhance manufacturability for effective clinical translation. In addition, there has been a relative lack of advances for nanostructured materials that use oligonucleotides to specifically deactivate immune cells, despite the promising potential of this approach to treat autoimmune diseases in humans.
Our group has pioneered the development of spherical nucleic acids (SNAs), a class of well-defined macromolecules, which are formed by organizing nucleic acids radially around a nanoparticle core (20, 21). These structures exhibit the ability to enter cells without the need for auxiliary delivery vehicles or transfection reagents, by engaging scavenger receptors and lipid rafts (22). Various SNAs have been evaluated for their potential to treat a wide variety of diseases and conditions that may not be easily addressable with conventional therapeutic strategies (23, 24). SNAs, especially ones containing natural phosphodiester nucleic acids, are internalized into various cells, mainly via caveolin-mediated endocytosis, leading to endosomal localization (25, 26). Once inside the cell, the nucleic acid components of SNAs resist nuclease degradation, leading to longer intracellular lifetimes. Moreover, SNAs, due to their multifunctional chemical structures, have the ability to bind their targets in a high-affinity multivalent fashion (25, 26). Therefore, in addition to demonstrating many of the characteristics that would be considered preferred for designing immunomodulatory therapies, SNAs also provide a chemical and structural scaffold for evaluating the structure–activity relationships for the two major components: the oligonucleotide shell and the nanoparticle core. Indeed, SNAs can be assembled in many different structural forms, with their cores consisting of inorganic compounds, such as gold, or organic materials such as <50-nm liposomes (27, 28).
Herein, we describe a previously unreported class of immunomodulatory SNAs, which can be used either for stimulating (immunostimulatory, IS-SNAs) or regulating (immunoregulatory, IR-SNA) immune responses (Fig. 1). The functional activity of these SNAs is defined by the type of oligonucleotide pharmacophores used to assemble the SNA structures—oligonucleotide TLR agonists or antagonists, respectively. In addition to this modularity, we demonstrate that the properties of SNAs lead to significant activity improvements compared with free oligonucleotide counterparts for both IS-SNAs and IR-SNAs in vitro and in vivo. Furthermore, through systematic modification of multiple components that comprise the SNA structure, including oligonucleotide sequence, sugar-phosphate backbone chemistry, orientation, and core template, we are able to elucidate the key structural factors that provide for enhanced immunomodulatory function.
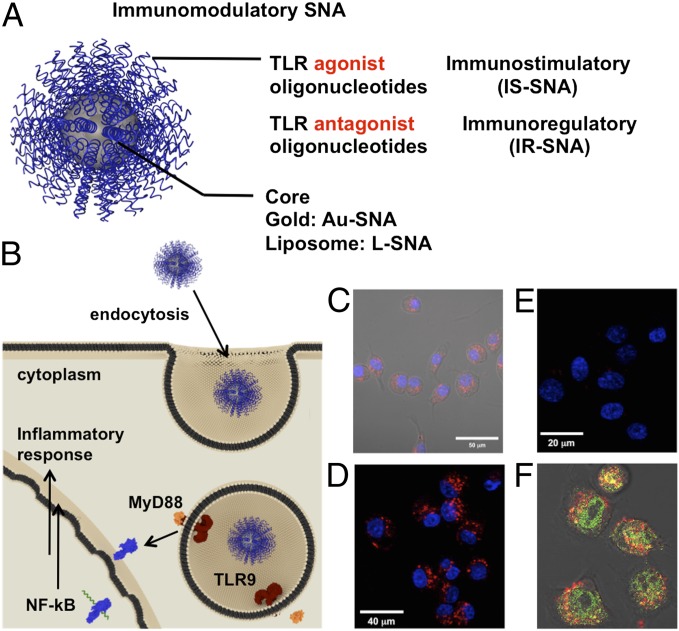
Fig. 1.
Design and properties of immunomodulatory spherical nucleic acids. (A) IS-SNAs, which contain a shell of TLR agonist oligonucleotides or IR-SNAs, which contain a shell of TLR antagonist oligonucleotides, (B) are both taken up into immune cells and reside in endosomes where they modulate toll-like receptor (TLR) activity. (C) Confocal microscopy image showing Cy-5–labeled Au-SNAs have been internalized. (D and E) Probing the importance of class A scavenger receptors in the uptake pathway for Cy-5–labeled (red signal) L-SNAs: (D) SNAs freely enter cells in the absence of SR-A receptor ligand fucoidan. (E) Cellular uptake of the same SNAs is inhibited in the presence of fucoidan. (F) Confocal microscopy image of Cy-5–labeled L-SNAs (red signal) colocalizing (yellow signal) with Alexa Flour 488–labeled EEA-1 marker (green signal) after 1 h of uptake. Cells were fixed before immunostaining and imaging.
Results and Discussion
Cellular Internalization Pathway for IS- and IR-SNAs.
