Significance
The assembly of chloroplast ATP synthase, a multiprotein complex made of subunits of mixed genetic origin, is essential for organelle biogenesis and organism survival. Assembly of multiprotein complexes relies not only on the proper action of universal folding chaperones but also on that of specific assembly chaperones that orchestrate the joining of individual components into higher-order complexes. In this work, we have identified a specific assembly chaperone for chloroplast ATP synthase and shown that it functions downstream of the Cpn60 chaperone to promote assembly of the catalytically active core of the chloroplast ATP synthase. Such collaboration between folding and assembly chaperones appears to be a widely used cellular strategy for the folding and assembly of nucleus-encoded proteins into multiprotein photosynthetic complexes.
Keywords: assembly, ATP synthase, chloroplast, Cpn60
Abstract
The chloroplast ATP synthase, a multisubunit complex in the thylakoid membrane, catalyzes the light-driven synthesis of ATP, thereby supplying the energy for carbon fixation during photosynthesis. The chloroplast ATP synthase is composed of both nucleus- and chloroplast-encoded proteins that have required the evolution of novel mechanisms to coordinate the biosynthesis and assembly of chloroplast ATP synthase subunits temporally and spatially. Here we have elucidated the assembly mechanism of the α3β3γ core complex of the chloroplast ATP synthase by identification and functional characterization of a key assembly factor, PAB (PROTEIN IN CHLOROPLAST ATPASE BIOGENESIS). PAB directly interacts with the nucleus-encoded γ subunit and functions downstream of chaperonin 60 (Cpn60)-mediated CF1γ subunit folding to promote its assembly into the catalytic core. PAB does not have any recognizable motifs or domains but is conserved in photosynthetic eukaryotes. It is likely that PAB evolved together with the transfer of chloroplast genes into the nucleus to assist nucleus-encoded CF1γ assembly into the CF1 core. Such coordination might represent an evolutionarily conserved mechanism for folding and assembly of nucleus-encoded proteins to ensure proper assembly of multiprotein photosynthetic complexes.
The F-type ATP synthase is a ubiquitous multisubunit membrane-bound complex found in the inner membrane of mitochondria, the thylakoid membrane of chloroplasts, and the plasma membrane of bacteria (1). As a central enzyme of energy metabolism in most organisms, it couples the transmembrane proton motive force to the production of ATP from ADP and orthophosphate via a rotation mechanism (1). The F-type ATP synthase is divided into two subcomplexes with distinct functionalities: The hydrophobic membrane-integrated F0 section (subunits ab2c10 in Escherichia coli) is involved in proton transport across the membrane, and the hydrophilic F1 section (subunits α3β3γδε) contains three catalytic nucleotide and phosphate binding sites. F0 and F1 are physically connected by a central stalk containing the γ and ε subunits and a peripheral one containing the δ and β subunits (1, 2). Although crystal structures of the main parts of the ATP synthase have been determined at atomic resolution (3–5), the assembly of this marvelous rotary engine from distinct subunits is not well-understood. The emerging consensus from several studies on yeast strains deficient in the mitochondrial ATP synthase is that it forms in a stepwise manner through the formation of subcomplexes or modules (6) and requires the assistance of chaperones that act sequentially with distinct recruitment and displacement activities during interdependent steps of ATP synthase biogenesis (7). Currently, little is known about the mechanisms by which assembly of the chloroplast ATP synthase is regulated.
In higher plants, two auxiliary proteins, Alb4 and AtCGL160, facilitating the assembly of chloroplast ATP synthase subunits have been identified (8, 9). Both display similarity to assembly factors of bacterial ATP synthase, suggesting conservation between ATP synthase assembly in bacteria and chloroplasts. However, unlike bacterial ATP synthase, some genes encoding chloroplast ATPase subunits have been transferred to the nuclear genome and therefore the ATP synthase of chloroplasts is assembled from a combination of nucleus- and organelle-encoded subunits. For instance, the α, β, and ε subunits of CF1 are encoded by the chloroplast genes atpA, atpB, and atpE, respectively, whereas subunits γ and δ are encoded by the nuclear genes atpC and atpD (10–12). The ATP synthase subunits encoded by nuclear genes are synthesized in the cytosol and imported posttranslationally into organelles via protein translocases localized in the envelope, after which their folding is facilitated by chaperones. They are sorted to the thylakoid membranes, where they associate with other subunits. Accordingly, the formation of a functional organellar ATP synthase requires cross-talk between two distinct genetic compartments to ensure not only the coordinated expression of genes encoded in nuclear and organelle genomes (13) but also the coordinated import, folding, and assembly of nuclear-encoded subunits in distinct subcompartments. It is therefore expected that some plant-specific auxiliary proteins might have evolved to support the assembly of nucleus-encoded subunits of the chloroplast ATP synthase. However, none has been reported.
Chaperones not only interact transiently with polypeptide chains, to prevent or reverse misfolding and thereby promote the adoption of functional tertiary structures; they also function in the assembly of multisubunit macromolecular complexes (14). In the latter case, the primary function of the chaperone is to orchestrate the joining of individual components into a higher-order complex. This type of chaperone is therefore referred to as an assembly chaperone (14). Although the mechanisms of chaperone action in protein folding are well-established (15), the mechanisms of assembly chaperone action are not well-understood, and only a few examples of specific assembly chaperones have been described (16–19). In chloroplasts, chaperonins, such as Cpn60, which is related to the GroEL-type chaperonins of E. coli, are important for protein folding. They function together with factors of the GroES (Escherichia coli chaperonin cpn10)/chaperonin 20 (Cpn20) family and represent a general chaperonin system facilitating the folding of a broad range of substrates (20, 21). Nevertheless, Cpn60 alone is not able to facilitate reconstitution of the active CF1 core, although it is indispensable for successful reconstitution (22). This suggests that as-yet unknown assembly chaperones might coordinate the action of folding chaperones to ensure efficient assembly of folded subunits into the ATP synthase complex.
Here we report the identification of a plant-specific assembly chaperone of the chloroplast ATP synthase PAB (PROTEIN IN CHLOROPLAST ATPASE BIOGENESIS). We demonstrate that PAB assists the assembly of the CF1γ subunit into the active CF1 core downstream of Cpn60-mediated folding, which is critical for the biogenesis of the chloroplast ATP synthase.
