Significance
Proteins in the chloroplast thylakoid membrane system are derived from both the nuclear and plastid genomes. Mechanisms that localize nucleus-encoded proteins to the thylakoid membrane have been studied intensively, but little is known about the analogous issues for plastid-encoded proteins. This genome-wide, high-resolution analysis of the partitioning of chloroplast ribosomes between membrane and soluble fractions revealed that approximately half of the chloroplast-encoded thylakoid proteins integrate cotranslationally and half integrate posttranslationally. Features in the nascent peptide that underlie these distinct behaviors were revealed by analysis of the position on each mRNA at which elongating ribosomes first become attached to the membrane.
Keywords: ribosome profiling, protein targeting, plastid, chloroplast, SecA
Abstract
Chloroplast genomes encode ∼37 proteins that integrate into the thylakoid membrane. The mechanisms that target these proteins to the membrane are largely unexplored. We used ribosome profiling to provide a comprehensive, high-resolution map of ribosome positions on chloroplast mRNAs in separated membrane and soluble fractions in maize seedlings. The results show that translation invariably initiates off the thylakoid membrane and that ribosomes synthesizing a subset of membrane proteins subsequently become attached to the membrane in a nuclease-resistant fashion. The transition from soluble to membrane-attached ribosomes occurs shortly after the first transmembrane segment in the nascent peptide has emerged from the ribosome. Membrane proteins whose translation terminates before emergence of a transmembrane segment are translated in the stroma and targeted to the membrane posttranslationally. These results indicate that the first transmembrane segment generally comprises the signal that links ribosomes to thylakoid membranes for cotranslational integration. The sole exception is cytochrome f, whose cleavable N-terminal cpSecA-dependent signal sequence engages the thylakoid membrane cotranslationally. The distinct behavior of ribosomes synthesizing the inner envelope protein CemA indicates that sorting signals for the thylakoid and envelope membranes are distinguished cotranslationally. In addition, the fractionation behavior of ribosomes in polycistronic transcription units encoding both membrane and soluble proteins adds to the evidence that the removal of upstream ORFs by RNA processing is not typically required for the translation of internal genes in polycistronic chloroplast mRNAs.
The chloroplast thylakoid membrane is a highly organized, protein-rich, and dynamic membrane system that is the site of the light reactions of photosynthesis (1). The majority of proteins in the thylakoid membrane are subunits of photosynthetic enzyme complexes: photosystem II (PSII), the cytochrome b6f complex, photosystem I (PSI), the ATP synthase, and the NADH dehydrogenase-like complex (NDH) (2). In land plants and green algae, roughly half of the subunits of these complexes are encoded by the plastid genome and half by the nuclear genome (3, 4). This genetic arrangement necessitates a coordination of protein synthesis and assembly among cooperating proteins that originate in two compartments.
Intensive study of the mechanisms underlying the thylakoid localization of nucleus-encoded proteins revealed the participation of four machineries of cyanobacterial ancestry: the cpSec, cpTAT, cpSRP, and ALB3 systems (reviewed in ref. 5). Whereas the cpTAT pathway operates independently to mediate the translocation of folded proteins across the membrane, the cpSRP, cpSec, and ALB3 machineries cooperate in the targeting and integration of certain substrates. The bacterial orthologs of cpSRP and ALB3, known as SRP and YidC, respectively, integrate proteins into the cytoplasmic membrane in a cotranslational manner (6, 7). However, the targeting of nucleus-encoded proteins to the thylakoid membrane is posttranslational, as they are synthesized in the cytosol and then imported into the chloroplast stroma before their membrane localization.
In contrast to the sophisticated understanding of mechanisms that localize nucleus-encoded proteins to the thylakoid membrane, little information is available about the analogous issues for plastid-encoded proteins. Pioneering studies demonstrated that some chloroplast ribosomes are attached to the thylakoid membrane by the nascent peptide, implying a cotranslational integration mechanism (8, 9). Several specific plastid-encoded proteins have been shown to integrate cotranslationally: the PSII subunits PsbA (also known as D1), PsbB, PsbC, and PsbD; the PSI subunits PsaA and PsaB; and the cytochrome b6f subunit PetA (also known as cytochrome f) (10–14). The insertion of PetA into the membrane requires cpSecA (12, 15, 16), whereas PsbA integrates independent of both the cpSecA and cpTAT systems (17). In vitro cross-linking experiments showed further that nascent PsbA is in proximity to both cpSRP54 (18) and cpSecY (19). However, it is not known whether the majority of chloroplast-encoded thylakoid proteins are co- or posttranslationally integrated, nor is it known which, if any, of the known thylakoid targeting machineries are involved in their targeting and integration.
In this work, we revisited these long-standing questions by taking advantage of technical advances that allow the precise mapping of ribosomes on mRNAs. A method termed ribosome profiling generates a genome-wide, quantitative map of ribosome positions in vivo by sequencing the ribonuclease-resistant “footprints” left by ribosomes (20). We adapted this method for the rapid analysis of chloroplast translation by substituting high-resolution tiling microarrays for the deep-sequencing step (21). In this work, we modified the microarray approach by profiling chloroplast ribosomes in separated membrane and soluble fractions of leaf tissue. The results provide a genome-wide and high-resolution view of the partitioning of chloroplast ribosomes between the soluble and membrane phase and provide insight into the signals that target proteins for cotranslational integration into the thylakoid membrane.
Results
Spatially Resolved Ribosome Profiling Distinguishes Plastid-Encoded Proteins That Are Co- and Posttranslationally Targeted to the Thylakoid Membrane.
The method we used to map membrane-bound and soluble chloroplast ribosomes is shown in Fig. 1. Leaf homogenates were initially treated with micrococcal nuclease to release ribosomes from membranes that were tethered only by mRNA; this treatment will release ribosomes that are bound to membranes due to their presence on an mRNA that is membrane-tethered via a different ribosome or via an RNA binding protein. Subsequently, membrane and soluble fractions were separated by centrifugation. Ribosome footprints were purified from each fraction, labeled with fluorescent dyes, and hybridized to a high-resolution tiling microarray covering all chloroplast ORFs, using the methods described previously (21). Due to the 20-nucleotide overlap of the 50-mers on the array, this procedure maps ribosome footprints with a resolution of ∼30 nucleotides.
Fig. 1.