Similarly to previous observations involving other cell lines (25, 26), immunomodulatory SNAs (Fig. 1A) are taken up by RAW 264.7 macrophages, a model antigen-presenting cell (APC) line, via endocytosis (Fig. 1B). Using confocal fluorescence microscopy, we observed rapid internalization of fluorophore-labeled SNAs bearing immunostimulatory motifs in RAW 264.7 macrophages (Fig. 1 C and D and Fig. S1). We also found that the SNA constructs associate with cells via class A scavenger receptors (SRA), as IS-SNA internalization was dramatically decreased in the presence of an SRA inhibitor, fucoidan (Fig. 1 D and E). Furthermore, immunostaining studies show that the SNAs are found primarily in the endosomes at early time points (Fig. 1F). These data suggest that IS-SNAs enter immune cells via receptor-mediated endocytosis and accumulate primarily in endosomes.
SNA 3D Structure Leads to Enhanced Immunomodulatory Activity in Vitro.
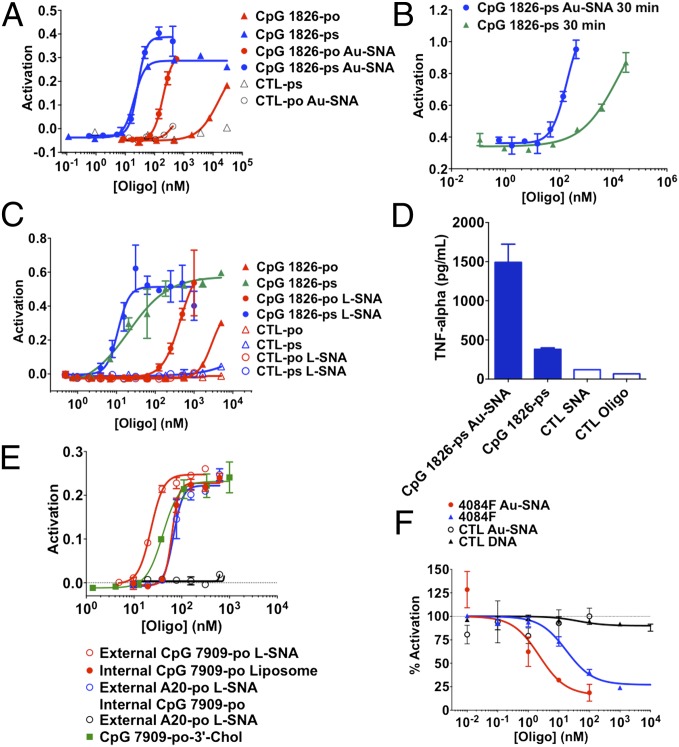
We next sought to evaluate whether 3D organization of IS oligonucleotides into SNAs substantially changes their baseline activity in vitro. To do this, we synthesized a family of compounds based on the TLR9 agonist oligonucleotide, CpG 1826, with either phosphodiester (PO) or phosphorothioate (PS) backbones, in free or SNA forms, using the conventional 13-nm gold core template (Au-SNA) (21). Following an overnight incubation of IS-SNAs with RAW-Blue macrophages, an 80-fold increase in potency (as measured by EC50 values) was observed for PO SNA CpG 1826 compared with the free oligonucleotide counterpart (Fig. 2A). For PS CpG 1826, the differences in activity of IS-SNAs compared with the corresponding free oligonucleotides were highly time-dependent and in all cases higher than the PO counterparts, as expected (29). At relatively short incubation times (30 min), a 94-fold increase in potency (EC50 value) was observed, which decreased to a 1.8-fold higher potency after 4 h of the treatment, ultimately becoming comparable in potency after overnight incubation (Fig. 2 A and B and Fig. S2). This marked kinetic difference, which likely is the result of differences in uptake rates, may be beneficial with regard to in vivo (vide infra) applications of these SNA constructs. We also synthesized the corresponding CpG 1826-based compounds with a liposomal core (L-SNA) instead of a gold core and found that the IS activity was nearly identical and independent of core, for both PO and PS backbones (Fig. 2C). We also found that free 3′-tocopherol oligonucleotides showed similar activity to the SNAs (Fig. S2G); this led us to hypothesize that the 3′-tocopherol oligonucleotides were forming small micellar structures under these conditions. We confirmed formation of the micellular structures in PBS by dynamic light scattering (see SI Materials and Methods for details).
Fig. 2.