Results
PAB Is Involved in the Assembly of the Chloroplast ATP Synthase.
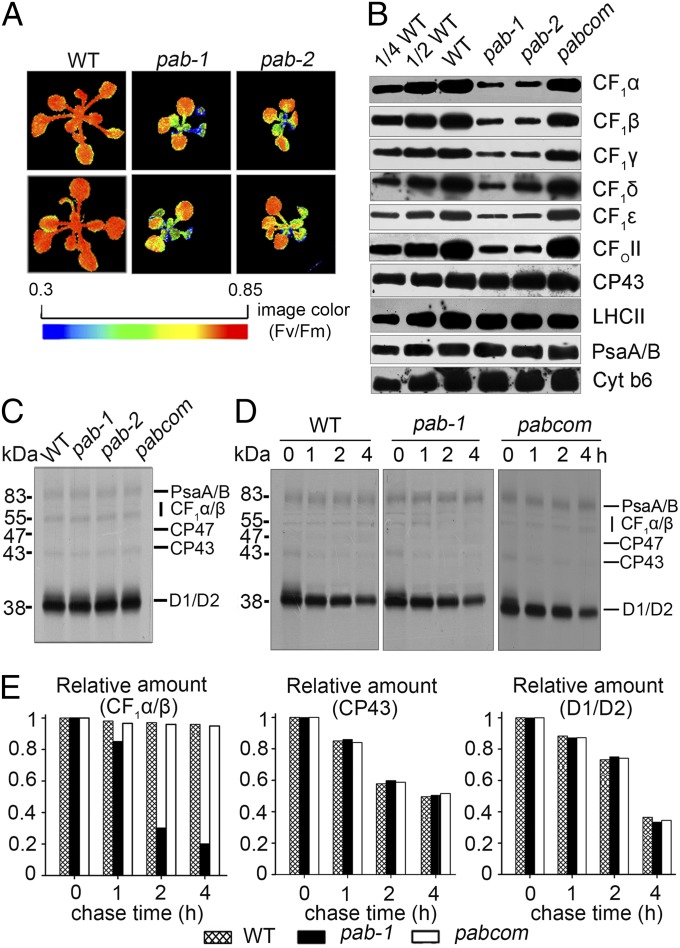
The pab mutants were isolated from the Scheible and Somerville T-DNA Arabidopsis lines due to their high–chlorophyll-fluorescence phenotype, as described previously (23) (Fig. 1A). The growth of two lines of pab (pab-1 and pab-2) was retarded, and their leaves were pale green when grown on Murashige and Skoog medium containing 1% sucrose (Fig. S1A). When grown under photoautotrophic conditions in soil, both mutant lines displayed more severe growth defects and did not flower and produce seeds. This indicates that PAB is essential for plant viability and photoautotrophic growth. Molecular cloning showed that pab-1 contained a T-DNA insertion in the tenth exon of the coding region of the At4g34090 gene, whereas pab-2 had a T-DNA insertion in the first exon of the same gene (Fig. S1B). Whereas expression of PAB at both the transcript and protein level was observed in wild-type plants, no expression was detected in the mutants (Fig. S1 C and D), suggesting that they are null mutants. Expression of full-length PAB cDNA under the control of the constitutive 35S promoter in the pab mutants fully restored the wild-type phenotype, and the level of PAB in total protein preparations of complemented plants was comparable to that in wild-type plants (Fig. S1 A and D). This functional complementation indicates that the inactivation of the At4g34090 gene is responsible for the pab phenotype. Both of the pab mutants displayed the same phenotypes, and pab-1 was used for further studies.
Fig. 1.
Phenotype and photosynthetic protein analyses in pab. (A) Chlorophyll fluorescence images of pab mutants and wild-type plants. After growth for 3 wk on separate plates, the WT and pab mutant plants were then transferred onto the same plate for chlorophyll fluorescence images. The Fv/Fm ratios were measured with a CF imager (Technologica) and visualized using a pseudocolor index, as indicated at the bottom. Dividing lines indicate noncontiguous seedlings in the picture. (B) Immunoblot analysis of thylakoid proteins with antibodies indicated on the right. (C) Pulse labeling of thylakoid membrane proteins from 10-d-old leaves. After 20-min pulse labeling in the presence of cycloheximide, the thylakoid membranes were isolated, separated by SDS/PAGE, and visualized by autoradiography. (D) Pulse–chase labeling of thylakoid membrane proteins. The 20-min pulse in 10-d-old Arabidopsis leaves was followed by a chase of unlabeled Met. The thylakoid membranes were then isolated, separated by SDS/PAGE, and visualized by autoradiography. (E) Quantification of the data reported in D for CF1α/β, CP43, and D1/D2. Ratio of the CF1α/β, CP43, and D1/D2 during the chase progress relative to the amount at the initial time (0 h). The CF1α/β, CP43, and D1/D2 at the initial time (0 h) was taken as 100%.
The high–chlorophyll-fluorescence phenotype suggested a block in photosynthetic electron transport, which might be the result of a defect in accumulation of photosynthetic protein complexes in pab mutants. To test this possibility, we investigated the steady-state levels of representative subunits of the distinct thylakoid membrane complexes (Fig. 1B). The ratios of the variable fluorescence to the maximum fluorescence (Fv/Fm; indicating the maximum potential capacity of the photochemical reactions of photosystem II) of the young leaves of the pab mutants were substantially lower than those of their wild-type counterparts (Fig. 1A). However, the Fv/Fm ratios gradually increased and approached wild-type levels (0.85) in more mature and older leaves. Thus, only young leaves with a high–chlorophyll-fluorescence phenotype were collected for analysis. The results showed that subunits of photosystem II (PSII; CP43 and LHCII), photosystem I (PSI; PsaA/B), and the cytochrome b6f complex (Cyt b6) accumulated to normal levels; however, the ATP synthase subunits (CF1α, CF1β, CF1γ, CF1δ, CF1ε, and CF0II) were reduced in pab-1 and pab-2 to 10–20% of wild-type levels. It is likely that PAB is critical for de novo ATP synthase biogenesis in young leaves. The abundance of ATP synthase subunits was restored to wild-type levels in the complemented transgenic plants. We conclude that PAB inactivation leads to a drastically reduced accumulation of the ATP synthase complex.
