Significance
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an arrhythmogenic syndrome characterized by life-threatening cardiac arrhythmias triggered by physical exercise or emotional stress. Although patients with CPVT have no abnormalities in cardiac structure, they present ventricular fibrillation or sudden death as the first symptom. Most CPVT cases are linked to mutations in cardiac ryanodine receptor (RyR2), an intracellular Ca2+ channel that provides the majority of Ca2+ that enables heart contraction. The current mechanism for CPVT arrhythmogenesis requires RyR2 affected by gain-of-function mutations. However, loss-of-function mutations of RyR2 have also been found in CPVT patients. We generated an animal model of CPVT that harbors a loss-of-function mutation that was originally described in humans and elucidated novel mechanisms by which hypoactive RyR2 channels trigger malignant arrhythmias.
Keywords: ryanodine receptor, heart, cardiac arrhythmias, CPVT, sarcoplasmic reticulum
Abstract
Current mechanisms of arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia (CPVT) require spontaneous Ca2+ release via cardiac ryanodine receptor (RyR2) channels affected by gain-of-function mutations. Hence, hyperactive RyR2 channels eager to release Ca2+ on their own appear as essential components of this arrhythmogenic scheme. This mechanism, therefore, appears inadequate to explain lethal arrhythmias in patients harboring RyR2 channels destabilized by loss-of-function mutations. We aimed to elucidate arrhythmia mechanisms in a RyR2-linked CPVT mutation (RyR2-A4860G) that depresses channel activity. Recombinant RyR2-A4860G protein was expressed equally as wild type (WT) RyR2, but channel activity was dramatically inhibited, as inferred by [3H]ryanodine binding and single channel recordings. Mice heterozygous for the RyR2-A4860G mutation (RyR2-A4860G+/−) exhibited basal bradycardia but no cardiac structural alterations; in contrast, no homozygotes were detected at birth, suggesting a lethal phenotype. Sympathetic stimulation elicited malignant arrhythmias in RyR2-A4860G+/− hearts, recapitulating the phenotype originally described in a human patient with the same mutation. In isoproterenol-stimulated ventricular myocytes, the RyR2-A4860G mutation decreased the peak of Ca2+ release during systole, gradually overloading the sarcoplasmic reticulum with Ca2+. The resultant Ca2+ overload then randomly caused bursts of prolonged Ca2+ release, activating electrogenic Na+-Ca2+ exchanger activity and triggering early afterdepolarizations. The RyR2-A4860G mutation reveals novel pathways by which RyR2 channels engage sarcolemmal currents to produce life-threatening arrhythmias.
In the heart, ryanodine receptor (RyR2) channels release massive amounts of Ca2+ from the sarcoplasmic reticulum (SR) in response to membrane depolarization, in turn modulating cardiac excitability and triggering ventricular contractions (1, 2). In their intracellular milieu, RyR2 channels are regulated by a variety of cytosolic and luminal factors so that their output signal (i.e., Ca2+) finely grades cardiac contractions (3). However, RyR2 channels operate within a limited margin of safety because conditions that demand higher RyR2 activity (such as sympathetic stimulation) also increase the vulnerability of the heart to life-threatening arrhythmias (4), and this risk is higher in hearts harboring mutant RyR2 channels. Indeed, point mutations in RYR2, the gene encoding for the cardiac RyR channel, are associated with catecholaminergic polymorphic ventricular tachycardia (CPVT) (5), a highly arrhythmogenic syndrome triggered by sympathetic stimulation that may lead to sudden cardiac death, especially in children and young adults (6).
To date, delayed afterdepolarizations (DADs) triggered by spontaneous Ca2+ release stand as the most accepted cellular mechanism to explain cardiac arrhythmias in CPVT. In this scheme, RyR2 channels destabilized by gain-of-function mutations release Ca2+ during diastole, generating a depolarizing transient inward current (Iti) as the sarcolemmal Na+-Ca2+ exchanger (NCX) extrudes the released Ca2+. This electrogenic inward current then causes DADs, which, if sufficiently large, reach the threshold to initiate untimely action potentials (APs) and generate triggered activity (6–8). Hence, hyperactive RyR2 channels eager to release Ca2+ on their own appear as essential components of this arrhythmogenic scheme. In fact, most RyR2-linked CPVT mutations characterized to date produce hyperactive RyR2 channels (9–12). This scheme therefore appears inadequate to explain lethal arrhythmias in patients harboring RyR2 channels destabilized by loss-of-function mutations (13).
How do hypoactive RyR2 channels trigger lethal arrhythmias? Here we studied the RyR2-A4860G mutation, which was initially detected in a young girl presenting idiopathic catecholaminergic ventricular fibrillation (VF) (14). When expressed in HEK293 cells, recombinant RyR2-A4860G channels displayed a dramatic depression of activity, manifested mainly as a loss of luminal Ca2+ sensitivity (13). However, this in vitro characterization was insufficient to elucidate the mechanisms by which these hypoactive channels generate cellular substrates favorable for cardiac arrhythmias. We thus generated a mouse model of CPVT harboring the RyR2-A4860G mutation. Inbreeding of mice heterozygous for the mutation (RyR2-A4860G+/−) yields only WT and heterozygous mice, indicating that the mutation is too strong to be harbored in the two RYR2 alleles. Ventricular myocytes from RyR2-A4860G+/− mice have constitutively lower Ca2+ release than WT littermates, and undergo apparently random episodes of prolonged systolic Ca2+ release upon β-adrenergic stimulation, giving rise to early afterdepolarizations (EADs). Thus, this unique RYR2 mutation reveals novel pathways whereby RyR2 channels engage sarcolemmal currents to trigger VF. Although exposed in the setting of CPVT, this mechanism may be extended to a variety of settings, including heart failure, atrial fibrillation, and other cardiomyopathies in which RyR2 down-regulation and posttranslational modifications depress RyR2 function.
Results
The RyR2-A4860G Mutation Dramatically Depresses Channel Activity.