IS-SNAs demonstrate increased potency vs. matched unformulated oligonucleotides in vitro. IS-SNAs formed with oligonucleotides (as indicated) and a 13-nm Au nanoparticle core were tested for their ability to induce NF-κB following incubation with RAW-Blue macrophages: (A) overnight or (B) 30 min incubation. (C) A similar set of IS-SNAs with small unilamellar liposomal vesicle cores (L-SNA) were tested following overnight incubation. (D) Production of TNF-α following 48-h incubation with RAW 264.7 cells at 0.4 μM concentration. (E) Evaluation of IS-SNA activity in Ramos-Blue cells compared with liposomes with TLR9 agonist oligonucleotides facing inward, with or without a d-A20 all-PO oligonucleotide (5′-d-A20-3′). Results are representative of two or more independent experiments; points show mean ± SD (F) Immunoregulatory SNAs (IR-SNA) demonstrate enhanced potency vs. matched unformulated oligonucleotides in vitro. IR-SNAs formed using the TLR9 antagonist sequence (4084F) were tested for their ability to inhibit activation of RAW-Blue macrophages in Au-SNA form (mean ± SD).
We corroborated our findings of enhanced NF-κB activation in the APC reporter system by IS-SNAs by demonstrating that (i) IS-SNAs in RAW 264.7 murine macrophages generally induce higher levels of several proinflammatory cytokines regulated by NF-κB (TNF-α, IL-12, and IL-6) compared with free oligonucleotides at the same dose (Fig. 2D and Fig. S2 B, C, and F); (ii) NF-κB activation by IS-SNAs was dependent on the presence of functional TLR9 (Fig. S2 D and E); (iii) TLR9 activation by IS-SNAs could be replicated in a similar human B-cell reporter system (Ramos-Blue cells; Fig. S2 D and G); (iv) inversion of the oligonucleotide orientation relative to SNA core by placement of the functionally important 5′-terminus (30) internally and immediately adjacent to the SNA core greatly reduced the compound’s activity (Fig. S2H); (v) use of a different TLR9 agonist oligonucleotides, all-PO CpG 7909 (more optimized for interacting with human TLR9), against human PBMCs, resulted in markedly higher cytokine activation in SNA form (∼1,400-fold higher production of TNF-α) compared with linear either all-PO or all-PS oligonucleotides (Fig. S2F). This improvement in activity in human cells (for CpG 7909 constructs) vs. murine cells (for CpG 1826 counterparts) may be due to the previously described differences in TLR receptor affinities and the preferences of oligonucleotide phosphodiester vs. phosphorothioate backbones for human and murine TLR ligands, respectively (31); and (vi) the studied IS-SNAs did not induce significant production of IFN-α in hPBMCs in vitro under the conditions studied (Fig. S2 J–L). Taken together, these results strongly suggest that the 3D structured oligonucleotide pharmacophore shell, and not the core, are the main determinant of IS activity.
Next, we sought to evaluate whether it is advantageous to organize IS-oligonucleotides into IS-SNAs vs. encapsulating them in liposomes of comparable size. To do this, we prepared an IS L-SNA (external CpG 7909-PO L-SNA) and a corresponding liposome with the IS-oligonucleotides encapsulated (internal CpG 7909-PO liposome) and compared them in terms of their ability to activate signaling in the NF-κB reporter cells. Our results show that the outward-facing L-SNA structure led to an approximate threefold improvement in potency following overnight incubation (Fig. 2E). To test whether this improvement was simply the result of greater SNA-mediated uptake, we took the internal CpG 7909-PO liposome structures and postfunctionalized them with immunologically inactive d-A20 all-PO oligonucleotide and tested their activity. The internal CpG/external d-A20 L-SNAs showed no difference compared with the oligonucleotide-encapsulated liposome, suggesting that external presentation of oligonucleotides in SNA format confers other advantages in addition to uptake. These advantages may include presentation of the oligonucleotide pharmacophore in a more available conformation for interactions with the target receptor.
Next, we tested the IR-SNAs (both Au-SNA and L-SNA) as potential negative regulators of TLR activity in vitro. IR-SNAs were designed based on the TLR9 antagonist oligonucleotide, 4084F (32). To evaluate TLR antagonist activity, cells were first activated by incubation with PS CpG 1826 (at 0.5 μM) for 2 h. The cells were then treated with various antagonists: 4084F-derivatized Au-SNA, free 4084F all-PS oligonucleotide, or mismatched oligonucleotide controls. The results demonstrate that 4084F-derivatized Au-SNAs show an approximate eightfold increase in potency compared with the free 4084F all-PS counterpart (Fig. 2F). Similar trend in the activity was observed with L-SNA antagonist constructs designed with 4084F sequences (Fig. S3A). These data further suggest that the outer oligonucleotide shell, and not the SNA core, is the defining factor for the increased IR properties. Last, we confirmed that the administration of IR-SNAs (Au-SNA) inhibited production of a key proinflammatory protein downstream of NF-κB, TNF-α (Fig. S3B).
IS-SNA 3D Structure Leads to More Potent and Durable Activation of Innate Immune Cells in Vivo.
Next, we sought to evaluate the potential of PS CpG 1826 Au-SNA (IS-SNA) constructs to activate innate immune cells in vivo. We first confirmed that Au-based IS-SNAs accumulate in draining lymph nodes in mice (LNs) at short time points following intradermal injection (t = 1, 4, and 24 h postinjection). The histological sections show evidence of significant accumulation of Au-based IS-SNAs in the cortical and medullary LN regions as early as 1 h postinjection (Fig. 3A). Progressive enlargement of lymph nodes with time was also observed, which is consistent with IS-SNA–induced local cellular activation (Fig. 3B and Fig. S4).