Diminished amounts of proteins may be due to their impaired translation. To address this possibility, the biosynthesis of chloroplast-encoded proteins was investigated in pab mutant seedlings by pulse-labeling experiments with [35S]methionine (Met) in the presence of cycloheximide, which inhibits the translation of nucleus-encoded proteins (24). As shown in Fig. 1C, the rates of translation of CP47, CP43, D1, and D2 of PSII, the PSI reaction center proteins PsaA and PsaB, and CF1α and CF1β of ATP synthase in pab-1 and pab-2 plants were comparable to those in wild-type and also in the complemented transgenic plants. However, whereas the turnover rates of PSII and PSI core subunits were relatively unaffected in the mutant, the turnover rates of ATP synthase CF1α and CF1β were greatly accelerated (Fig. 1 D and E). The decreased accumulation of ATP synthase in pab could also potentially result from the accelerated degradation of its subunits. Immunoblot analysis showed no obvious changes in the pab-1 mutant in terms of the stabilities of assembled thylakoid membrane complexes (PSII, PSI, Cyt b6f, and ATP synthase; Fig. S2). In Chlamydomonas, synthesis of the nucleus-encoded γ subunit is required for sustained translation of the chloroplast-encoded β subunit, which in turn promotes the expression of the chloroplast-encoded α subunit (13). However, the degree to which such a translational autoregulation mechanism of ATP synthase biogenesis operates in higher plants remains unclear (25, 26). Indeed, inactivation of the chloroplast γ subunit of the ATP synthase in Arabidopsis did not affect the synthesis of α and β subunits but resulted in a greatly reduced level of CF1 (27). Thus, it is likely that assembly-dependent protein stability may be responsible for the reduced accumulation of CF1, as rapid degradation of excess unassembled subunits is a general mechanism ensuring constant stoichiometry and apparently synchronous biogenesis of protein complexes (28–32).
PAB Promotes Reconstitution of the CF1 Catalytic Core.
The full-length cDNA of PAB encodes a protein consisting of 390 amino acids with a deduced mass of ∼44 kDa. A second Arabidopsis PAB homolog is predicted to be localized in the mitochondria by TargetP (www.cbs.dtu.dk/services/TargetP). Database searches and BLAST analysis revealed neither recognizable motifs nor transmembrane domains in PAB. The presence of a transit peptide of 35 amino acids at its N terminus suggests that PAB is targeted to chloroplasts. Chloroplast localization of PAB was confirmed by fluorescence microscopy of transformed Arabidopsis protoplasts expressing PAB-GFP (Fig. S3A). Fractionation and immunoblot analysis showed that the PAB protein is localized in the chloroplast stroma in plant cells (Fig. S3B). In addition to vascular plants, homologs of PAB exist in moss (Physcomitrella patens) as well as in several photosynthetic algae, including Chlamydomonas (Chlamydomonas reinhardtii), Ostreococcus lucimarinus, and Thalassiosira pseudonana. However, no homologs of PAB were found in cyanobacteria (Fig. S3C).
To determine the abundance of PAB and CF1γ in wild-type plants, titrations of PAB and CF1γ were performed using corresponding recombinant proteins as reference. The results showed that CF1γ (∼0.89 mmol/mol chlorophyll; Chl) is considerably more abundant than PAB (∼0.06 mmol/mol Chl) (Fig. S3D), suggesting that PAB is not a subunit of the ATP synthase complex.
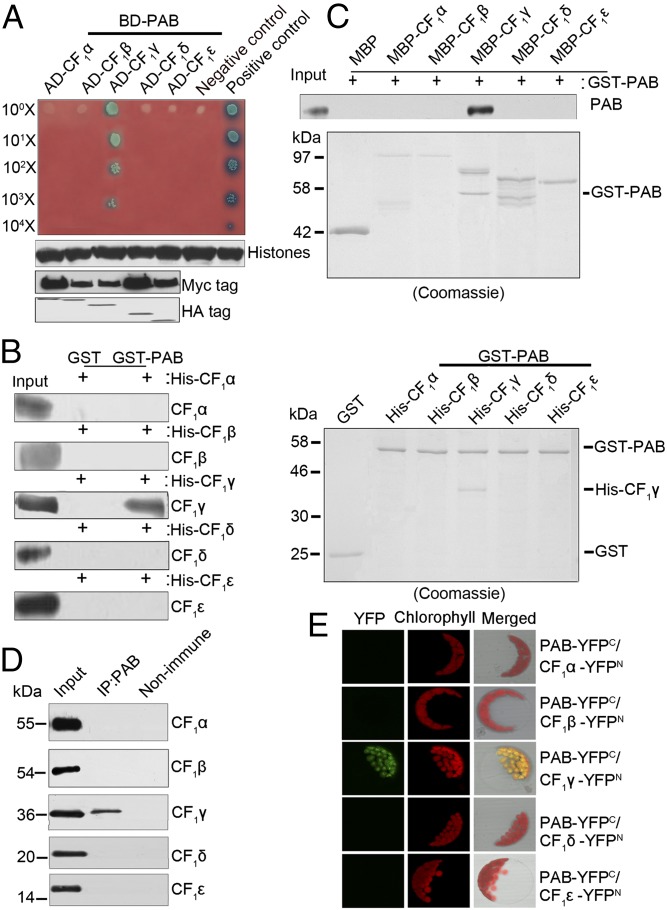
If PAB is involved in the assembly of ATP synthase, one would expect it to interact with at least one subunit of the ATP synthase complex. To address this, we performed yeast two-hybrid analysis to examine the interactions between PAB and CF1 subunits (Fig. 2A). As shown in Fig. 2A, only when PAB was coexpressed with CF1γ could the yeast transformants grow on the medium and the X-α-galactosidase be activated. These results indicate that PAB directly interacts with CF1γ but not with other subunits of CF1. The interaction of PAB with CF1γ was further confirmed by pull-down assays, which showed that PAB was able to pull down CF1γ but not CF1α, CF1β, CF1δ, or CF1ε (Fig. 2 B and C). Pull-down assays also showed the interaction of CF1γ with the CF1αβ subcomplex (Fig. S4). Coimmunoprecipitation and bimolecular fluorescence complementation (BiFC) analyses further confirmed the interactions between CF1γ and PAB in vivo (Fig. 2 D and E).