Method for profiling chloroplast ribosome positions in separated membrane and soluble fractions. The method is similar to that used previously (21), except that separated membrane and soluble fractions are used as the source of ribosome footprints. Leaves are flash frozen in liquid nitrogen, and homogenates are prepared in the absence of detergents and presence of chloramphenicol to stall translation. Lysates are treated with micrococcal nuclease to release ribosomes that are tethered to membranes solely by mRNA. Membrane and soluble fractions are then separated by centrifugation. Ribosome footprints purified from the two fractions are differentially labeled with fluorescent dyes, combined, and hybridized to a tiling microarray spanning all chloroplast ORFs. Array probes are 50 nt in length and overlap by 20 nt, providing a resolution of ∼30 nucleotides. In some experiments, the lysates were not pretreated with nuclease before pelleting membranes; these are denoted “– nuclease pretreatment” in subsequent figures.
Normalized signals from thylakoid-attached and soluble ribosome footprints were plotted according to position on the chloroplast genome (Fig. 2B) either as a ratio (upper plot) or separately (lower plot). All ORFs encoding proteins that were shown previously to integrate cotranslationally into the thylakoid membrane (PsbA, PsbB, PsbC, PsbD, PsaA PsaB, and PetA) (10–13, 22) are represented by prominent membrane-associated peaks, validating the method. Additional peaks revealed the thylakoid-associated translation of 12 proteins that had not been assayed in prior studies: AtpF, AtpI, PetB, NdhA, NdhB, NdhC, NdhD, NdhE, NdhF, NdhG, Ycf4, and CcsA (Fig. 2B). Each of these proteins has at least one transmembrane segment (TMS). By contrast, the ratios of membrane to soluble ribosome footprints for all chloroplast proteins lacking a TMS (e.g., ribosomal proteins, RbcL, AtpB) were very low; in fact, many such ORFs lacked any detectable signal in the membrane fraction (marked by red diamonds in the Upper panel of Fig. 2B). When lysates were not treated with nuclease before membrane pelleting (Fig. 2C), similar trends were observed; differences, however, highlight regions in which ribosomes were tethered to the membrane solely by RNA (see arrows in Fig. 2 B and C).
Fig. 2.
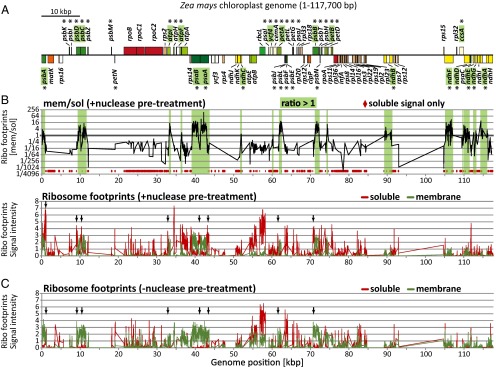
Overview of spatially resolved profiling of chloroplast ribosomes. The plotted signal intensities are the normalized values × 10−4 and are medians from two biological replicates, each with three replicate spots per array element. The data are provided in Dataset S1. Arrows mark ribosome footprints whose association with the membrane was reduced markedly when lysates were treated with nuclease before membrane pelleting (compare panels B and C). (A) Map of the maize chloroplast genome showing only protein coding regions and only one of the two large inverted repeats. Asterisks mark genes coding for proteins that contain TM segments. Genes highlighted in green are represented by abundant membrane-bound ribosome footprints in the + nuclease experiments (B). Genome position refers to the reference maize chloroplast genome (51). The map was created with OGDraw (52). (B) Ribosome footprints in membrane and soluble fractions from assays that included nuclease treatment before membrane pelleting. With this protocol, ribosomes that are tethered to membranes solely by mRNA are recovered in the soluble fraction. The ratio of signal in the membrane relative to soluble fraction is shown in the Upper panel using a log scale; green shaded regions represent ORFs whose transiting ribosomes are attached to the membrane even after nuclease treatment. Array elements with no detectable signal in the membrane fraction are marked by red diamonds at the bottom of the plot. The individual signals for the membrane and soluble fractions are plotted below (green and red lines, respectively). (C) Ribosome footprints in membrane and soluble fractions from assays that did not include nuclease pretreatment. This protocol recovers ribosomes in the membrane fraction when they are tethered either by mRNA or by protein. The individual signals for the membrane and soluble fractions are represented with green and red lines, respectively.
These results show that ribosomes transiting many chloroplast ORFs encoding integral thylakoid proteins are bound to the thylakoid membrane in a nuclease-resistant manner, implying a cotranslational targeting mode. However, ribosomes synthesizing many other transmembrane (TM) proteins are not bound to the membrane under these conditions. Proteins in the latter group include the multispanning proteins PetD and AtpH and proteins with a single TMS, such as PetL and PsbH (Fig. 2B and SI Appendix, Table S1). These proteins must integrate into the membrane posttranslationally. The basis for the distinct behaviors of these two sets of membrane proteins was clarified in the subsequent analyses.
Synthesis of Cotranslationally Targeted Proteins Initiates on Stromal Ribosomes and Transitions to Thylakoid-Bound Ribosomes.