Fig. 3.
IS-SNAs induce systemic responses. IS-SNAs (Au-SNA) were administered into the footpad and the draining (popliteal) LN was collected aseptically at (A) t = 1 h and (B) t = 24 h and placed into RPMI-1640 growth medium for overnight incubation. The LNs were silver stained to reveal the presence of the Au nanoparticle core and counterstained with eosin. (C) The supernatants from LNs collected at t = 4 h and incubated overnight were probed for cytokines, including IL-12 at multiple doses. (D) Full cytokine panel showing specificity for TLR9 oligonucleotide motifs. (E) Cytokine panel collected from serum at t = 4 h post footpad administration. (F) Elevated and prolonged induction of IL-12 following systemic administration (n = 6 mice per group pooled to n = 3 to increase sample volume, points or bars show mean ± SD).
To test which formulation of nucleic acid induces more robust draining LN responses, we compared the levels of cytokines produced by the aseptically collected LNs at varying oligonucleotide doses. The results demonstrate that IS-SNA formulation of PS CpG 1826 produces a ∼10-fold increase in the amounts of several secreted proinflammatory cytokines compared with free IS-oligonucleotide, particularly for IL-12 and IFN-γ (Fig. 3 C and D and Fig. S5). Importantly, the increased IS potency did not appear to be an intrinsic feature of the SNA 3D structure itself, because a control SNA (containing TLR9 inactive GpC replacing CpG motif) was essentially inactive (Fig. 3D); given the robust local LN cytokine response, we sought to evaluate whether this led to elevations in a representative set of cytokines in the serum. Despite the profound IS effect in the draining LN, we saw no notable increases in serum levels for a panel of 12 different cytokines (Fig. 3E).
Next, we sought to determine whether IS-SNA structures could be used to increase the activity of systemically administered (i.v. administration) oligonucleotides. To evaluate the effect of IS-SNA compounds, we administered PO or PS CpG 1826 in either free or in Au-SNA form, and then measured the serum cytokines over a 6-h period. The results show that SNAs, particularly PS-containing SNAs, induce up to 10-fold higher levels of serum proinflammatory cytokines, particularly for IL-12 (Fig. 3F and Fig. S6). In addition, the duration of the stimulatory effect appeared to be extended by several hours compared with free oligonucleotides.
IS-SNAs Elicit Enhanced Humoral and Cellular Immune Responses to Model Antigens in Vivo.
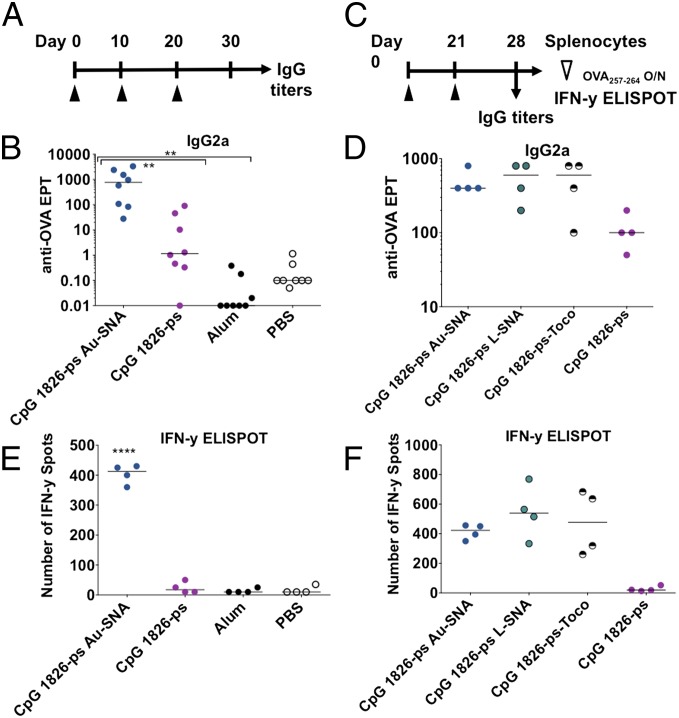
We next sought to examine whether the observed increases in the key cytokines could adjuvant improved humoral and/or cellular immune responses to a model antigen. We selected the model antigen ovalbumin (OVA) due to its widespread use in vaccine efficacy and antibody production studies. To maximize the immune response, we have attached the antigen to the surface of the IS-SNA ([Ag]IS-SNA); to achieve this, OVA or SIINFEKL peptides were conjugated to the oligonucleotide sequence complementary to the adjuvant oligonucleotide strands on the nanoparticle. The obtained peptide–oligonucleotide conjugates were hybridized to the surface of an SNA to achieve loading of ∼30 OVA or SIINFEKL per particle (see SI Materials and Methods for details). We tested by immunizing mice into the footpad with [OVA]IS-SNA or matched unformulated oligonucleotide mixed with OVA, using alum as a benchmark for potent antibody responses and evaluated both IgG1 and IgG2a titers at day 30 postimmunization (Fig. 4A). Remarkably, formulations containing IS-SNA–based adjuvants with OVA antigen led to ∼700-fold higher median IgG2a serum titers than unformulated CpG oligonucleotide at the same dose (Fig. 4B), with comparable levels of IgG1 compared with alum and unformulated CpG oligonucleotide (Fig. S7). The latter observation demonstrates that IS-SNA–based adjuvants induce significantly higher Th1-like memory response to antigen peptide, in contrast to free CpG oligonucleotide or alum under the conditions tested.