The residue RyR2-A4860 is highly conserved among species and RyR isoforms (Fig. S1), and new structural data place it forming part of segment S6 (15), a transmembrane helical segment forming part of the conduction pathway but outside the selectivity filter (16, 17). We performed site-directed mutagenesis and introduced the CPVT mutation RyR2-A4860G in the murine RYR2 gene. Western blots analysis indicated that HEK293 cells expressed WT RyR2 and RyR2-A4860G proteins equally effectively (Fig. S2A), but [3H]ryanodine binding experiments, an index of RyR2 channel activity (18), revealed a dramatic depression of RyR2-A4860G channel activity (Fig. S2B). With Ca2+ as the only agonist, [3H]ryanodine binding to solubilized RyR2-A4860G was barely greater than nonspecific binding, but could be increased by caffeine (10 mM) and amplified even more by the combined addition of caffeine and the specific RyR agonist imperacalcin (1 μM) (19). Thus, the RyR2-A4860G mutation severely impairs but does not abolish channel activity. This was directly confirmed by single RyR2 channel recordings in lipid bilayers (Fig. S2C). Whereas WT channels displayed characteristically high open probability (Po) in the presence of ∼5 μM cytosolic [Ca2+], RyR2-A4860G channels gated briefly and sporadically under identical conditions. As a consequence, the diary of channel openings (Fig. S2D) for WT channels displayed periods of steady activity interspaced by bursts of high Po and almost no sweeps of inactivity, but the latter were the characteristic event of RyR2-A4860G channels.
Generation of Mice Heterozygous for the RyR2-A4860G Mutation.
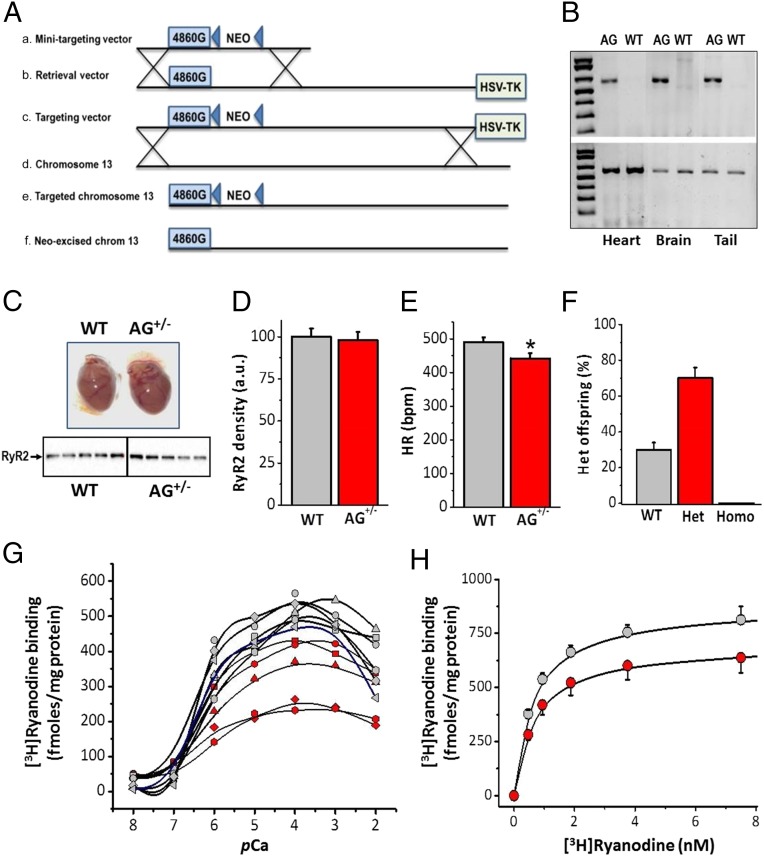
The aforementioned results revealed the intrinsic molecular alterations brought about by the RyR2-A4860G mutation, but our conditions mimicked a homozygous mutation (as opposed to the heterozygous nature of the clinical presentation) in an expression system that poorly models cardiac cells. We generated a knock-in mouse line harboring the RyR2-A4860G mutation. Fig. 1A shows the strategy used to generate the targeting vector and the resultant targeted allele. PCR of RyR2-WT and RyR2-A4860G+/− tissues yielded the expected products (Fig. 1B). Hearts from mice heterozygous for the mutation showed no gross structural alterations and expressed RyR2 proteins at the same level as WT (Fig. 1 C and D). Major hemodynamic parameters [e.g., fractional shortening (FS), ejection fraction) were normal for mice heterozygous for the mutation (RyR2-A4860G+/−), but, interestingly, they presented bradycardia at baseline (Fig. 1E and Table S1). Remarkably, inbreeding of RyR2-A4860G+/− mice generated offspring that were ∼30% WT, ∼70% heterozygotes, and 0% homozygotes (Fig. 1F). This non-Mendelian propagation of the mutant allele is present in another (but not all) phenotypically strong CPVT mutation (RyR2-V2475F+/−) (12) and strongly suggests that the mutation is too severe to be harbored in the two alleles. Correspondingly, there are no reported cases yet of patients with CPVT homozygous for a given RyR2 mutation, and thus the heterozygous mice serve as excellent models to study this unique loss-of-function mutation. Although Western blots of RyR2 protein using whole heart homogenates revealed that expression of RyR2 channels was similar for WT and RyR2-A4860G+/− mice (Fig. 1 C and D), the amplitude of the [3H]ryanodine binding curves varied widely among heterozygous specimens. [3H]Ryanodine binding to each of the heart homogenates (Fig. 1G) showed uniform activity among WT samples (gray symbols), with minimum and maximum binding at pCa (−lg[Ca2+], M) 8–7 and 4, respectively, and ED50 for Ca2+ activation of 0.94 ± 0.15 μM (n = 6). By contrast, RyR2-A4860G+/− homogenates, while conserving the minimum and maximum pCas and similar ED50 for Ca2+ activation (0.88 ± 0.12 μM; n = 5), displayed variable absolute binding (Fig. 1G, red symbols). Some RyR2-A4860G+/− homogenates fell within the range of WT values, whereas others had as much as a 50% reduction in binding activity. The results suggest variable penetrance of the RyR2-A4860G+/− mutation phenotype. Because permutation analysis shows that equal contribution of WT and mutant alleles to the formation of a tetrameric RyR2 channel yields variable populations of WT and mutation-containing channels (Fig. S3), it is possible that penetrance is related to the proportion of dysfunctional channels harbored by a given specimen, among other factors (20, 21). We grouped WT and RyR2-A4860G+/− heart homogenates together and conducted [3H]ryanodine binding under conditions that maximize activity of RyR2 channels (1 M NaCl, 100 μM Ca2+, pH 7.4). Equilibrium binding isotherms under these conditions (Fig. 1H) were still lower for RyR2-A4860G+/− homogenates (Bmax = 810 ± 55 and 625 ± 52 fmol/mg protein; Kd = 0.31 ± 0.02 and 0.42 ± 0.03 nM, respectively) suggesting that, even under maximal stimulation, there is a discrete number of RyR2 channels that remain resilient to activation or contribute minimally to overall activity.