Fig. 2.
Interaction of PAB with CF1γ. (A) Yeast two-hybrid analysis of the interaction between PAB and CF1 subunits. The mature form of PAB was fused to the GAL4 DNA-binding domain (BD-PAB) as bait. CF1α, CF1β, CF1γ, CF1δ, and CF1ε were individually fused to the GAL4 activation domain (AD-CF1α, AD-CF1β, AD-CF1γ, AD-CF1δ, and AD-CF1ε) as prey. Yeast cells transformed with pGBKT7-53 and pGADT7-T were used as a positive control whereas those with pGBKT7-lam and pGADT7-T were a negative control. Immunoblot analysis of Myc and HA in yeast extracts was used to indicate the protein expression in the bait and prey plasmids, respectively. Immunoblot analysis of histone protein in yeast extracts was used as the estimation of yeast cell amounts. (B and C) Pull-down assays of the interaction between PAB and CF1 subunits. In B, PAB–glutathionine S-transferase (GST) fusion protein was constructed as bait. CF1α, CF1β, CF1γ, CF1δ, and CF1ε were individually fused to His as prey. In C, PAB-GST fusion protein was constructed as prey. CF1α, CF1β, CF1γ, CF1δ, and CF1ε were individually fused to maltose-binding protein (MBP) as bait. The bound proteins were eluted and analyzed by immunoblot analysis or Coomassie staining. (D) Coimmunoprecipitation (co-IP) analysis of the interaction between PAB and CF1 subunits. Arabidopsis total proteins were immunoprecipitated with nonimmune serum (right lane) or with antibodies against PAB (middle lane) and then analyzed by immunoblot with antibodies indicated on the right. Input (left lane) indicates that 100 μg total proteins was loaded on the gel. (E) Bimolecular fluorescence complementation analysis of the interaction between PAB and CF1 subunits. Plasmids encoding fusion constructs with the N- or C-terminal part of YFP (YFPN or YFPC, respectively) were transiently expressed in Arabidopsis protoplasts. YFP fluorescence indicates a direct interaction in planta localized to the chloroplasts.
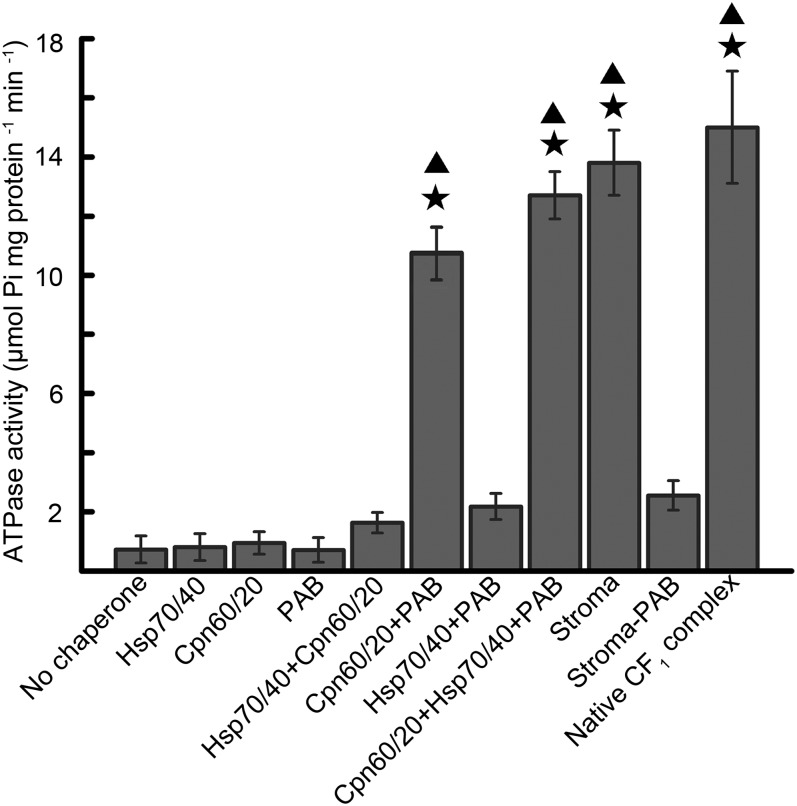
To further dissect the function of PAB in the folding and assembly of ATP synthase, we performed in vitro reconstitution of the CF1 catalytic core from individual subunits with chloroplast stroma proteins. Because chloroplast chaperone Cpn60/Cpn20 and heat shock protein 70 (Hsp70)/heat shock protein 40 (Hsp40) systems have been shown to be implicated in reconstitution reactions of the chloroplast CF1 core (22), both were included in our reconstitution assays. As shown in Fig. 3, no ATPase activity was detected when the reconstitution of CF1α, CF1β, and CF1γ was attempted without additional protein factors, suggesting that the CF1 catalytic core cannot assemble spontaneously. When stroma (containing the total complement of chloroplast molecular chaperones) was included in the reconstitution reaction, the ATPase activity reached 92% of that detected with native Arabidopsis CF1 core ATPase, which is comparable to that obtained from reconstituted enzyme with spinach stroma (22). However, when the stromal extract was replaced by Cpn60/Cpn20, Hsp70/Hsp40, or both or the stromal extract depleted of PAB (the level of PAB depletion from stroma is shown in Fig. S5), ATPase activity was barely detectable. Similarly, PAB alone did not support the reconstitution of active CF1 core (Fig. 3). However, the combination of PAB and Cpn60 increased the reconstitution efficiency significantly, and the ATPase activity was increased to 73% of that of the stroma-assisted reconstitution. The combination of Cpn60, Hsp70, and PAB led to an ATPase activity almost as high as that of the stroma-assisted reconstitution (∼95%). Moreover, the combination of Hsp70 and PAB did not significantly improve the reconstitution efficiency. These results suggest that PAB promotes CF1 core reconstitution that is assisted by Cpn60.
Fig. 3.