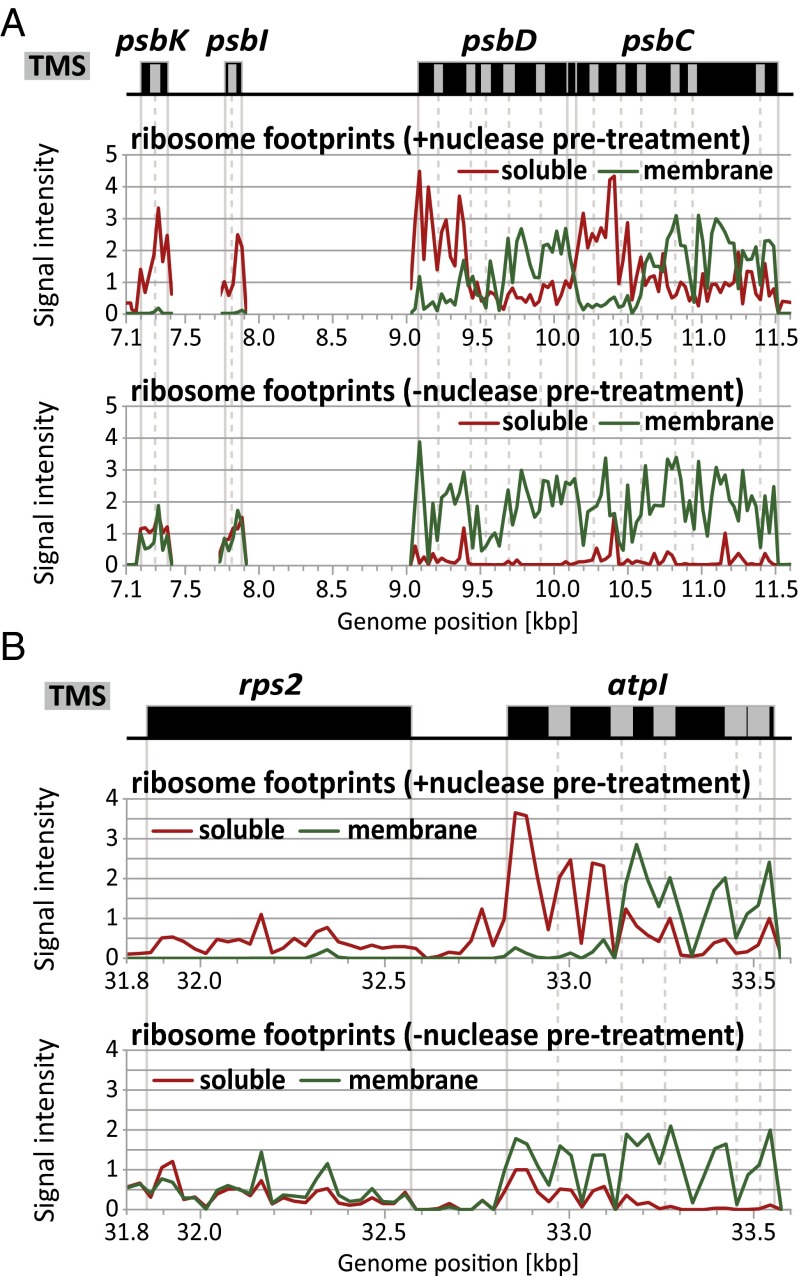
The high resolution of the data revealed the spatial dynamics of protein synthesis as chloroplast ribosomes move along each mRNA. Experiments involving nuclease treatment before membrane pelleting were most informative in this regard. In these assays, ribosome footprints near the start of all chloroplast ORFs were found predominantly in the soluble fraction (Figs. 2B and 3 and SI Appendix, Fig. S1), indicating that initiating ribosomes are not bound to the membrane. However, elongating ribosomes relocate to the membrane at a particular point along each ORF that encodes a cotranslationally targeted protein (Figs. 2B and 3 and SI Appendix, Fig. S1). Consider, for example, the psaA and psaB ORFs, which are separated by only 25 nucleotides on the same polycistronic mRNA. Ribosomes at the end of the psaA ORF remain bound to the membrane after ribonuclease treatment, whereas ribosomes at the start of the psaB ORF do not (Fig. 3A). Similar phenomena are apparent for the cotranscribed psbD and psbC genes (Fig. 3B), psbB and petB genes (Fig. 3C), and atpI and atpF genes (Fig. 3D). Other examples are presented in SI Appendix, Fig. S1. This position-dependent relocation of ribosomes from the soluble to membrane fraction was confirmed by slot-blot hybridization analysis of ribosome footprints, using probes specific for the 5′ and 3′ regions of selected ORFs (SI Appendix, Fig. S2).
Fig. 3.
Zoom-in images of data for several polycistronic transcription units. Data and annotations are as in Fig. 2. Each TMS is represented by a gray rectangle within an ORF. Gray vertical lines mark the boundaries of ORFs and introns, and dashed lines mark each TMS. Gaps in the data correspond to large intergenic regions and introns, which were not represented on the array. Analogous images for additional genes are shown in SI Appendix, Fig. S1 and Fig. 5. TMS positions are based either on experimental data or prediction, as summarized in SI Appendix, Table S1. (A) The psaA transcription unit. The psaA and psaB ORFs are always represented on the same polycistronic mRNA, whereas rps14 is also found in a monocistronic RNA isoform (53–55). (B) The psbD transcription unit. The psbD and psbC ORFs are found together on polycistronic mRNAs and also on separate processed transcripts (53). (C) The psbB transcription unit. A primary transcript spanning psbB–psbT–psbH–petB–petD is processed to yield numerous processed RNAs with intercistronic termini (31). The psbN gene is encoded by the opposite strand. (D) The atpI transcription unit. A primary transcript spanning atpI–atpH–atpF–atpA is processed to yield numerous processed RNAs with intercistronic termini (56). (E) Model for the relocation of ribosomes from the soluble to membrane fraction. Ribosomes become attached to the membrane in a nuclease-resistant fashion after the nascent peptide stably engages the thylakoid membrane, either directly or via a thylakoid-bound protein. Ribosomes whose nascent peptide is not sufficiently long to expose the signal for membrane attachment are released to the soluble fraction by the nuclease pretreatment. Scissors represent nuclease cleavage sites. The association of the nascent peptide with a hypothetical channel in the membrane is shown for illustration only and is not intended to imply a particular mechanism.
These results suggest that ribosomes become attached to the membrane in a nuclease-resistant fashion after a particular feature in the nascent peptide has emerged from the ribosome’s exit channel. According to this view, ribosomes that have not yet passed the point at which this signal is exposed are released to the soluble fraction when membranes are treated with ribonuclease (see cartoon in Fig. 3E). As predicted by this model, omission of the ribonuclease treatment before pelleting the membranes reduced the recovery of 5′-proximal ribosome footprints in the soluble fraction and increased their recovery in the membrane fraction (Figs. 2 and 3, compare + and – nuclease pretreatment). Hence, ribosomes at the start of each of the 19 membrane-translated ORFs are tethered to the thylakoid membrane by mRNA, whereas the elongating ribosomes downstream are attached via the nascent peptide. These results imply that a first round of translation anchors the mRNA to the thylakoid membrane via the nascent peptide and that subsequent rounds initiate in the vicinity of the membrane due to mRNA tethering.
Emergence of Either a TMS or cpSecA-Dependent Signal Sequence from the Ribosome Correlates with Cotranslational Membrane Anchoring.
Next, we sought to understand (i) the features that determine which chloroplast-encoded TM proteins are cotranslationally targeted to the thylakoid membrane and (ii) the point at which elongating ribosomes acquire a nuclease-resistant attachment to the membrane. All 19 membrane-translated ORFs contain at least one TMS (SI Appendix, Table S1), whereas those proteins lacking a TMS were invariably translated off the membrane (Fig. 2B). Additionally, the transition from soluble to membrane-attached translation occurred downstream of sequences encoding the first TMS (Fig. 3 and SI Appendix, Fig. S1 and Table S1), with the sole exception of PetA (discussed below). These results suggested that the emergence of a TMS in the nascent peptide triggers nuclease-resistant attachment to the membrane, as occurs for SRP-mediated targeting of signal-anchor proteins to the endoplasmic reticulum (ER) and to the bacterial cytoplasmic membrane (23, 24).