Fig. 1.
Generation of mice harboring the RyR2-A4860G mutation and functional assessment of their RyR2 activity. (A) Strategy for generation of the knock-in mice by homologous recombination. Lines a and b show minitargeting vector and retrieval vector generated the targeting vector (line c) by homologous recombination (crosses). Line c shows the RYR2 targeting vector containing the A4860G mutation and the loxP flanked NEO cassette and the HSV-TK cassette. Line d shows the homologous recombination (crosses) between the endogenous RyR2 locus and the A4860G knock-in targeting vector. Line e shows the targeted chromosome 13 containing the 4860G mutation. Line f shows the floxed allele after Cre excision of the Neo cassette. (B) PCR confirmation of WT and heterozygous mice. The absence of the 620-bp (Upper) indicates the mutated allele. (C) Whole hearts of WT and RyR2-A4860G+/− heterozygous mice are structurally indistinguishable by gross appearance and echocardiographic parameters. (Bottom) Western blot of five different animals from each group. (D) The density of the RyR2 protein was similar for both groups. (E) Heart rate was measured in anesthetized animals (n = 8 for each group) during echocardiography. (F) Non-Mendelian propagation of RyR2-A4860G heterozygous mice yields ∼30% WT, ∼70% heterozygotes, and 0% homozygotes. (G) [3H]Ryanodine binding to whole heart homogenates of WT (gray symbols) or RyR2-A4860G+/− (red symbols) mice yield variable binding activity in the latter group only. (H) [3H]Ryanodine binding isotherms for pooled WT (gray symbols) and RyR2-A4860G+/− (red symbols) heart homogenates under conditions that maximize RyR2 activity. *P < 0.05 vs. WT.
Abnormal Ca2+ Release and EADs in RyR2-A4860G+/− Ventricular Cells.
We next conducted electrophysiology experiments to assess the Ca2+ handling and excitation–contraction (e–c) coupling parameters of RyR2-A4860G+/− cardiomyocytes and tested their arrhythmogenic potential. We voltage-clamped single WT and RyR2-A4860G+/− ventricular myocytes and simultaneously measured L-type Ca2+ current (ICaL) over a wide range of voltages and their corresponding intracellular Ca2+ ([Ca2+]i) transient (Fig. 2A). The current–voltage (I–V) relation for ICaL was similar for WT and RyR2-A4860G+/− myocytes (n = 10 from n = 8 mice in each group; Fig. 2B), but the maximum rise rate (Fig. 2C) and amplitude (Fig. 2D) of their evoked [Ca2+]i transient were depressed in RyR2-A4860G+/− myocytes. As a result, the e–c coupling “gain,” the ratio of [Ca2+]i transient amplitude vs. peak ICaL density (Fig. 2E), was lower in RyR2-A4860G+/− cells too. Because e–c coupling gain represents the efficiency by which L-type Ca2+ channels of the sarcolemma elicit Ca2+ release from RyR2 channels of SR, the results suggest that the Ca2+ release machinery of RyR2-A4860G+/− cells remains partially ineffective to the triggering ICaL stimuli.
Fig. 2.
Assessment of e–c coupling gain in WT and RyR2-A4860G+/− ventricular myocytes. (A) Representative ICaL (Top), laser scanning confocal Ca2+ image (Middle), and fluorescence intensity plot of the Ca2+ image (Bottom) from a stimulation step to 0 mV. (B) I–V plot of ICaL density (pA/pF) was similar for WT (black symbols) and RyR2-A4860G+/− (red symbols) cells. Rise rate (C), as well as amplitude (D) of the intracellular Ca2+ transient, was lower for RyR2-A4860G+/− cells (*P < 0.05, **P ≤ 0.01, and ***P ≤ 0.001). (E) e–c coupling gain, calculated as the ratio of [Ca2+]i transient amplitude (ΔF/F0) vs. ICaL density (pA/pF), was significantly lower for RyR2-A4860G+/− cells, especially at positive test potentials (n = 10 cells from n = 8 mice for each group).
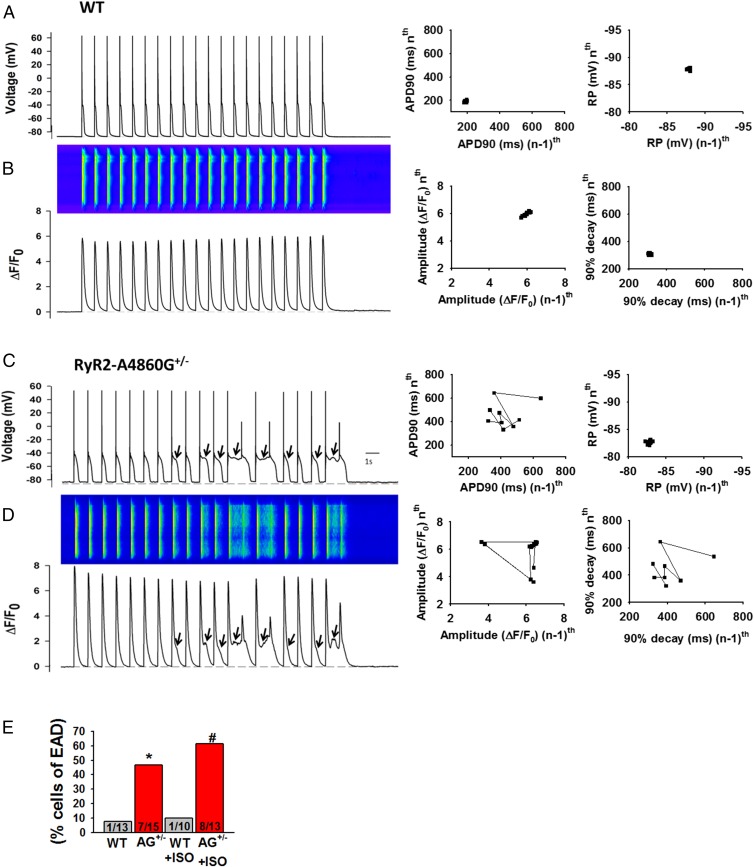
We also measured APs and their evoked [Ca2+]i transients in ventricular cells stimulated with isoproterenol (ISO; 300 nM) and paced at 1 Hz under current-clamp mode (Fig. 3). RyR2-WT cardiomyocytes exhibited typical AP amplitude and waveform and normal [Ca2+]i transients (Fig. 3 A and B). In contrast, RyR2-A4860G+/− cells showed random occurrence of EADs (Fig. 3C, arrows), which appeared at depolarized potentials (mean takeoff potential = −44.7 ± 2.6 mV) and distorted the AP waveform. [Ca2+]i transients were altered accordingly, showing random occurrence of a sustained, low-amplitude phase of Ca2+ release (Fig. 3D, arrows). Remarkably, there was a strict correlation between the prolonged phase of Ca2+ release and the induction of EADs: the longer the sustained phase of Ca2+ release, the longer the action potential duration at 90% decay (APD90) and the greater incidence of EADs. Poincaré plots (22) of APD90 and resting potential for WT cells (Fig. 3A, Middle and Right, respectively) showed no variability of either parameter during the corresponding 20-s train of stimulation, but the former was typically erratic in RyR2-A4860G+/− cells (Fig. 3C, Middle) and correlated with high variability in the [Ca2+]i transient decay (Fig. 3D, Right). Because some [Ca2+]i transients took off before complete recovery from the previous pulse, Ca2+ transient amplitude was also variable during the train of stimulation (Fig. 3D, Middle). Overall, the incidence of this type of behavior was significantly greater in RyR2-A4860G+/− cells: only 1 in 13 (no ISO) and 1 in 10 (with ISO) WT cells displayed EADs, whereas 7 in 15 (no ISO) and 8 in 13 (with ISO) RyR2-A4860G+/− cells showed EADs and abnormal Ca2+ release (Fig. 3E). EADs as a hallmark of electrical disturbance in CPVT (as opposed to DADs) reveal a novel cellular mechanism of RyR2 arrhythmogenesis.