Reconstitution of the CF1 core. Individually expressed urea-solubilized CF1α, CF1β, and CF1γ subunits were incubated together with different combinations of protein factors, and the magnesium-dependent ATPase activity was determined. The ATPase activity (13.7 ± 0.3 µmol Pi⋅mg−1⋅min−1) of the reconstitution reaction with the stroma (containing the total complement of chloroplast molecular chaperones) was set to 100%. Error bars represent SEM (n = 6). Stars indicate significant differences from the “no chaperone” value determined by Student’s t test (P < 0.01). Triangles indicate significant differences from the “Hsp70/40+Cpn60/20” value determined by Student’s t test (P < 0.01).
Cpn60 Captures and Assists Refolding of Unfolded CF1γ.
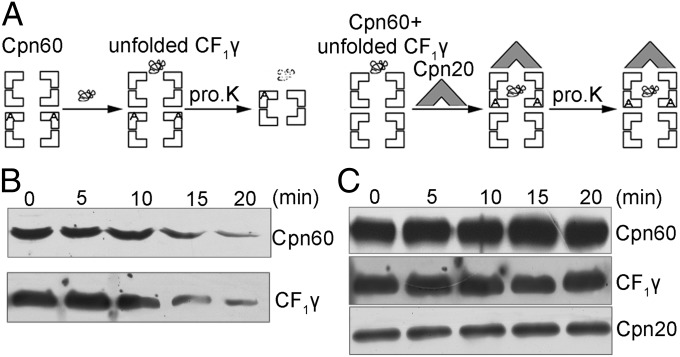
It has been shown that the catalytically active CF1 core was only obtained when the CF1α and CF1β were prefolded together (22). Folding of CF1γ is imperative for its binding to the CF1αβ complex; however, how this occurs is still unknown. Several studies have suggested that Cpn60 and Cpn20 may carry out housekeeping chaperonin functions by assisting the folding of a wide range of proteins (33, 34). To address whether folding of CF1γ is assisted by the Cpn60/Cpn20 folding machinery, we performed proteinase K assays. These assays take advantage of the formation of highly stable cis-ternary complexes of components of the GroEL/GroES system in the presence of ADP (35, 36) (Fig. 4). Addition of GroES after the substrate results in the formation of this cis-ternary complex and the sequestering of the GroEL-bound substrate, which protects the substrate protein from digestion by proteinase K. On the contrary, if GroES is not added to the substrate, the substrate will not be protected by the cis-ternary complex and will be sensitive to proteinase K (35, 36). In addition, proteinase K treatment results in removal of 16 amino acid residues from the C terminus of GroEL subunits that are not in contact with GroES, whereas GroEL molecules that are in contact with GroES remain intact (37).
Fig. 4.
Proteinase K digestion analysis of Cpn60/Cpn20-assisted refolding of CF1γ. (A) Schematic representation of molecular interactions during the experiment. The Cpn60 and unfolded CF1γ were incubated in the absence (Left) or presence (Right) of Cpn20 and ADP for 10 min. Following these steps, the protein mixtures were treated with proteinase K for 0–20 min. (B) Immunoblot analysis of CF1γ after proteinase K treatments corresponding to the procedure described in A, Left. The proteins were separated by 15% (wt/vol) SDS/PAGE, and the Cpn60 degradation product can be detected on 7% (wt/vol) SDS/PAGE. (C) Immunoblot analysis of digestion of CF1γ by proteinase K corresponding to the procedure described in A, Right. The experimental analysis was the same as in B.
In our proteinase K assays, Cpn60 and unfolded CF1γ were incubated for 10 min without or with Cpn20 (Cpn60 and Cpn20 are homologs of GroEL and GroES, respectively). The mixtures were then treated with proteinase K for 20 min followed by immunoblot analysis. As shown in Fig. 4B, in the absence of Cpn20, Cpn60 and CF1γ could be digested by proteinase K, which may be due to a binary structure formed between Cpn60 and CF1γ, as described for the GroEL/GroES system (35, 36).
However, in the presence of Cpn20, almost all of the Cpn60 and CF1γ was protected from digestion by proteinase K (Fig. 4C), which is likely due to the formation of a stable cis-ternary complex in the presence of Cpn20. Taken together, these results suggest that the unfolded CF1γ could be captured and folded by the Cpn60/Cpn20 folding machinery.
PAB Functions Downstream of Cpn60-Mediated CF1γ Folding to Promote the Formation of the CF1 Catalytic Core.
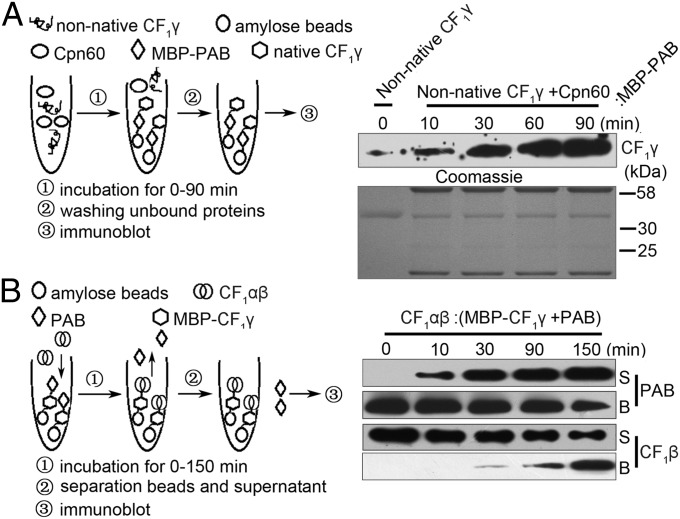
Our above results demonstrated an association between PAB and CF1γ (Fig. 2), and we further investigated the binding ability of PAB to native and nonnative CF1γ. As shown in Fig. 5A, unfolded CF1γ was first incubated with Cpn60/Cpn20 and then with MBP-PAB as well, followed by immunoblot analysis of PAB-bound CF1γ. At the beginning of the CF1γ refolding process, only a small amount of CF1γ was found to bind to PAB, whereas the amount of PAB-bound CF1γ dramatically increased as the incubation period of CF1γ and Cpn60/Cpn20 was extended (Fig. 5A). These results indicate that PAB likely binds to the folded form of CF1γ rather than to the unfolded form.
Fig. 5.