Fig. 4.
[Ag]IS-SNAs enhance humoral and cellular immune responses to antigen, independent of core template. (A) Mice were immunized as shown on day 0, 10, and 20 and (B) IgG2a endpoint titers were determined on day 30. (C) Vaccination on day 0 and 21 was followed by collection of (D) IgG2a titers on day 28 and (E) splenocytes, which were restimulated overnight with OVA257–264 (SIINFEKL) then probed for cellular activity by IFN-γ ELISPOT. (F) Comparison among Au-SNA, L-SNA, 3′-lipid, and unmodified oligonucleotide adjuvants. Line indicates median observation (n = 4–8 mice per group, **P < 0.01, ****P < 0.0001).
Next, we sought to evaluate the adjuvant properties of [Ag]IS-SNAs. We compared the overall cellular response by immunizing mice with PS CpG 1826 containing [OVA]IS-SNAs, and free OVA antigen in the presence of unformulated linear 1826 PS CpG oligonucleotide, or alum as adjuvants. Phosphate buffer saline was used as an adjuvant control in this experiment. At the end of the immunization, mice were killed and the collected splenocytes were restimulated with the major antigenic H-2Kb–restricted peptide determinant OVA257–264 (SIINFEKL) in an IFN-γ ELISPOT assay ex vivo (Fig. 4C). The results demonstrate that IS-SNA–based adjuvants induce significant desired memory response to SIINFEKL peptide, in contrast to free CpG oligonucleotide or alum under the conditions tested (Fig. 4D).
We then sought to examine whether the observed increases in activity in vivo by the SNAs depend on the type of SNA core. For this, we compared the ability of PS CpG 1826 [Ag]Au-SNA and [Ag]L-SNAs for their ability to boost humoral or cellular immune responses to OVA antigen. The results show that both [Ag]Au-SNAs and [Ag]L-SNAs induce production of comparable levels of IgG2a antibody titers, as well as comparable secretion of IFN-γ following restimulation with SIINFEKL peptide ex vivo (Fig. 4 E and F). In addition, we found that immunizations with OVA peptide in the presence of 3′-tocopherol–modified CpG oligonucleotide or with [Ag]IS-SNAs resulted in comparable humoral and cellular immune responses. However, the general ability of 3′-tocopherol–modified CpG DNA, PS 1826 [Ag]Au-SNA, and PS 1826 [Ag]L-SNA to boost the anti-OVA responses was higher than that of the free PS CpG 1826 oligonucleotide adjuvant (Fig. 4 E and F). Again we confirmed that the 3′-tocopherol–modified CpG oligonucleotides were forming SNA-like micellar structures in situ via dynamic light scattering. These data provide further evidence that the oligonucleotide spatial 3D organization and the pharmacophore orientation play key roles in providing the highly beneficial immunologic properties of IS-SNAs.
IS-SNAs Reduce Tumor Growth Rates and Improve Animal Survival in Lymphoma Model.