Fig. 3.
EADs and anomalous [Ca2+]i transients in RyR2-A4860G+/− ventricular myocytes stimulated with ISO. Representative APs of WT (A) and RyR2-A4860G+/− (C) cells and their associated [Ca2+]i transients (B and D). Poincaré plots show uniform APD90 and resting potential (A, Middle and Right, respectively) between stimulating pulses and uniform amplitude and decay time for their associated [Ca2+]i transients (B, Middle and Right, respectively) for WT cells, but variability in the same parameters (C and D, Middle and Right, respectively) for RyR2-A4860G+/− cells. (E) WT ventricular myocytes rarely exhibited EADs during the stimulation protocol, but they were frequent in RyR2-A4860G+/− cells, especially after ISO stimulation. Incidence of EADs: only 1 in 13 (no ISO) and 1 in 10 (with ISO) WT cells displayed EADs, whereas 7 in 15 (no ISO) and 8 in 13 (with ISO) RyR2-A4860G+/− cells showed EADs and abnormal Ca2+ release (*P < 0.05 vs. WT; #P < 0.05 vs. WT with ISO; WT, n = 6 mice; heterozygous, n = 8 mice).
Cellular Mechanisms That Generate EADs in RyR2-A4860G+/− Cardiomyocytes.
The presence of a sustained, low-amplitude component of Ca2+ release during the systolic phase of the AP suggests that the phenotypic alterations of RyR2-A4860G+/− channels first induce abnormal Ca2+ release and then EADs. An alternative explanation is that electrical alterations of the sarcolemma first engender EADs, which then evoke prolonged Ca2+ release. If the latter is true, EADs should persist in cells in which the [Ca2+]i transient is abolished or attenuated. We applied the protocol of Fig. 3 to ISO-stimulated RyR2-A4860G+/− cells, but included in the pipette 10 mM of the slow Ca2+ chelator EGTA, which gradually decreased [Ca2+]i transient amplitude and duration as it dialyzed into the cell (Fig. S4). Resting potential and AP amplitude did not change in EGTA-containing cells (Fig. S4 B and C), but APD shortened as a function of [Ca2+]i transient amplitude, especially during phases 2 and 3 (Fig. S4D). As a result, the incidence of EADs decreased dramatically, with 0 EADs in 11 different EGTA-containing cells vs. four of seven cells without EGTA displaying EADs (Fig. S4E). Thus, SR Ca2+ release and global [Ca2+]i transients are essential to maintain an arrhythmogenic substrate in RyR2-A4860G+/− cardiomyocytes.
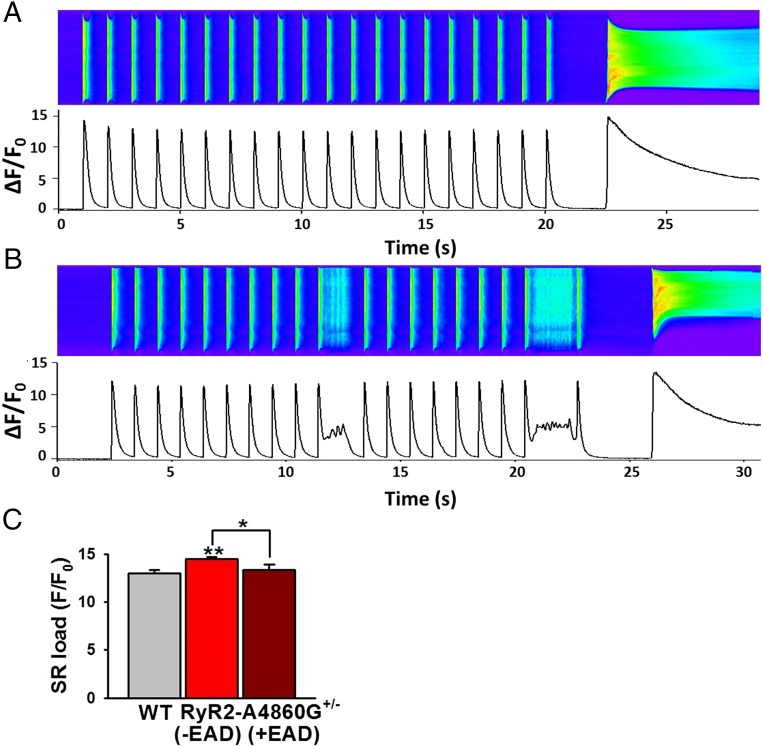
What prompts RyR2-A4860G+/− cardiomyocytes to alter their Ca2+ release dynamics and thus induce EADs? We tested whether variable SR Ca2+ load was responsible for the apparently random appearance of the prolonged Ca2+ release (Fig. 4 and Fig. S5). We reasoned that, because [Ca2+]i transient amplitude was decreased in RyR2-A4860G+/− cells in response to normal ICaL (decreased e–c coupling gain; Fig. 2), there could be a gradual increase of Ca2+ inside the SR with each pulse until a threshold was reached in which RyR2-A4860G+/− channels could be activated. This could also explain why EADs were more readily apparent under the Ca2+ overload setting of adrenergic stimulation (Fig. 3E). We measured SR Ca2+ load by measuring the amplitude of the caffeine-induced Ca2+ transient right after a 20-s train of stimulation at 1 Hz. At baseline, SR Ca2+ load was higher in RyR2-A4860G+/− cells (Fig. S5), but the incidence of EADs was low (Fig. 3E), suggesting that SR load alone was insufficient to bring mutant channels to threshold. In ISO-stimulated cells, incidence of EADs increased and SR Ca2+ load was also higher in RyR2-A4860G+/− cells, but only in that incidence of cells that exhibited no EADs during the train of stimulation (Fig. 4A); if EADs were present during the pulsing protocol, SR load was similar between WT and RyR2-A4860G+/− cells (Fig. 4 B and C). These findings suggest that sympathetic stimulation brings about a critical number of factors that collectively trigger EADs. In addition, the prolonged phase of Ca2+ release that gives rise to EADs helps to unload Ca2+ from the SR, preventing SR overfilling.