Functional analysis of PAB in the assembly of CF1γ into the ATP synthase CF1 catalytic core. (A) Pull-down assay of the interaction between PAB and unfolded or Cpn60-assisted refolded CF1γ. The experimental procedure is diagrammed (Left). The unfolded CF1γ was incubated with Cpn60/Cpn20 for 0, 10, 30, 60, and 90 min in the presence of ATP and then subjected to pull-down assay with PAB. The Coomassie-stained gel is shown below the immunoblot (Right, Lower). (B) Analysis of substitution during CF1γ assembly with CF1αβ. PAB and CF1γ-MBP formed a PAB–CF1γ complex bound to amylose beads via the MBP tag on CF1γ, and then the CF1αβ complex was added, followed by incubation for 0, 10, 30, 90, and 150 min. Proteins in the supernatant (S) and bound to the amylose beads (B) were recovered and subjected to immunoblot analysis.
To address further the molecular mechanism through which PAB mediates the integration of CF1γ into CF1, we performed substitution experiments between the CF1αβ and PAB–CF1γ complexes. As shown in Fig. 5B, CF1γ-MBP protein was incubated with His-PAB to form the CF1γ–PAB complex, which was bound to amylose beads through the MBP tag on CF1γ. After washing away the unbound His-PAB, the PAB–CF1γ complex was incubated with the CF1αβ subcomplex, followed by immunoblot analysis of the proteins in the supernatant and amylose fractions. At the beginning of the incubation of the PAB–CF1γ complex with CF1αβ, almost all of the PAB was found in the amylose fraction, whereas most of the CF1β was present in the supernatant. With increased incubation time, however, the level of PAB in the amylose resin fraction decreased whereas that in the supernatant fractions gradually increased (Fig. 5B). The level of CF1β displayed the opposite pattern during this process (Fig. 5B). These results indicate that the PAB proteins originally retained in the amylose resin fraction through interaction with CF1γ-MBP had been replaced by the CF1αβ complex, suggesting that the PAB–CF1γ interaction is dynamic, facilitating displacement of PAB from CF1γ by the CF1αβ complex to produce the CF1 catalytic core.
To directly test the ordered assembly, we performed the reconstitution assay using different combinations of individual subunits in the presence of the chloroplast molecular chaperones. No ATPase activity was observed when individual subunits were folded with the help of the chaperones followed by the addition of the other two subunits (Table S1). It was only when the α and β subunits were incubated together followed by the addition of the γ subunit that ATPase activity was close to that reconstituted with all three subunits together (Table S1). Reconstitution using MBP-CF1γ fusion protein instead of His-CF1γ occurred with the same efficiency (Fig. S6). Thus, the bulky MBP did not affect the reconstitution process. To obtain further insights into this reconstitution process, we examined the state of oligomerization of the CF1αβ subcomplex before its assembly into the active core. Immunoblot analysis of blue native (BN) gel-separated protein complexes showed that the CF1α and CF1β proteins are detected around 120 kDa. It is therefore likely that these two proteins form heterodimers before assembly with CF1γ (Fig. S7). Hence, we suggest that the active enzyme can only be reconstituted when the α and β subunits are folded together followed by the assembly of the γ subunit.
Discussion
Whether in prokaryotic or eukaryotic cells, most if not all proteins function within the context of multimeric or supramolecular assemblies. Based on this, spatial and temporal assembly of multiprotein complexes is critical for the viability of cellular organisms (38). Growing bodies of evidence indicate that molecular chaperones, functioning in diverse aspects of protein homeostasis, occupy a central place in the assembly of multimeric complexes (15, 38). Here we have reported the identification and functional characterization of a chloroplast protein, PAB, that acts as an assembly chaperone in the formation of the CF1α3β3γ core complex. PAB functions downstream of Cpn60-mediated folding and promotes the assembly of CF1γ into the active CF1 core, which sheds new light on the mechanisms of folding and assembly of nucleus-encoded photosynthetic components.
PAB is likely not to be involved in the folding of CF1γ, as PAB appears to bind folded, rather than unfolded, CF1γ (Fig. 5A). However, complex formation between PAB and native CF1γ was dynamic and PAB was readily displaced from native CF1γ by the CF1αβ complex (Fig. 5B). Thus, PAB seems to serve as a pivotal factor coupling CF1γ folding to its subsequent assembly. However, how PAB really acts during this process remains unknown. In E. coli, GroEL/GroES releases the substrate protein in a form that tends to aggregate and can rebind the chaperone (39). A pronounced tendency of RbcL to rebind to GroEL after folding is also reported in Synechocystis, and RbcX, an assembly chaperone, acts as a molecular staple to overcome the rebinding ability of RbcL (18, 40). Rebinding of substrate proteins to Cpn60/Cpn20 has generally not been reported in chloroplasts; however, unassembled RbcL was found to accumulate in chloroplasts in a complex with chaperonin (41). This suggests that similar mechanisms may also operate in the biogenesis and assembly of photosynthetic protein complexes within chloroplasts of higher plants. The mechanism used by RbcX in the assembly of hexadecameric Rubisco may also be adopted by PAB in assisting the assembly of the ATP synthase. Upon release of CF1γ from the Cpn60/Cpn20 chaperone, PAB immediately binds CF1γ (Fig. 5 A and B), which may be critical in preventing rebinding to the Cpn60/Cpn20 chaperone. Once the CF1αβ complex binds CF1γ, an as-yet unknown conformational change of CF1γ, like that of RbcL, may be triggered, facilitating PAB release and the formation of the CF1αβγ complex. Alternatively, the interaction between PAB and CF1γ may protect CF1γ from undergoing aberrant interactions with other proteins rather than CF1αβ and/or promote the insertion of CF1γ into the CF1αβ complex (see the postulated mechanism for PAB action schematically depicted in Fig. S8). Although CF1αβ subcomplexes are known to better resist proteolytic degradation than CF0 subunits (10), 10–20% of all ATP synthase subunits accumulated in the pab mutants, which indicates successful ATP synthase assembly. This phenomenon was also observed in another ATP synthase assembly mutant, AtCGL160 (9). It is possible that some residual PAB protein is sufficient for assembly of the complex at a lower but still significant yield. Another likely possibility is that the assembly of 10–20% of the ATP synthase in the absence of PAB occurs. Nevertheless, a role for Hsp70/Hsp40 in the folding of the CF1 core subunits cannot be excluded, because Hsp70/Hsp40 improved the reconstitution of the catalytically active CF1 core mediated by Cpn60/Cpn20 and PAB (Fig. 3). It seems that Hsp70/Hsp40 might facilitate Cpn60/Cpn20-assisted folding and/or PAB-assisted assembly in some way.