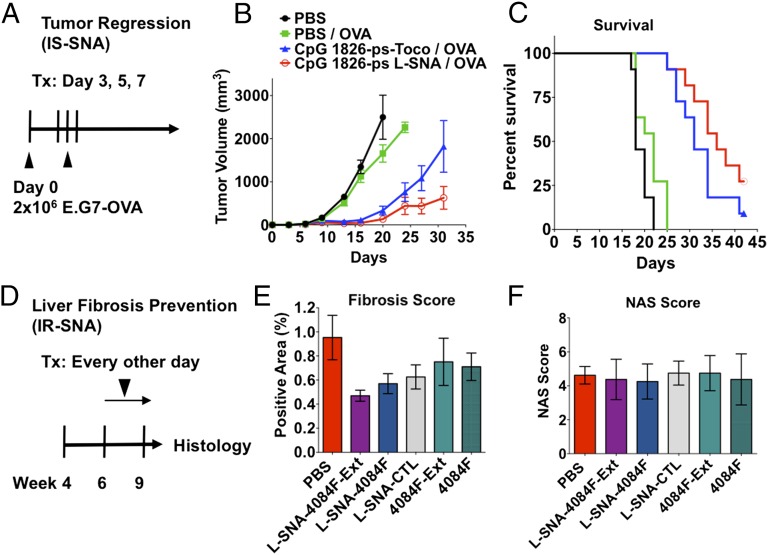
We next sought to determine whether [Ag]IS-SNAs could demonstrate improved efficacy in cancer immunotherapy disease model. For this purpose, we used the well-established E.G7-OVA lymphoma syngeneic flank model as a model for a cancer expressing a well-defined antigen (OVA). To produce OVA-expressing tumors, we injected 2 × 106 E.G7-OVA lymphoma cells into the flank of C57BL/6 mice. Following the tumor cell injection (on day 0), we treated mice with either [Ag]IS-SNAs or appropriate controls on days 3, 5, and 7 (Fig. 5A). The results show that SNA-boosted anti-OVA responses were able to induce profound and durable tumor growth remission (Fig. 5B) that translated into a doubling of mouse survival rates (Fig. 5C). Use of the full OVA protein or the major antigenic determinant SIINFEKL peptide in vaccination appeared to show no difference in either tumor growth rates or survival under these conditions (Fig. S8). Interestingly, OVA-free formulations also exhibited a significant reduction in tumor growth rates and improvements in mouse survival. The latter observation could be attributed to the induction of innate immune responses. Moreover, significant differences were observed in tumor growth rates in vaccinations containing L-SNAs and 3′-tocopherol–modified oligonucleotides. Thus, results show a significant reduction in tumor growth rates by the 3D structured L-SNAs compared with free 3′-tocopherol–modified CpG oligonucleotides, which also correlates with positive differences in mouse survival. Importantly, for all adjuvant formulations, and in all animal groups, no signs of gross toxicity were observed (Fig. S8C).
Fig. 5.
SNAs demonstrate immunomodulatory properties in vivo. (A) [Ag]IS-SNAs enhance tumor clearance. Mice were inoculated with 2 × 106 E.G7-OVA cells into the right flank on day 0, then treated as indicated with one treatment on days 3, 5, and 7. (B) Tumor growth curves (n = 11 mice per group, error bars show mean ± SEM) and (C) corresponding survival endpoints. (D) IR-SNAs show enhanced treatment of liver fibrosis in mice with NASH. STAM mice were treated every other day from 6 to 9 wk of age. At 9 wk, the liver was collected and processed for (E) fibrosis score by Sirius Red staining and (F) nonalcoholic fatty liver disease activity (NAS) score by H&E (n = 8 mice per group; points show mean ± SEM).
IR-SNAs Demonstrate Enhanced Antifibrotic Activity in a Mouse Model of NASH.
To further elucidate therapeutic potential of IR-SNAs, we sought to determine whether SNA structure exhibited advantages over sequence- and chemistry-matched free unformulated TLR antagonist oligonucleotides in vivo. Nonalcoholic steatohepatitis (NASH) is a fatty liver disease with prevalence as high as 3–5% in the United States. It is characterized by pathologic changes in livers of patients, including steatosis and inflammation, which ultimately progress in a subset of patients to nodular fibrosis, full-blown cirrhosis, and hepatocellular carcinoma (HCC). Currently, there are no FDA-approved treatments for this disease.
The STAM mouse model has been developed as a small animal model that recapitulates important features of the human disease (33). Nearly 100% of STAM mice follow disease progression from steatosis to NASH to fibrosis to HCC, making this a model that is well suited to evaluate the ability of compounds to prevent disease progression. In this model, mice that are 6 wk old demonstrate evidence of NASH, and from 6 to 9 wk old progress to histological evidence of fibrosis.
To assess whether IR-SNAs could affect disease progression, we began treatment at week 6 with IR-SNAs (L-SNA-4084F-Ext, L-SNA-4084F), sequence-matched unformulated free oligonucleotides (4084F-Ext, 4084F), or controls (L-SNA-CTL, PBS) and continued treatment following an every-other-day schedule for the 3-wk period to week 9 (Fig. 5D). After 9 wk, the mice in all groups were killed, and their livers were collected, processed for histology (H&E and Sirius Red), and scored with regards to fibrosis (Fig. 5E and Fig. S9) and NASH (Fig. 5F and Fig. S9). The results demonstrate that IR-SNA administration leads to a 40–51% reduction in fibrosis relative to control; this is in contrast to unformulated free oligonucleotides, which showed more modest antifibrotic effects (21–25% reduction). Neither IR-SNAs nor unformulated oligonucleotides showed any significant effect on nonalcoholic fatty liver disease score in this study. These results suggest that IR-SNAs can potentially be developed into compounds that modify rates of NASH progression to fibrosis, an advance that could reduce mortality from this disease.