Fig. 4.
SR Ca2+ load in RyR2-A4860G+/− cells decreases after EADs. SR Ca2+ load (estimated by measuring the peak of the caffeine-induced Ca2+ release) was higher in RyR2-A4860G+/− cells that did not exhibit EADs during the stimulation protocol (A) than in those exhibiting EADs during identical protocols (B). Bars in C represent the SR Ca2+ load from a group of 30 WT cells, 28 RyR2-A4860G+/− cells without EADs, and 11 RyR2-A4860G+/− cells with EADs. A total of 300 nM ISO was applied in all cells (WT, n = 5 mice; heterozygous, n = 7 mice). **P < 0.01 vs. WT; *P < 0.05 between the two RyR2-A4860G+/− groups.
The ionic mechanisms of EADs are diverse and can involve Ca2+-dependent and Ca2+-independent processes (23, 24). Because Ca2+ release is decreased in RyR2-A4860G+/− cells (Fig. 2), we first tested the role of ICaL inactivation, which is greatly dependent on voltage and SR Ca2+ release (25). The fast component of ICaL inactivation was slightly slower in RyR2-A4860G+/− cells under basal conditions, especially at positive holding potentials (Fig. S6 A and B), but the difference was minimized in cells stimulated with ISO (Fig. S6C). We thus investigated whether blocking NCX currents could decrease the incidence of EADs (Fig. 5). We used the amiloride derivative CB-DMB to block NCX currents in forward mode (26). In the nanomolar range, CB-DMB blocks NCX current with high selectivity and does not interfere with other Ca2+ currents (including ICaL) (26). Resting potential and AP amplitude in RyR2-A4860G+/− cells were not modified by 300 nM CB-DMB (Fig. 5 A–C), but APD was significantly decreased, especially at 75–90% decay (Fig. 5D). As APD shortened with CB-DMB, the incidence of EADs also decreased (Fig. 5E).
Fig. 5.
Inhibition of the sarcolemmal NCX abolishes EADs in RyR2-A4860G+/− cells. (A) Ventricular myocyte APs during [Ca2+]i transients with prolonged phase of Ca2+ release before (black line) and after (red line) perfusion of 300 nM CB-DMB. Cells were stimulated with 300 nM ISO for at least 15 min before patch-clamping. Resting potential (B) and AP amplitude (C) were not altered by CB-DMB (300 nM) treatment, but APD (D) substantially decreased. (E) The percentage of cells displaying EADs decreased with CB-DMB treatment, from 8 of 14 (57%) to 3 of 11 (27%) to 0 of 2 (0%) after 0, 300 nM, and 3 µM CB-DMB, respectively. Data are from six mice.
VF in RyR2-A4860G+/− Hearts.
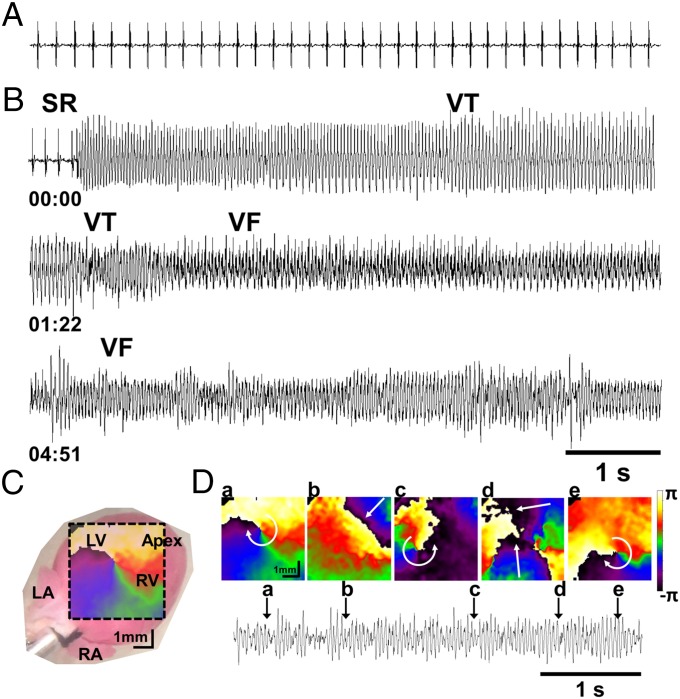
We tested whether the cellular alterations described earlier predispose RyR2-A4860G+/− hearts to VF, the main electrocardiographic event presented by the young girl affected by the mutation (14). We performed simultaneous ECG recordings and epicardial ventricular optical mapping in WT (n = 4) and RyR2-A4860G+/− (n = 6) hearts stimulated with ISO (200 nM). There were no spontaneous arrhythmias in either group under baseline conditions. Surprisingly, coadministration of caffeine (1 mM), a reliable trigger of arrhythmias in other CPVT mouse models (7, 12, 27), failed to induce arrhythmias in both groups (n = 2 and n = 3 hearts, respectively). However, increasing the extracellular [Ca2+] from 1.8 to 3.6 mM during adrenergic stress induced spontaneous long-lasting (>15 s average) VF in RyR2-A4860G+/−, which, in the optical movies, showed as meandering rotors, some of which lasted only one or more rotations (Fig. 6 and Movie S1). Thus, the RyR2-A4860G+/− mouse recapitulates the main proarrhythmogenic features originally described in the human patient.
Fig. 6.