The chloroplasts of vascular plants originated from an ancestral cyanobacterial endosymbiont. It is reasonable to speculate that the machinery functioning in the biogenesis of multiprotein complexes also originated from the cyanobacterial ancestor. The Cpn60/Cpn20 refolding machine could be one such example, as it is derived from a prokaryotic counterpart, GroEL/GroES (42, 43). However, the photosynthetic protein complexes are composed of approximately equal numbers of nucleus- and chloroplast-encoded proteins. The transfer of genes encoding components of the photosynthetic apparatus to the nucleus after endosymbiosis, together with the integration of the organelles into plants, which have distinct developmental stages and cell types, has necessitated a novel machinery to coordinate the biosynthesis and assembly of chloroplast- and nucleus-encoded subunits. Indeed, the protein translocation machinery located in the outer and inner envelope membranes has evolved for the import of nucleus-encoded proteins into chloroplasts (44). It seems possible that specific assembly factors also evolved to facilitate the folding and assembly of nucleus-encoded proteins into photosynthetic protein complexes. Indeed, PAB appears to be such an assembly factor, evolving together with the transfer of genes into the nucleus to assist CF1γ assembly into the CF1 core. The function of PAB and Cpn60 provides an example of the complex interplay of prokaryotic and eukaryotic components that occurs in endosymbiotic organelles. Additionally, it might represent an evolutionarily conserved strategy for the folding and assembly of nucleus-encoded proteins to ensure the proper assembly of multiprotein complexes.
Materials and Methods
A detailed description of materials and methods is given in SI Materials and Methods. In vivo protein labeling was performed as described (24) using 10-d-old leaves with [35S]Met. The ATPase CF1 active core was reconstituted in vitro as previously described (22) using subunits expressed in E. coli. Proteinase K protection assay of Cpn60–substrate complexes was performed according to methods previously described (35, 36).
Supplementary Material
Acknowledgments
We thank the Arabidopsis Biological Resource Center for the seed stocks. We thank Prof. Cuimin Liu for helpful discussions and Prof. Jean-David Rochaix for comments. This work was supported by the National Natural Science Foundation of China (31130059), the Major State Basic Research Development Program (2012CB917300), and the Solar Energy Initiative and Cooperative Team Project of the Chinese Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413392111/-/DCSupplemental.
References
- 1.von Ballmoos C, Wiedenmann A, Dimroth P. Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem. 2009;78:649–672. doi: 10.1146/annurev.biochem.78.081307.104803. [DOI] [PubMed] [Google Scholar]
- 2.Nakamoto RK, Baylis Scanlon JA, Al-Shawi MK. The rotary mechanism of the ATP synthase. Arch Biochem Biophys. 2008;476(1):43–50. doi: 10.1016/j.abb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370(6491):621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 4.Stock D, Leslie AG, Walker JE. Molecular architecture of the rotary motor in ATP synthase. Science. 1999;286(5445):1700–1705. doi: 10.1126/science.286.5445.1700. [DOI] [PubMed] [Google Scholar]
- 5.Groth G, Pohl E. The structure of the chloroplast F1-ATPase at 3.2 Å resolution. J Biol Chem. 2001;276(2):1345–1352. doi: 10.1074/jbc.M008015200. [DOI] [PubMed] [Google Scholar]
- 6.Rak M, Gokova S, Tzagoloff A. Modular assembly of yeast mitochondrial ATP synthase. EMBO J. 2011;30(5):920–930. doi: 10.1038/emboj.2010.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rak M, Zeng X, Brière JJ, Tzagoloff A. Assembly of F0 in Saccharomyces cerevisiae. Biochim Biophys Acta. 2009;1793(1):108–116. doi: 10.1016/j.bbamcr.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benz M, et al. Alb4 of Arabidopsis promotes assembly and stabilization of a non chlorophyll-binding photosynthetic complex, the CF1CF0–ATP synthase. Mol Plant. 2009;2(6):1410–1424. doi: 10.1093/mp/ssp095. [DOI] [PubMed] [Google Scholar]
- 9.Rühl T, et al. The Arabidopsis protein CONSERVED ONLY IN THE GREEN LINEAGE160 promotes the assembly of the membranous part of the chloroplast ATP synthase. Plant Physiol. 2014;165(1):207–226. doi: 10.1104/pp.114.237883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemaire C, Wollman FA. The chloroplast ATP synthase in Chlamydomonas reinhardtii. II. Biochemical studies on its biogenesis using mutants defective in photophosphorylation. J Biol Chem. 1989;264(17):10235–10242. [PubMed] [Google Scholar]
- 11.Inohara N, et al. Two genes, atpC1 and atpC2, for the γ subunit of Arabidopsis thaliana chloroplast ATP synthase. J Biol Chem. 1991;266(12):7333–7338. [PubMed] [Google Scholar]
- 12.Herrmann RG, Steppuhn J, Herrmann GS, Nelson N. The nuclear-encoded polypeptide CF0-II from spinach is a real, ninth subunit of chloroplast ATP synthase. FEBS Lett. 1993;326(1–3):192–198. doi: 10.1016/0014-5793(93)81789-3. [DOI] [PubMed] [Google Scholar]
- 13.Drapier D, Rimbault B, Vallon O, Wollman FA, Choquet Y. Intertwined translational regulations set uneven stoichiometry of chloroplast ATP synthase subunits. EMBO J. 2007;26(15):3581–3591. doi: 10.1038/sj.emboj.7601802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis RJ. Molecular chaperones: Assisting assembly in addition to folding. Trends Biochem Sci. 2006;31(7):395–401. doi: 10.1016/j.tibs.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;276(2):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 16.Hirano Y, et al. Cooperation of multiple chaperones required for the assembly of mammalian 20S proteasomes. Mol Cell. 2006;24(6):977–984. doi: 10.1016/j.molcel.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Le Tallec B, et al. 20S proteasome assembly is orchestrated by two distinct pairs of chaperones in yeast and in mammals. Mol Cell. 2007;264(17):660–674. doi: 10.1016/j.molcel.