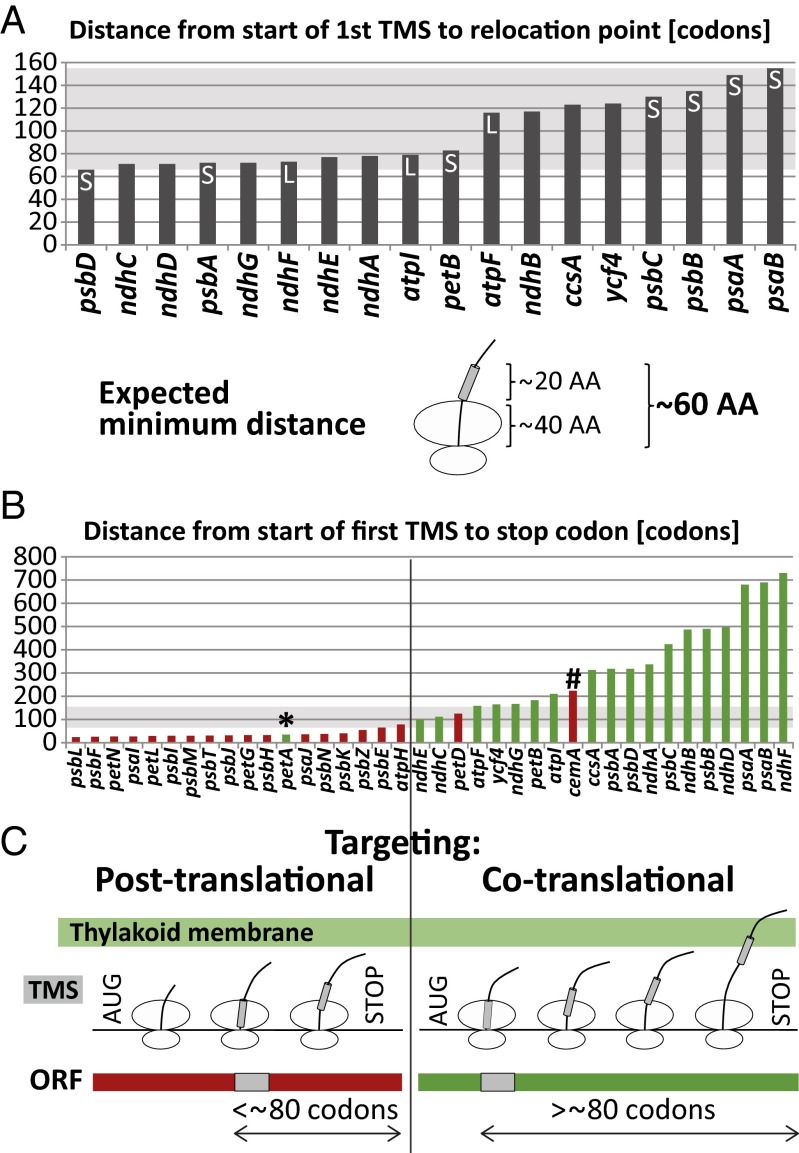
To evaluate this possibility, we calculated the distance between the start of the first TMS and the point at which ribosomes synthesizing each protein relocate from the soluble to membrane fraction (Fig. 4A). If emergence of the first TMS is a requirement for cotranslational membrane integration, a minimum distance of ∼60 amino acids is expected: This corresponds to the length of a TMS (∼20 amino acids) added to the length of the nascent chain that is obscured by the exit tunnel of the ribosome (∼40 amino acids) (25). The actual distances observed here vary between ∼66 and 155 amino acids. Thus, the first TMS in each nascent peptide will have emerged fully from the ribosome before the relocation of elongating ribosomes to the membrane. That being said, there was considerable variation in the positioning of this relocation event: In some cases, it occurred soon after the predicted emergence of the first TMS (e.g., PsbD), whereas in others considerably more nascent peptide was synthesized before the relocation (e.g., PsaB). This difference did not correlate with protein topology (N terminus in the Stroma or Lumen marked in Fig. 4A) or hydrophobicity of the first TMS (SI Appendix, Table S1). The variation in the positioning of membrane engagement following emergence of the first TMS might be influenced by the kinetics of ribosome movement or peptide-specific association with chaperones. A recent similar analysis of cotranslational targeting to the ER (26) revealed a bimodal distribution of the position at which ribosomes engage the membrane, centered at ∼60 and 120 amino acids after the start of the first TMS. This correlated with distinct requirements for components of the ER targeting machineries and was suggested to reflect either “head first” or looped insertion mechanisms. Our results hint at a similar bimodal distribution (Fig. 4A).
Fig. 4.
Full exposure of the first TMS in the nascent peptide correlates with nuclease-resistant attachment of ribosomes to the thylakoid membrane. (A) Distance from start of first TMS to the position where ribosomes remain bound to the membrane after nuclease treatment. All ORFs encoding proteins that are cotranslationally targeted to the membrane are shown, except petA, whose N-terminal signal peptide mediates membrane contact before appearance of the first TMS (Results). Proteins with experimentally validated topologies are annotated with S (N terminus in stroma) or L (N terminus in lumen). The diagram illustrates the minimum distance between the start of a TMS and its full emergence from the ribosome. The data and criteria used to define the point of relocation to the membrane are provided in SI Appendix, Table S1 and Materials and Methods. Note that the resolution of our assay is ∼10 amino acids. (B) Distance between the start of the first TMS and the stop codon for plastid-encoded TM proteins. Red and green bars denote soluble and membrane-attached translation, respectively, as shown in this study (Fig. 2). The two exceptional ORFs discussed in Results (petA and cemA) are marked with an asterisk and hashmark, respectively. The shaded region marks the range of distances following the first TMS in which nascent peptides engage the membrane (from panel A). (C) Model for targeting of plastid-encoded proteins to the thylakoid membrane. ORFs whose first TMS is fully exposed before translation termination (greater than ∼80 amino acids upstream of the stop codon) are cotranslationally targeted via engagement of the first TMS by a thylakoid-bound component of the targeting machinery. ORFs whose first TMS is not fully exposed before termination are translated on ribosomes that are not attached to the membrane and are posttranslationally targeted. The 80-amino-acid demarcation is an estimate that is based on the data summarized in Fig. 4B and SI Appendix, Table S1. This is not intended to imply a strict rule, as proteins whose first TMS maps between 79 and 125 amino acids upstream of the stop codon exhibit variable behavior.