Optical mapping reveals VF in RyR2-A4860G+/− hearts. Volume-conducted ECG from a RyR2-A4860G+/− heart at sinus baseline (A) and after perfusion with 200 nM ISO and 3.6 mM CaCl2 (B). SR, sinus rhythm; VT, ventricular tachycardia. Elapsed time (min) is indicated underneath the traces. (C) Phase map of a rotor on the anterior ventricular epicardial surface superimposed on a digital snapshot of a mouse heart. (D) Sequential 2D phase maps of meandering rotors maintaining VF in a RyR2-A4860G+/− heart. Colors indicate progressive increase in phase over space covering a complete cycle from −π to +π. All phases (colors) converge on a phase singularity (rotor) near the center of each map. Curved arrows indicate sense of rotation. Vertical arrows on ECG trace (Bottom) indicate the time points corresponding to phase maps a–e. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Discussion
The classical mechanism of RyR2-triggered arrhythmias in CPVT requires the participation of mutation-destabilized RyR2 channels eager to release Ca2+ during diastole and ignite cellular processes that lead to DADs (6–9). This scheme, therefore, necessarily rests on intrinsically hyperactive RyR2 channels that are primed for activation by any of a number of adrenergically induced exacerbating factors (e.g., increased ICaL, RyR2 phosphorylation, increased SR load). However, there is no a priori reason to discard RyR2 loss-of-function mutations in CPVT, and, because their defining characteristic is a lethargic, not a hyperactive, RyR2 channel, they presumably follow alternative mechanisms to generate arrhythmias. In this study, we generated, to our knowledge, the first CPVT animal model harboring hypoactive RyR2 channels. The RyR2-A4860G mutation was initially detected in a 7-y-old girl presenting idiopathic catecholaminergic VF who was treated with β-blockers, carried an implantable cardiac defibrillator, and had family history of unexplained sudden death (14). Previous work by Chen and coworkers showed that recombinant RyR2-A4860G channels expressed in HEK293 cells displayed a dramatic depression of activity, manifested mainly as a loss of luminal Ca2+ sensitivity (13). However, this in vitro characterization was insufficient to elucidate the mechanisms by which these channels trigger cardiac arrhythmias. Our work here allowed the discernment of novel pathways by which RyR2 channels engage sarcolemmal currents to trigger VF.
The crystal structure of RyR proteins has been hampered by the sheer size of the channel complex, but advances in single-particle electron cryomicroscopy have produced a 3D map of the rabbit skeletal RyR (RyR1) at 3.8-Å resolution that locates RyR1-A4930 (the residue analogous to RyR2-A4860) in S6, a transmembrane helical segment that forms part of the conduction pathway and supports the channel’s selectivity filter (15). These data essentially validate the 3D model of the RyR pore produced previously by homology to the KcsA channel (Fig. S1) (16, 17). In KcsA channels, the inner α-helix equivalent to S6 undergoes a hinge-type movement that allows channel gating (28); it is therefore possible that substitution of RyR2-A4860 by the helix-breaker residue Gly may directly interfere with channel opening. In fact, experimental tests of the 3D model also indicate that mutations close to the A4860 residue and forming part of the same α-helix (I4867A, F4870A) profoundly affect channel gating (29). We found that the naturally occurring RyR2-A4860G mutation does not affect channel expression (Fig. S2A) but severely reduces the number of openings and their mean open time, in essence producing a mostly mute channel that could, nonetheless, be partially activated by specific agonists (Fig. S2B). The scant and ultrafast flickering events of RyR2-A4860G channels made it difficult to estimate with precision the channel’s unitary conductance, but fully resolved openings were of similar amplitude than those of WT channels (Fig. S2C), in agreement with the 3D model and electron cryomicroscopy data that indicate A4860 resides within a domain highly sensitive to perturbations but outside the field that influences channel conductance.
The aforementioned results, obtained in homogeneous RyR2-G4860 mutant channels (mimicking a homozygous carrier), predict a severe, probably lethal, dysfunctional phenotype in humans. Indeed, inbreeding of mice heterozygous for the mutation (RyR2-A4860G+/−) produced WT and heterozygous mice only (Fig. 1F). On the contrary, [3H]ryanodine binding assays performed in homogenates of WT and RyR2-A4860G+/− hearts (Fig. 1G) showed variable reduction of binding capacity in the latter group, despite similar RyR2 protein expression (Fig. 1 C and D). As [3H]ryanodine binding may be used as an index of channel activity (3, 18), this indicates that a distinct number of RyR2 channels in RyR2-A4860G+/− hearts remain resilient to Ca2+ activation, and their variable reduction of binding capacity in turn suggests that there is variable penetrance of the dysfunctional phenotype. The randomness in the assemblage of tetrameric channels containing a critical number of mutant monomers (Fig. S3), a disproportional transcript level of the mutant gene, among other factors (12, 20, 21), may account for the variable penetrance detected here, but, whatever the roots, binding isotherms under conditions of maximal stimulation (Fig. 1H) showed that the majority of RyR2-A4860G+/− hearts contain a discrete set of functionally depressed channels. Consequently, quantitative tests of coupling fidelity between L-type Ca2+ channels (ICaL) and RyR2 channels (SR Ca2+ release) were consistently lower in RyR2-A4860G+/− ventricular myocytes (Fig. 2). The rate of rise and the amplitude of the [Ca2+]i transient were decreased in RyR2-A4860G+/− cardiomyocytes despite similar ICaL (reduced e–c coupling gain).
The deleterious consequences of harboring in a single cell a population of functionally divergent RyR2 channels were probed in current-clamped, ISO-stimulated ventricular myocytes with fast and simultaneous recording of APs and [Ca2+]i (Fig. 3). As expected, WT cardiomyocytes expressing homogeneous RyR2 channels displayed normal and uniform APs and [Ca2+]i transients whose dynamics varied little during a train of stimulating pulses (Fig. 3 A and B). By contrast, APs were strikingly longer in RyR2-A4860G+/− cells, but the prolongation occurred only in those APs whose evoked [Ca2+]i transient was anomalous, that is, it was composed of the normal peak of Ca2+ release (“peak”) and a lower but prolonged phase of Ca2+ release (“pedestal”; Fig. 3 C and D). The correlation between the prolonged phase of Ca2+ release and APD was qualitatively and quantitatively strict, i.e., the longer the pedestal, the longer the APD, and discrete EADs with takeoff potentials close to −45 mV followed pedestals that surpassed a critical time. Precise time alignment of EADs and pedestals strongly suggested that the latter preceded the former, but the most convincing argument in favor of Ca2+ distorting the AP waveform came from experiments in which EADs were abolished by intracellular EGTA (Fig. S4). The latter decreased the peak and eliminated altogether the pedestal of [Ca2+]i transients, both of which reduced APD significantly. This would not be expected if alterations inherent to sarcolemmal currents and independent of [Ca2+]i were inducing the EADs. Finally, the prominent role of the NCX in the Ca2+ → AP cross-talk was uncovered by experiments in which CB-DMB, a specific blocker of the NCX with little effect on L-type Ca2+ channels at the submicromolar doses used here (26), reduced EADs (Fig. 5).