Conclusion
The results presented in this manuscript are significant for the following reasons. The data convincingly show that SNAs can be used as constructs for sequence-specific, potent, and therapeutically relevant immunostimulation and immunoregulation. Importantly, such constructs outperform linear nonlipidated phosphodiester deoxynucleic acids due to a rapid cellular uptake, predominant accumulation in endosomes, and increased resistance to nucleases. Moreover, the data suggest that many of the attractive SNA properties are defined primarily by the 3D arrangement and orientation of the oligonucleotide shell for both IS and IR applications. Taken together, the presented data underscore the importance of spatial orientation of the immunomodulatory pharmacophores, and highlight the modular nature of the SNA therapeutic constructs.
Materials and Methods
Spherical Nucleic Acids, Adjuvants, Antigens, and Reagents.
Oligonucleotides were synthesized using automated solid support phosphoramidite synthesis (sequences for activating and regulating immunity are included in SI Materials and Methods). Ovalbumin (Sigma-Aldrich) or SIINFEKL (GenScript) were purchased at their highest purity and used as-is without additional purification. The 13-nm Au-SNAs were prepared as described (21) with important modifications (SI Materials and Methods) and formulated to contain 5.2% (wt/wt) (L-SNA) or 6.3% (wt/wt) (Au-SNA) of oligonucleotide. L-SNAs were synthesized as described (27) with modifications (SI Materials and Methods and Fig. S10).
Antibody Titers.
All animal studies were conducted according to protocols approved by the local Institutional Animal Care and Use Committees of Explora BioLabs, SRI Biosciences, Stelic Institute & Co., Inc., and Ricerca Biosciences, and by the local Ethical Review Process committee of KWS BioTest. Anti-OVA antibody endpoint titers were determined by ELISA. In brief, mice were bled as indicated in the figure captions either on day 28 or day 30 following the first immunization. Either OVA323–339 peptide (Fig. 5B and Fig. S6) or ovalbumin protein (Fig. 5E) was used as the capture antigen, and an IgG or IgG isotype-specific (IgG1 or IgG2) was used as the secondary antibody linked to HRP. The endpoint titer was determined via linear regression analysis using a curve with six or more points.
Ex Vivo ELISPOT.
The OVA-specific cellular response was evaluated by IFN-γ ELISPOT. In brief, at the conclusion of the in-life portion (day 28), mice were killed and spleens were processed to splenocytes by mechanical disruption of the spleen and removal of RBC by hypotonic lysis. Splenocytes were seeded at 4 × 105 cells per well of IFN-γ ELISPOT plates together with media alone (negative control), 1 µM OVA257–264, or PMA/ionomycin (positive control). Following incubation overnight, the number of IFN-γ–secreting cells was quantified by an automated ELISPOT counter.
In Vivo E.G7-OVA Tumor Growth Study.
The 2 × 106 E.G7-OVA (American Type Cell Culture Collection) cells were inoculated into the right flank of 4- to 6-wk-old C57BL/6 mice on day 0. On days 3, 5, and 7 post tumor induction, 200 µL of compound were administered. Tumor volumes were measured twice a week by quantifying the length and width of the tumor and applying the formula V = L × W × W/2. If a second tumor occurred in a given mouse, the second tumor was measured via the same method and the two volumes were added together. The general health of animals was monitored daily. Mice were euthanized if and when tumor volume reached or exceeded 2,000 mm3 or if found to be moribund.
Liver Fibrosis Prevention Study.
The activity of IR-SNAs was tested in the STAM mouse model (Stelic Institute & Co., Inc.) of NASH. In brief, pathogen-free, 15-d pregnant C57BL/6 mice were obtained from Japan SLC. NASH was induced by s.c. injection of streptozotocin (Sigma-Aldrich) after birth followed by feeding with a high fat diet (CLEA Japan) ad libitum after 4 wk of age. At 6 wk old, mice were randomized into groups of eight and treated every other day from 6 to 9 wk old (3 wk). Compounds or control (PBS) were administered (40 µM oligonucleotide equivalent concentration) in 100 µL by i.p. administration. The impact of treatment administration on NASH and liver fibrosis was determined by collecting livers at 9 wk of age and assessing NASH disease and fibrosis histologically in blinded fashion. In brief, H&E-stained liver sections were scored for steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2), after which a composite nonalcoholic fatty liver disease score was assigned (scale: 0–8). Sections were also stained with Sirius Red for fibrosis and scored as percent positive area according to established protocols.
Supplementary Material
Acknowledgments
We thank Andrew Schook, Blake Tutterow, and Shweta Iyer for technical assistance. This material is based upon work supported by the Center for Cancer Nanotechnology Excellence initiative of the National Institutes of Health Award U54 CA151880 and the Defense Advanced Research Projects Agency Grant HR0011-13-2-0018.
Footnotes
Conflict of interest statement: C.A.M. is a cofounder and A.F.R.-M., S.N., R.S.K., C.H.R., S.A., M.B., and S.M.G. are employees of AuraSense Therapeutics, LLC.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502850112/-/DCSupplemental.