The results above suggested that the exposure of the first TMS from the ribosome is required to attach ribosomes to thylakoid membranes in a nuclease-resistant fashion. This hypothesis predicts that any protein whose first TMS is so close to the stop codon that it would remain hidden in the ribosome until translation terminates would be translated off the membrane. The placement of the first TMS in those TM proteins that are posttranslationally targeted support this prediction. To illustrate this point, the distance between the first TMS and the stop codon was plotted for each plastid-encoded TM protein (Fig. 4B). All proteins for which this distance is less than the minimal 60 amino acids required to expose the first TMS before termination are translated off the membrane (with the exception of PetA, the special case discussed below). As noted above, there is a range of distances past the first TMS within which ribosomes engage the membrane (between ∼66 and 155 amino acids) (Fig. 4A). Within this range of distances with respect to the stop codon, some proteins are cotranslationally bound to the membrane (NdhE and NdhC), whereas others are not (AtpH and PetD). This difference might reflect distinct kinetics of ribosome movement or “head first” versus looped insertion mechanisms, as discussed above.
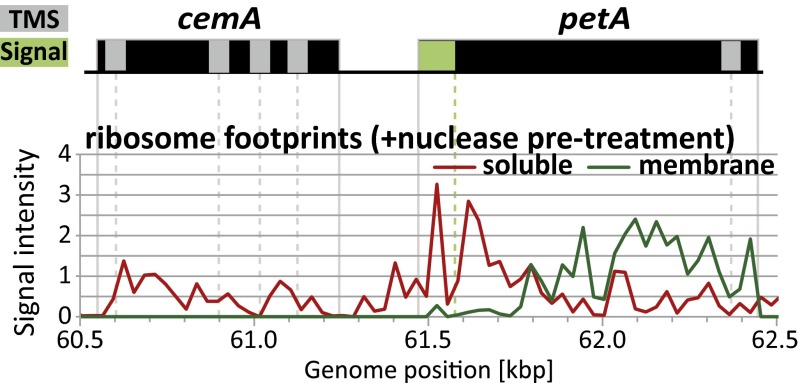
Two ORFs, petA and cemA, were exceptions to these trends. Each is a special and informative case. CemA is the sole plastid-encoded protein in maize that localizes to the inner envelope membrane and is discussed below. PetA is the sole plastid-encoded protein harboring a cleavable N-terminal cpSecA-dependent signal sequence, which mediates its targeting to the thylakoid membrane (12, 15, 16). The petA ORF engages the membrane after synthesis of ∼100 amino acids (Fig. 5). This is well before the single TMS in PetA has been synthesized, but it is 65 amino acids after the signal peptide cleavage site (27). Thus, the PetA signal peptide likely anchors the translating ribosome to the thylakoid membrane cotranslationally in vivo.
Fig. 5.
Zoom-in image of data for the cotranscribed cemA and petA genes. Data representations and annotations are as described in Fig. 3. Ribosomes transiting petA are recovered in the membrane fraction shortly after the cpSecA-dependent signal sequence emerges. Ribosomes transiting the cemA ORF are poorly recovered in the membrane fraction, even though two TMSs are predicted to be exposed before translation termination. CemA is the only chloroplast-encoded protein that localizes to the inner envelope in maize.
Taken together, the results described above provide strong evidence that exposure of either a full TMS or cpSecA-dependent signal sequence is necessary for the cotranslational targeting of chloroplast-encoded proteins to the thylakoid membrane. It seems likely that these features in the nascent peptide anchor the ribosome to the membrane by engaging a translocon. Those chloroplast-encoded proteins whose translation terminates before the emergence of a full TMS from the ribosome (roughly half of the plastid-encoded TMS proteins) are posttranslationally targeted to the membrane (Fig. 4C).
Spatial Dynamics of Plastid Ribosomes Synthesizing Envelope Proteins.
CemA’s first and second predicted TMS map far upstream of the stop codon (SI Appendix, Table S1) and are expected to be accessible for cotranslational membrane attachment. Nonetheless, ribosomes transiting the cemA ORF were recovered predominantly in the soluble fraction (Fig. 5). This unusual behavior correlates with the fact that CemA localizes to the inner envelope membrane (28) and is the only plastid-encoded protein in maize to do so.
To determine whether plastid ORFs encoding other inner envelope proteins behave similarly, we took advantage of the fact that the tobacco chloroplast genome encodes a second integral inner envelope protein, Ycf1/TIC214 (29). Ribosome footprints were prepared from membrane and soluble fractions of tobacco leaves as shown in Fig. 1. The partitioning of ribosome footprints between the two fractions was assessed by slot-blot hybridization using probes for specific ORFs (Fig. 6). Ribosomes transiting tobacco rbcL and psaA behaved as they did in maize (see SI Appendix, Fig. S2 for the maize data): Those in rbcL were largely in the soluble fraction, whereas those in psaA started in the soluble fraction but relocated to membranes within the ORF. Ribosomes synthesizing the integral inner envelope proteins CemA and Ycf1 did not fall into either of these categories: They were equally distributed between the soluble and membrane fractions, even at the 3′ ends of the ORFs where multiple TMSs are expected to have emerged. The analogous slot-blot assay for maize cemA gave similar results (SI Appendix, Fig. S2). During our fractionation protocol, a marker for the inner envelope was recovered primarily in the “soluble” fraction (SI Appendix, Fig. S2), presumably due to the low density of envelope membranes. Thus, our data cannot distinguish between the possibilities that CemA and Ycf1 integrate co- or posttranslationally into the inner envelope. Nonetheless, these results indicate that the TMSs in CemA and Ycf1 either lack a signal needed to engage the thylakoid membrane or have a feature that prevents them from doing so.
Fig. 6.
Spatial dynamics of ribosomes transiting several chloroplast ORFs in tobacco. Ribosome footprint RNA (300 ng) obtained from membrane and soluble fractions (after nuclease pretreatment) was applied to nylon membranes and hybridized to radiolabeled DNA probes covering 5′ or 3′ located segments of the indicated ORFs. Probe positions are given in SI Appendix, Table S2. The results were quantified with a phosphorimager and are plotted below. Analogous slot-blot data for maize are provided in SI Appendix, Fig. S2.
Ribosome Dynamics in Polycistronic Transcription Units Provide Evidence That Intercistronic RNA Processing Is Not Generally Required for Translation.
Chloroplast genes in land plants are typically organized in polycistronic transcription units that give rise to processed RNAs with termini between ORFs. It has been suggested that intercistronic RNA processing is a mechanism to improve translational efficiency, but there is conflicting evidence in this regard (reviewed in 30). The results presented here provide insight into this issue. Consider, for example, the petD gene in the psbB transcription unit (Fig. 3C). The petD ORF is found on processed mRNAs with a proximal 5′ end as well as on transcripts that include the petB ORF upstream (31). Ribosomes transiting petD were similarly abundant in membrane and soluble fractions when lysates were not pretreated with nuclease (Fig. 3C). However, nuclease pretreatment removed the petD ribosome footprints from the membrane, but not those from petB. These results imply that ribosomes transiting petD are tethered to the membrane via the nascent peptide on the upstream petB ORF. In other words, translation initiates on petD in the context of polycistronic mRNAs even though monocistronic transcripts are available. These results are consistent with those in a prior study, in which mRNA isoforms engaged in synthesizing PetB and PetD were identified by immunoprecipitation with antibodies to the nascent peptides (31).