Overall, then, the reduced peak of Ca2+ release despite normal Ca2+ entry plus the random appearance of a prolonged phase of Ca2+ release suggest a distinct mechanism for RyR2-A4860G+/− arrhythmogenesis, as follows (Fig. 7). (i) AP-evoked Ca2+ entry (i.e., ICaL) triggers SR Ca2+ release, which is decreased in RyR2-A4860G+/− cells (Fig. 2) as a result of the presence of a defined number of hyporesponsive RyR2 channels (Fig. 1). (ii) Consecutive pulses of ICaL gradually increase SR Ca2+ load (Fig. 4). (iii) When a threshold has been reached, SR Ca2+ is unloaded in the form of decreased but prolonged Ca2+ release (pedestal; Figs. 3 and 4). The participation of heterogeneous and functionally divergent RyR2 channels is essential to bring about the distorted [Ca2+]i transient waveform: we propose that hyporeactive RyR2-A4860G channels are activated during the pedestal by a combination of high SR load and Ca2+-induced Ca2+ release (CICR) by normal RyR2 channels, and they in turn activate normal channels, reinforcing CICR. Although activation of mutant channels by luminal Ca2+ appears counterintuitive based on their lack of luminal Ca2+ sensitivity as originally reported by Chen and coworkers (13), it is possible if other activating factors converge simultaneously with luminal Ca2+ to overcome the resilience of these channels to open. (iv) The prolonged phase of Ca2+ release supercharges the electrogenic NCX, generating an inward current as it extrudes Ca2+, and inducing EADs. (v) If SR Ca2+ release during the pedestal returns SR load to below threshold, a new equilibrium ensues, and EADs will be absent until SR load increases again. In this scheme, sympathetic stimulation and activation of its downstream effector PKA favors RyR2-A4860G+/− arrhythmogenesis: in addition to phosphorylating RyR2 and promoting Ca2+ release, PKA increases ICaL and accelerates SR Ca2+ uptake (1, 3, 4), all of which would encourage activation of RyR2-A4860G channels. Nonetheless, because the mutation intrinsically hinders the activity of the RyR2 channel, the scheme predicts that EADs could be detected even in the absence of adrenergic stimulation, albeit at a lower rate. Fig. 3E shows higher incidence of EADs in ISO-stimulated RyR2-A4860G+/− cells.
Fig. 7.
Hypothetical mechanisms generating EADs in RyR2-A4860G+/− ventricular myocytes. In a single RyR2-A4860G+/− cardiomyocyte containing functionally divergent RyR2 channels (WT shown in violet, AG shown in bronze), a normal ICaL first elicits a [Ca2+]i transient that displays a single phase of Ca2+ release and turns off normally, although its amplitude is lower than that of WT cells (first and second pulses). The lower amplitude of the [Ca2+]i transient despite normal ICaL (decreased e–c coupling gain) leaves a residual amount of Ca2+ inside the SR, which gradually builds up. This process is repeated n times until the SR Ca2+ load reaches a threshold that is sufficient to activate hyporesponsive RyR2 channels, which then release Ca2+ and further activate neighboring RyR2 channels, enhancing CICR. The activation of hyporesponsive RyR2 channels immediately after an initial burst of Ca2+ release (peak) generates a second, protracted and low-amplitude phase of Ca2+ release (pedestal) that supercharges the sarcolemmal NCX, generating in turn an Iti that depolarizes the membrane further, creating an EAD. If SR Ca2+ release during the pedestal returns SR load to below threshold, a new equilibrium ensues, and EADs will be absent until SR load increases again.
Thus, unlike the spontaneous and diastolic Ca2+ release that characterizes RyR2 gain-of-function mutations, an induced and systolic Ca2+ release appears as the defining feature of this loss-of-function mutation. In both cases, however, the electrogenic NCX appears to be the main instrument used by RyR2s to trigger extemporaneous APs (with a lesser role played by the L-type Ca2+ channel; Fig. S6), but, because the anomalous RyR2 Ca2+ signals occur at different phases of the AP, the former trigger DADs whereas the latter lead to EADs. Also, because EADs appear to occur at random and only in a small proportion of evoked APs, there seems to be no constitutive alteration of the AP waveform. This may explain the absence of significant alteration in the QT interval, as expected if the prolonged phase of Ca2+ release were to cause uniform and constant prolongation of the APD. Mouse hearts are remarkably resilient to VF even if harboring CPVT mutations, hence necessitating the RyR2 sensitizer caffeine as part of a standard arrhythmogenic mixture (7, 12, 27). Interestingly, caffeine was unable to trigger tachyarrhythmias in RyR2-A4860+/− hearts, which is in line with its rather modest activation of recombinant RyR2-A4860 channels (Fig. S2). Instead, doubling of the external [Ca2+] was effective to bring about VF in RyR2-A4860G+/− hearts, which is also consistent with the critical role that SR Ca2+ load plays in our proposed arrhythmogenic scheme for this mutation. Thus, although hypoactive or hyperactive RyR2 mutations engender different cellular mechanisms of arrhythmias, they both trigger in mice common ECG alterations, mainly VF and cardiac arrest. The electrocardiographic similarity across species caused by Ca2+-triggered arrhythmias suggests common pathways, regardless of the molecular mechanisms. Induction of triggered activity by EADs (loss of function) and DADs (gain of function) are possibly the converging points. The underlying basis for bradycardia in the RyR2-A4860G+/− mouse is presently unknown, but, because RyR2 Ca2+ signals play a prominent role in the Ca2+ clock that regulates SA node pacemaking (30), it is tempting to speculate that the hypoactive RyR2-A4860G+/− channels fail to entrain membrane currents of SA node cells effectively. Interestingly, some patients with CPVT present bradycardia (31), but it is unknown yet whether this subset of patients has a common RyR2 mutation phenotype.