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Saschenbrecker S, et al. Structure and function of RbcX, an assembly chaperone for hexadecameric Rubisco. Cell. 2007;129(6):1189–1200. doi: 10.1016/j.cell.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Chari A, et al. An assembly chaperone collaborates with the SMN complex to generate spliceosomal snRNPs. Cell. 2008;135(3):497–509. doi: 10.1016/j.cell.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Frydman J. Folding of newly translated proteins in vivo: The role of molecular chaperones. Annu Rev Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 21.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295(5561):1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 22.Chen GG, Jagendorf AT. Chloroplast molecular chaperone-assisted refolding and reconstitution of an active multisubunit coupling factor CF1 core. Proc Natl Acad Sci USA. 1994;135(3):11497–11501. doi: 10.1073/pnas.91.24.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi W, et al. The pentatricopeptide repeat protein DELAYED GREENING1 is involved in the regulation of early chloroplast development and chloroplast gene expression in Arabidopsis. Plant Physiol. 2008;147(2):573–584. doi: 10.1104/pp.108.116194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meurer J, Plucken H, Kowallik KV, Westhoff P. A nuclear-encoded protein of prokaryotic origin is essential for the stability of photosystem II in Arabidopsis thaliana. EMBO J. 1998;17(18):5286–5297. doi: 10.1093/emboj/17.18.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barkan A. Expression of plastid genes: Organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol. 2011;155(4):1520–1532. doi: 10.1104/pp.110.171231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoschke R, Watkins KP, Barkan A. A rapid ribosome profiling method elucidates chloroplast ribosome behavior in vivo. Plant Cell. 2013;25(6):2265–2275. doi: 10.1105/tpc.113.111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dal Bosco C, et al. Inactivation of the chloroplast ATP synthase γ subunit results in high non-photochemical fluorescence quenching and altered nuclear gene expression in Arabidopsis thaliana. J Biol Chem. 2004;279(2):1060–1069. doi: 10.1074/jbc.M308435200. [DOI] [PubMed] [Google Scholar]
- 28.Biekmann S, Feierabend J. Synthesis and degradation of unassembled polypeptides of the coupling factor of photophosphorylation CF1 in 70S ribosome-deficient rye leaves. Eur J Biochem. 1985;152(3):529–535. doi: 10.1111/j.1432-1033.1985.tb09228.x. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Vermaas W. Synthesis and turnover of photosystem II reaction center polypeptides in cyanobacterial D2 mutants. J Biol Chem. 1993;268(10):7407–7413. [PubMed] [Google Scholar]
- 30.Wollman FA, Minai L, Nechushtai R. The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochim Biophys Acta. 1999;1411(1):21–85. doi: 10.1016/s0005-2728(99)00043-2. [DOI] [PubMed] [Google Scholar]
- 31.Peng LW, et al. LOW PSII ACCUMULATION1 is involved in efficient assembly of photosystem II in Arabidopsis thaliana. Plant Cell. 2006;18(4):955–969. doi: 10.1105/tpc.105.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, et al. PsbP-domain protein1, a nuclear-encoded thylakoid lumenal protein, is essential for photosystem I assembly in Arabidopsis. Plant Cell. 2012;24(12):4992–5006. doi: 10.1105/tpc.112.106542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerner MJ, et al. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell. 2005;122(2):209–220. doi: 10.1016/j.cell.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Fujiwara K, Ishihama Y, Nakahigashi K, Soga T, Taguchi H. A systematic survey of in vivo obligate chaperonin-dependent substrates. EMBO J. 2010;29(9):1552–1564. doi: 10.1038/emboj.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weissman JS, et al. Mechanism of GroEL action: Productive release of polypeptide from a sequestered position under GroES. Cell. 1995;83(4):577–587. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 36.Bakkes PJ, Faber BW, van Heerikhuizen H, van der Vies SM. The T4-encoded cochaperonin, gp31, has unique properties that explain its requirement for the folding of the T4 major capsid protein. Proc Natl Acad Sci USA. 2005;102(23):8144–8149. doi: 10.1073/pnas.0500048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langer T, et al. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. EMBO J. 1992;11(13):4757–4765. doi: 10.1002/j.1460-2075.1992.tb05581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chari A, Fischer U. Cellular strategies for the assembly of molecular machines. Trends Biochem Sci. 2010;35(12):676–683. doi: 10.1016/j.tibs.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Taguchi H, Yoshida M. Chaperonin releases the substrate protein in a form with tendency to aggregate and ability to rebind to chaperonin. FEBS Lett. 1995;359(2-3):195–198. doi: 10.1016/0014-5793(95)00041-7. [DOI] [PubMed] [Google Scholar]
- 40.Liu CM, et al. Coupled chaperone action in folding and assembly of hexadecameric Rubisco. Nature. 2010;463(7278):197–202. doi: 10.1038/nature08651. [DOI] [PubMed] [Google Scholar]
- 41.Barraclough R, Ellis RJ. Protein synthesis in chloroplasts. IX. Assembly of newly-synthesized large subunits into ribulose bisphosphate carboxylase in isolated intact pea chloroplasts. Biochim Biophys Acta. 1980;608(1):19–31. doi: 10.1016/0005-2787(80)90129-x. [DOI] [PubMed] [Google Scholar]
- 42.Bertsch U, Soll J, Seetharam R, Viitanen PV. Identification, characterization, and DNA sequence of a functional “double” groES-like chaperonin from chloroplasts of higher plants. Proc Natl Acad Sci USA. 1992;89(18):8696–8700. doi: 10.1073/pnas.89.18.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viitanen PV, et al. Functional characterization of the higher plant chloroplast chaperonins. J Biol Chem. 1995;270(30):18158–18164. doi: 10.1074/jbc.270.30.18158. [DOI] [PubMed] [Google Scholar]
- 44.Soll J, Schleiff E. Protein import into chloroplasts. Nat Rev Mol Cell Biol. 2004;5(3):198–208. doi: 10.1038/nrm1333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.