Other particularly informative gene sets are psbK–psbI–psbD and rps2–atpI (Fig. 7). In each case, the downstream ORF is represented on processed transcripts with a proximal 5′ end as well as on polycistronic RNAs that include the upstream ORF. Ribosomes transiting the rps2 and psbK/I ORFs are released from the membrane by nuclease pretreatment, but remain attached to the membrane in the absence of nuclease pretreatment. These results imply that ribosomes transiting each upstream ORF are tethered to the membrane by the nascent peptides arising from the downstream ORF. In other words, the downstream ORF is, in each case, translated in the context of polycistronic mRNAs despite the fact that each is also encoded by processed mRNAs with a proximal 5′ end. After extending this logic to all polycistronic transcription units, we saw no evidence that removal of upstream ORFs is a prerequisite for translation. However, this assay cannot eliminate the possibility that RNA processing can, in some instances, enhance translational efficiency.
Fig. 7.
Evidence for translation of downstream ORFs in polycistronic transcription units that are subject to intercistronic RNA processing. (A) RNA-mediated membrane attachment of ribosomes on psbI and psbK implies that the downstream psbD ORF is translated on polycistronic RNAs. The psbK–psbI–psbD–psbC genes are represented on polycistronic RNAs spanning all four genes, and by transcripts with a 5′ end between psbI and psbD (53, 57). (B) RNA-mediated membrane attachment of ribosomes on rps2 implies that the downstream atpI ORF is translated on polycistronic RNAs. Transcripts arising from the rps2–atpI region include polycistronic transcripts with both ORFs, as well as RNAs with a 5′ end in the intergenic region (58).
Discussion
Results presented here provide a comprehensive description of which plastid-encoded proteins are cotranslationally targeted to the thylakoid membrane, and they elucidate the signals that trigger cotranslational targeting. Nineteen of the 37 plastid-encoded integral thylakoid proteins in maize are cotranslationally targeted; ribosomes synthesizing these proteins initiate translation off the membrane and then become attached to the membrane in a nuclease-resistant manner shortly after a complete TMS or cpSecA-dependent signal sequence emerges from the exit channel. Those membrane proteins whose translation terminates before exposure of one of these signals are translated off the membrane and must be posttranslationally targeted.
Early studies in Chlamydomonas and pea concluded that chloroplast translation always initiates in the stroma and that some ribosomes subsequently become coupled to the thylakoid membrane via the nascent peptide (32, 33). Later reports identified several chloroplast mRNAs that are translated in association with the thylakoid membrane, but the results were sometimes conflicting (22, 34, 35). The most thorough study of this type (22) concluded that the psaA, psbB, psbC, psbD, and petA gene products integrate into the membrane cotranslationally, whereas the psbE, petD, and atpH gene products do not. The genome-wide analysis presented here corroborates and extends those findings by identifying all ORFs that attach to the membrane via the nascent peptide, by mapping the position at which this transition occurs, and by revealing principles that dictate which path is taken by each protein. Conflicting reports about membrane-localized translation of several ORFs in prior studies (34, 35) are likely due to their presence on polycistronic RNAs that encode both co- and posttranslationally targeted proteins. The ability of ribosome profiling to resolve different ORFs within polycistronic transcripts is a great advantage for studies of translation in chloroplasts and in bacteria.
Cotranslational Targeting to the Thylakoid Membrane.
Results presented here provide strong evidence that the first TMS is both necessary and sufficient to trigger the cotranslational targeting of the vast majority of chloroplast proteins to the thylakoid membrane. This view is supported by two reciprocal correlations: (i) Ribosomes invariably become attached to the membrane in a nuclease-resistant fashion shortly after the first TMS is fully emerged from the exit channel, and (ii) proteins that terminate translation before that point are translated off the membrane (with the exception of PetA, discussed below). It seems likely that the first TMS is recognized by the same machineries that promote the posttranslational targeting of nucleus-encoded proteins (5). For example, cpSRP54 might bind the first TMS cotranslationally, as do its orthologs in bacteria and the ER. In vitro crosslinking data support that possibility for the PsbA protein (18), but the mild phenotype of Arabidopsis mutants lacking cpSRP54 (36) indicates that this interaction is not essential for the targeting of most plastid-encoded proteins. The establishment of a stable interaction between the nascent peptide and the membrane might be mediated by membrane extrinsic proteins such as cpFtsY or cpSecA or could require the TMS to enter the cpSecY/E, ALB3, or TAT translocon. The close proximity of cpSecY to the PsbA nascent peptide in isolated chloroplasts (19) implicates cpSecY in the integration of PsbA, but these issues have not been addressed for other proteins. Extension of the approach described here to mutants lacking specific components of the thylakoid targeting machineries should clarify the early events in thylakoid targeting for the 19 cotranslationally targeted thyakoid proteins.
PetA (cytochrome f) presents a special case, as it is the only plastid-encoded protein to harbor a cleavable, cpSecA-dependent signal sequence at its N terminus (12, 15, 16). SecA-mediated targeting to the bacterial cytoplasmic membrane is considered to be a posttranslational process (6, 37), but our results show unambiguously that the PetA signal sequence engages the thylakoid membrane shortly after it emerges from the ribosome. Bacterial SecA is bound to the ribosome (38) and could potentially bind signal sequences cotranslationally. Assays similar to those used here could be used to address whether this in fact occurs.
Recent analyses of ribosome profiling data have revealed mRNA-programmed ribosome pauses that enhance SRP binding and faithful membrane integration (39, 40). In yeast, these events are mediated by rare codons, whereas in Escherichia coli they are mediated by ORF-internal Shine–Dalgarno elements. Classic experiments showed that chloroplast ribosomes in barley pause at discrete sites in the psbA ORF, and it was suggested that these pauses facilitate membrane integration (41). It is intriguing that the major pause detected in that study maps ∼50 nucleotides downstream of the point that we see the nascent peptide attach to the membrane. We did not, however, detect unambiguous ribosome pauses correlating with membrane attachment in our data. However, our microarray-based assay is not ideal for this purpose due to its inability to map ribosome positions to greater than 30-nucleotide resolution and to the fact that short ribosome footprints that reflect certain stages of the elongation cycle may not be detected (42). Use of deep sequencing to analyze spatially resolved ribosome footprints should allow the interplay between ribosome kinetics and thylakoid targeting/integration to be thoroughly addressed.