References
- 1.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 2.Autran B, Carcelain G, Combadiere B, Debre P. Therapeutic vaccines for chronic infections. Science. 2004;305(5681):205–208. doi: 10.1126/science.1100600. [DOI] [PubMed] [Google Scholar]
- 3.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 5.Melero I, et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat Rev Clin Oncol. 2014;11(9):509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RJ, et al. A self-adjuvanting vaccine induces cytotoxic T lymphocytes that suppress allergy. Nat Chem Biol. 2014;10(11):943–949. doi: 10.1038/nchembio.1640. [DOI] [PubMed] [Google Scholar]
- 7.Fraser CC. Nanoparticle therapy for allergic and inflammatory disease. Anti-Inflammatory Anti-Allergy Agents Med Chem. 2010;9(1):54–70. [Google Scholar]
- 8.Creticos PS, et al. Immune Tolerance Network Group Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355(14):1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 9.Getts DR, et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014;6(219):ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kontos S, Kourtis IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci USA. 2013;110(1):E60–E68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper C, Mackie D. Hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine: A review of HEPLISAV™ safety and efficacy. Expert Rev Vaccines. 2011;10(4):417–427. doi: 10.1586/erv.10.162. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5:10–25. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang W, et al. A Toll-like receptor 7, 8, and 9 antagonist inhibits Th1 and Th17 responses and inflammasome activation in a model of IL-23-induced psoriasis. J Invest Dermatol. 2013;133(7):1777–1784. doi: 10.1038/jid.2013.57. [DOI] [PubMed] [Google Scholar]
- 14.Zhu FG, et al. A novel antagonist of Toll-like receptors 7, 8 and 9 suppresses lupus disease-associated parameters in NZBW/F1 mice. Autoimmunity. 2013;46(7):419–428. doi: 10.3109/08916934.2013.798651. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee IH, et al. Imageable antigen-presenting gold nanoparticle vaccines for effective cancer immunotherapy in vivo. Angew Chem Int Ed Engl. 2012;51(35):8800–8805. doi: 10.1002/anie.201203193. [DOI] [PubMed] [Google Scholar]
- 17.Lin AY, et al. Gold nanoparticle delivery of modified CpG stimulates macrophages and inhibits tumor growth for enhanced immunotherapy. PLoS ONE. 2013;8(5):e63550. doi: 10.1371/journal.pone.0063550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Titta A, et al. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc Natl Acad Sci USA. 2013;110(49):19902–19907. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen DN, et al. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc Natl Acad Sci USA. 2012;109(14):E797–E803. doi: 10.1073/pnas.1121423109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382(6592):607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 21.Cutler JI, Auyeung E, Mirkin CA. Spherical nucleic acids. J Am Chem Soc. 2012;134(3):1376–1391. doi: 10.1021/ja209351u. [DOI] [PubMed] [Google Scholar]
- 22.Patel PC, et al. Scavenger receptors mediate cellular uptake of polyvalent oligonucleotide-functionalized gold nanoparticles. Bioconjug Chem. 2010;21(12):2250–2256. doi: 10.1021/bc1002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen SA, et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5(209):ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng D, et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci USA. 2012;109(30):11975–11980. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi CH, Hao L, Narayan SP, Auyeung E, Mirkin CA. Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proc Natl Acad Sci USA. 2013;110(19):7625–7630. doi: 10.1073/pnas.1305804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu XA, Choi CH, Zhang C, Hao L, Mirkin CA. Intracellular fate of spherical nucleic acid nanoparticle conjugates. J Am Chem Soc. 2014;136(21):7726–7733. doi: 10.1021/ja503010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banga RJ, Chernyak N, Narayan SP, Nguyen ST, Mirkin CA. Liposomal spherical nucleic acids. J Am Chem Soc. 2014;136(28):9866–9869. doi: 10.1021/ja504845f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JS, Lytton-Jean AK, Hurst SJ, Mirkin CA. Silver nanoparticle-oligonucleotide conjugates based on DNA with triple cyclic disulfide moieties. Nano Lett. 2007;7(7):2112–2115. doi: 10.1021/nl071108g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krieg AM, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 30.Yu D, Zhao Q, Kandimalla ER, Agrawal S. Accessible 5′-end of CpG-containing phosphorothioate oligodeoxynucleotides is essential for immunostimulatory activity. Bioorg Med Chem Lett. 2000;10(23):2585–2588. doi: 10.1016/s0960-894x(00)00537-0. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164(2):944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 32.Lenert P, et al. DNA-like class R inhibitory oligonucleotides (INH-ODNs) preferentially block autoantigen-induced B-cell and dendritic cell activation in vitro and autoantibody production in lupus-prone MRL-Fas(lpr/lpr) mice in vivo. Arthritis Res Ther. 2009;11(3):R79–R95. doi: 10.1186/ar2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujii M, et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol. 2013;46(3):141–152. doi: 10.1007/s00795-013-0016-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.