In summary, we have used molecular, cellular, heart, and intact animal studies to unravel the mechanisms by which the RyR2-A4860G mutation causes a profound depression of RyR2 activity and leads to life-threatening arrhythmias. Although exposed in the setting of CPVT, the mechanisms by which RyR2 loss-of-function mutations generate lethal arrhythmias may be extended to heart failure, atrial fibrillation, and other cardiomyopathies in which RyR2 down-regulation or posttranslational modifications may depress RyR2 function.
Materials and Methods
Animal experiments were performed in accordance with the protocol approved by the institutional animal care and use committees of the University of Michigan and by the Association for Assessment and Accreditation of Laboratory Care International. Detailed descriptions of materials and methods are provided in SI Materials and Methods.
Statistical analysis was performed by SigmaPlot (version 10.0; Systat) integrated with SigmaStat (version 3.5; Systat). All data are presented as mean ± SEM. Student t test, rank-sum test, two-way repeated-measures ANOVA, χ2 test, and Fisher exact test were applied when appropriate to determine statistical differences. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to Dr. Shi-Qiang Wang for critical comments on this manuscript and to Dr. Wayne Chen for providing the RyR2-A4860G clone. This work was supported by National Institutes of Health Grants R01-HL055438 (to H.H.V.), R01-HL108175 (to H.H.V.), P01-HL094291 (to R.L.M. and H.H.V.), and R01-HL122352 (to J.J.), and by the Leducq Foundation (J.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419795112/-/DCSupplemental.
References
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Van Petegem F. Ryanodine receptors: Structure and function. J Biol Chem. 2012;287(38):31624–31632. doi: 10.1074/jbc.R112.349068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capes EM, Loaiza R, Valdivia HH. Ryanodine receptors. Skelet Muscle. 2011;1(1):18. doi: 10.1186/2044-5040-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Grandi E, Puglisi JL, Sato D, Bers DM. β-adrenergic stimulation activates early afterdepolarizations transiently via kinetic mismatch of PKA targets. J Mol Cell Cardiol. 2013;58:153–161. doi: 10.1016/j.yjmcc.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priori SG, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103(2):196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 6.Leenhardt A, Denjoy I, Guicheney P. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012;5(5):1044–1052. doi: 10.1161/CIRCEP.111.962027. [DOI] [PubMed] [Google Scholar]
- 7.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108(7):871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venetucci L, Denegri M, Napolitano C, Priori SG. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat Rev Cardiol. 2012;9(10):561–575. doi: 10.1038/nrcardio.2012.93. [DOI] [PubMed] [Google Scholar]
- 9.George CH, Higgs GV, Lai FA. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res. 2003;93(6):531–540. doi: 10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]
- 10.Jiang D, et al. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97(11):1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 11.Uchinoumi H, et al. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106(8):1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loaiza R, et al. Heterogeneity of ryanodine receptor dysfunction in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2013;112(2):298–308. doi: 10.1161/CIRCRESAHA.112.274803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci USA. 2007;104(46):18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Priori SG, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106(1):69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 15.Yan Z, et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature. 2015;517(7532):50–55. doi: 10.1038/nature14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welch W, Rheault S, West DJ, Williams AJ. A model of the putative pore region of the cardiac ryanodine receptor channel. Biophys J. 2004;87(4):2335–2351. doi: 10.1529/biophysj.104.044180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramachandran S, et al. Structural determinants of skeletal muscle ryanodine receptor gating. J Biol Chem. 2013;288(9):6154–6165. doi: 10.1074/jbc.M112.433789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev. 1997;49(1):53–98. [PubMed] [Google Scholar]
- 19.Gurrola GB, Capes EM, Zamudio FZ, Possani LD, Valdivia HH. Imperatoxin A, a cell-penetrating peptide from scorpion venom, as a probe of Ca-release channels/ryanodine receptors. Pharmaceuticals (Basel) 2010;3(4):1093–1107. doi: 10.3390/ph3041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Werf C, et al. Familial evaluation in catecholaminergic polymorphic ventricular tachycardia: Disease penetrance and expression in cardiac ryanodine receptor mutation-carrying relatives. Circ Arrhythm Electrophysiol. 2012;5(4):748–756. doi: 10.1161/CIRCEP.112.970517. [DOI] [PubMed] [Google Scholar]
- 21.Hoit BD. Murine physiology: Measuring the phenotype. J Mol Cell Cardiol. 2004;37(2):377–387. doi: 10.1016/j.yjmcc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Thomsen MB, et al. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004;110(16):2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- 23.Karagueuzian HS, Nguyen TP, Qu Z, Weiss JN. Oxidative stress, fibrosis, and early afterdepolarization-mediated cardiac arrhythmias. Front Physiol. 2013;4:19. doi: 10.3389/fphys.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clusin WT. Calcium and cardiac arrhythmias: DADs, EADs, and alternans. Crit Rev Clin Lab Sci. 2003;40(3):337–375. doi: 10.1080/713609356. [DOI] [PubMed] [Google Scholar]
- 25.Grandi E, Morotti S, Ginsburg KS, Severi S, Bers DM. Interplay of voltage and Ca-dependent inactivation of L-type Ca current. Prog Biophys Mol Biol. 2010;103(1):44–50. doi: 10.1016/j.pbiomolbio.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Secondo A, et al. Molecular pharmacology of the amiloride analog 3-amino-6-chloro-5-[(4-chloro-benzyl)amino]-n-[[(2,4-dimethylbenzyl)-amino]iminomethyl]-pyrazinecarboxamide (CB-DMB) as a pan inhibitor of the Na+-Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in stably transfected cells. J Pharmacol Exp Ther. 2009;331(1):212–221. doi: 10.1124/jpet.109.152132. [DOI] [PubMed] [Google Scholar]
- 27.Cerrone M, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96(10):e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 28.Choe S, Grabe M. Conformational dynamics of the inner pore helix of voltage-gated potassium channels. J Chem Phys. 2009;130(21):215103. doi: 10.1063/1.3138906. [DOI] [PubMed] [Google Scholar]
- 29.Mason SA, et al. The contribution of hydrophobic residues in the pore-forming region of the ryanodine receptor channel to block by large tetraalkylammonium cations and Shaker B inactivation peptides. J Gen Physiol. 2012;140(3):325–339. doi: 10.1085/jgp.201210851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaniv Y, Stern MD, Lakatta EG, Maltsev VA. Mechanisms of beat-to-beat regulation of cardiac pacemaker cell function by Ca2+ cycling dynamics. Biophys J. 2013;105(7):1551–1561. doi: 10.1016/j.bpj.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postma AV, et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42(11):863–870. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.