Posttranslational Targeting of Plastid-Encoded Proteins to the Thyakoid Membrane.
Our data revealed that half of the integral thylakoid membrane proteins encoded by the plastid genome are targeted to the membrane after their synthesis is complete. The posttranslationally integrating proteins include the multispanning proteins PetD, AtpH, PsbK, and PsbZ and 14 proteins consisting of little more than a single TMS. These may integrate into the thylakoid membrane without the aid of a proteinaceous machinery, as has been shown for several single-spanning nucleus-encoded proteins (reviewed in ref. 43). On the other hand, YidC is required to integrate the AtpH ortholog in bacteria, Foc (44), and it has been suggested that many short membrane proteins with C-terminal signal anchor sequences are posttranslationally integrated by the YidC translocon (45). The degree to which ALB3 (the chloroplast YidC ortholog) participates in the posttranslational integration of plastid-encoded proteins remains to be determined.
Targeting of Plastid-Encoded Proteins to the Inner Envelope.
Most chloroplast genomes encode one or two proteins that integrate into the inner envelope (CemA and Ycf1). How these proteins find their way to the inner envelope is not known. Interestingly, ribosomes transiting the cemA ORF in maize behaved differently from those synthesizing thylakoid proteins: Despite the fact that two TMSs are predicted to emerge from the ribosome before termination, cemA ribosome footprints were recovered predominantly in the soluble fraction. Tobacco cemA and ycf1 behaved similarly, albeit with roughly equal representation of ribosome footprints in the membrane and soluble fraction.
These observations raise an interesting question: What prevents the TMSs in CemA and Ycf1 from engaging the thylakoid membrane cotranslationally? Some nucleus-encoded envelope proteins are imported into the stroma and subsequently “exported” to the inner envelope (46). TIC40, a well-studied example, has a serine/proline-rich domain that is crucial for inner envelope targeting (47). When TIC40 was expressed from a chloroplast transgene, it was targeted to the inner envelope (48), suggesting that similar mechanisms can target nucleus- and plastid-encoded proteins to the inner envelope. CemA, however, lacks a serine/proline-rich region. Interestingly, the CemA N terminus does resemble a bacterial signal sequence: a lysine-rich segment followed by a predicted TMS (MKKKKALPSFLYLVFIVLLPWGVSFSF…). An appealing possibility is that the novel Sec translocase discovered recently in the inner envelope (49) mediates CemA and Ycf1 targeting. Regardless of the machineries responsible, the distinct behavior of ribosomes synthesizing inner envelope and thylakoid proteins indicates that the sorting signals are distinguished cotranslationally. Lysine-rich stretches do not precede the first TMS in any of the 19 cotranslationally targeted thylakoid membrane proteins. A testable hypothesis is that the lysine-rich stretch at the CemA N terminus either interferes with the engagement of thylakoid translocons or is quickly bound by a protein that masks the TMS from the thylakoid targeting machineries.
In situ assays in Chlamydomonas chloroplasts revealed that psbA mRNA is bound to thylakoid membranes via the nascent peptide during PSII repair, but is bound independent of translation to distinct “biogenic membranes” shortly after a dark-to-light shift (14, 50). Our assays were performed 1 h into the light cycle on seedling leaf tissue at a stage with a young but assembled photosynthetic apparatus. It is difficult to compare the results of these two studies due to the very different organisms and assays used. We saw no apparent nuclease-resistant membrane association of ribosome footprints at the start of any chloroplast ORF, but this does not eliminate the possibility that RNA binding proteins might tether some RNAs to membranes for localized translation. Future studies that combine more refined membrane fractionation approaches with ribosome profiling should be useful for dissecting the interplay between membrane biogenesis and localized translation in the chloroplasts of plants and algae.
Materials and Methods
Plant Material.
Zea mays (inbred line B73) was grown in soil in cycles of 16 h light (∼300 µmol·m−2·s−1) at 28 °C and 8 h dark at 26 °C. The second and third leaf were harvested 1 h into the light cycle on the eighth day after sowing. Tobacco (Nicotiana tabacum cultivar Petit Havana) was grown in soil in cycles of 16 h light (∼350 µmol·m−2·s−1) at 23 °C and 8 h dark at 22 °C. Leaves were harvested 1 h into the light cycle on the 25th day after sowing. Tissue was snap-frozen in liquid nitrogen and stored at –80 °C.
Preparation of Ribosome Footprints from Membrane and Soluble Fractions.
All steps were performed at 4 °C unless otherwise noted. Leaf tissue (∼1 g fresh weight) was ground in liquid nitrogen with a mortar and pestle and thawed in 5 mL ribosome extraction buffer (0.2 M Sucrose, 0.2 M KCl, 40 mM Tris-Acetate pH 8.0, 10 mM MgCl2, 10 mM 2-mercaptoethanol, 100 µg/mL chloramphenicol, and 100 µg/mL cycloheximide). For + nuclease pretreatment experiments, 750 U micrococcal nuclease (Roche, 10107921001) was added and the homogenate was incubated on a rotator at 23 °C for 15 min. The suspension was centrifuged for 20 min at 15,000 × g in a JA-20 rotor (Beckman). The supernatant (soluble fraction) was transferred to a new tube, and the pellet was washed by resuspension in 5 mL extraction buffer and recentrifugation. The supernatant was discarded, and the pellet (the membrane fraction) was solubilized in 5 mL extraction buffer with added detergents [2% (vol/vol) Polyoxyethylene tridecyl ether, 1% Triton X-100]. After pelleting insoluble material (20 min at 15,000 × g), the supernatant contained virtually all of the chlorophyll and was transferred to a new tube. Monosomes were generated from each fraction by the addition of 25 µL·1 M CaCl2 and 750 U micrococcal nuclease and incubation on a rotator at 23 °C for 1 h. Monosomes were purified by ultracentrifugation through a sucrose cushion, and RNA fragments of ∼22–38 nt (“ribosome footprints”) were purified by polyacrylamide gel electrophoresis as described previously (21).
Microarray Hybridization and Data Analysis.
Ribosome footprints derived from membrane and soluble fractions (∼3 µg of each gel purified sample) were labeled with Cy5 and Cy3, respectively, using the ULS aRNA labeling kit (Kreatech Diagnostics). The labeled footprints were combined and hybridized to custom microarrays (Mycroarray) consisting of overlapping 50 mers representing all chloroplast ORFs (in triplicate), as previously described (21). Ribosome footprint signal intensity is the intensity of fluorescence resulting from hybridization of Cy-labeled ribosome footprints to each spot on the array. The presented analyses combine data from two biological replicates. Probe spots with background subtracted signals <0 were assigned values of 0. Note that the colors used to plot the data (green for membrane and red for soluble) were chosen to make the figures more intuitive and do not reflect the actual fluorescence wavelengths of the dyes.
The single channel data from each dataset were normalized based on the median values of the 233 probes in each dataset with the highest signal (median of the top 10% of probes in each of the two channels in each of the two biological replicates): The median value of each single channel dataset was set to this value by a multiplication factor. The ratio of membrane to soluble signal within each dataset was then adjusted to mimic that in vivo as follows: (i) For experiments that included a nuclease pretreatment, five published datasets from unfractionated wild-type seedling leaf tissue (21) were used to calculate the median ratio of ribosome footprint signals in the first 200 nt relative to the last 200 nt of the psbD, psbC, psaA, psaB, and psbB ORFs (excluding the psbC/D overlap). The observed ratio was 1.24. The analogous ratio was calculated for the normalized + nuclease pretreatment dataset, using the soluble signal for the 5′ region and the membrane signal for the 3′ region; this method is appropriate, as virtually all of the signals in the 5′ and 3′ regions of the selected ORFs came from the soluble and membrane fractions, respectively. This ratio was then adjusted to 1.24: the soluble values were multiplied and the membrane values were divided by the same factor, to bring their ratio to 1.24. (ii) For experiments that excluded the nuclease pretreatment, the overall ratio of membrane to soluble signal was adjusted based on ribosome footprint signal in the last 500 nt of the membrane-translated psbD, psbC, psaA, psaB, and psbB ORFs relative to that in the last 500 nt of the soluble atpB and rbcL ORFs. In the published data for unfractionated wild-type leaf (21), this ratio was ∼0.58. This ratio in the – nuclease dataset was adjusted to 0.58 by multiplying all soluble values and dividing all membrane values as described above. This normalization mode is appropriate because of the virtual absence of soluble and membrane signals at the 3′ and 5′ ends of membrane-translated ORFs (psbD, psbC, psaA, psaB, and psbB) and soluble-translated ORFs (atpB and rbcL), respectively. We chose different normalization approaches for experiments that did or did not include nuclease pretreatment due to the different nature of the data; although these gave slightly different overall ratios of soluble to membrane signal, this does not impact any of the conclusions made in this study.
The position at which ribosomes relocate to the membrane fraction was estimated with two methods, both of which are tabulated in SI Appendix, Table S1. In one method, the relocation point was defined as the midpoint of the position at which two consecutive array elements have values that are half the maximal membrane-associated value for that ORF. In the second method, the relocation was defined as the point at which the normalized membrane signal first exceeded the normalized soluble signal. The two methods generally gave similar results and corresponded well with a qualitative evaluation of the point at which the membrane signal had unambiguously increased from the background level. However, in several instances, there was considerable discrepancy, due either to noisiness in the soluble signals or to defective array elements (i.e., no signal at all) at critical positions. The method used for each ORF to obtain the data plotted in Fig. 4 is highlighted in bold in SI Appendix, Table S1.
Slot-Blot Hybridizations.
Slot-blot hybridizations were performed as described previously (21) using the PCR-generated probes described in SI Appendix, Table S2.
Supplementary Material
Acknowledgments
We thank Tiffany Kroeger, Susan Belcher, and Rosalind Williams-Carrier for excellent technical support; Kenneth Watkins for the Western blot analysis of IM35 partitioning; and Ralph Bock for his generous support while we completed a final experiment at the Max Planck Institute of Molecular Plant Physiology. We gratefully acknowledge Danny Schnell for providing the antibody to IM35; Kenneth Watkins and Kevin McNaught for helpful discussions; and Kenneth Watkins and Non Chotewutmontri for comments on the manuscript. This work was supported by a postdoctoral fellowship from the German Research Foundation (Grants Zo 302/1-1 and Zo 302/2-1; to R.Z.) and by National Science Foundation Grant IOS-1339130 (to A.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424655112/-/DCSupplemental.
References
- 1.Pribil M, Labs M, Leister D. Structure and dynamics of thylakoids in land plants. J Exp Bot. 2014;65(8):1955–1972. doi: 10.1093/jxb/eru090. [DOI] [PubMed] [Google Scholar]
- 2.Nelson N, Ben-Shem A. The complex architecture of oxygenic photosynthesis. Nat Rev Mol Cell Biol. 2004;5(12):971–982. doi: 10.1038/nrm1525. [DOI] [PubMed] [Google Scholar]
- 3.Lyska D, Meierhoff K, Westhoff P. How to build functional thylakoid membranes: From plastid transcription to protein complex assembly. Planta. 2013;237(2):413–428. doi: 10.1007/s00425-012-1752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen JF, de Paula WB, Puthiyaveetil S, Nield J. A structural phylogenetic map for chloroplast photosynthesis. Trends Plant Sci. 2011;16(12):645–655. doi: 10.1016/j.tplants.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Celedon JM, Cline K. Intra-plastid protein trafficking: How plant cells adapted prokaryotic mechanisms to the eukaryotic condition. Biochim Biophys Acta. 2013;1833(2):341–351. doi: 10.1016/j.bbamcr.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie K, Dalbey RE. Inserting proteins into the bacterial cytoplasmic membrane using the Sec and YidC translocases. Nat Rev Microbiol. 2008;6(3):234–244. doi: 10.1038/nrmicro3595. [DOI] [PubMed] [Google Scholar]
- 7.Driessen AJ, Nouwen N. Protein translocation across the bacterial cytoplasmic membrane. Annu Rev Biochem. 2008;77:643–667. doi: 10.1146/annurev.biochem.77.061606.160747. [DOI] [PubMed] [Google Scholar]
- 8.Chua NH, Blobel G, Siekevitz P, Palade GE. Attachment of chloroplast polysomes to thylakoid membranes in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1973;70(5):1554–1558. doi: 10.1073/pnas.70.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto T, Burke J, Autz G, Jagendorf AT. Bound ribosomes of pea chloroplast thylakoid membranes: Location and release in vitro by high salt, puromycin, and RNase. Plant Physiol. 1981;67(5):940–949. doi: 10.1104/pp.67.5.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrin D, Michaels A. The chloroplast 32 kDa protein is synthesized on thylakoid-bound ribosomes in Chlamydomonas reinhardtii. FEBS Lett. 1985;184(1):90–95. [Google Scholar]
- 11.Kim J, Eichacker LA, Rudiger W, Mullet JE. Chlorophyll regulates accumulation of the plastid-encoded chlorophyll proteins P700 and D1 by increasing apoprotein stability. Plant Physiol. 1994;104(3):907–916. doi: 10.1104/pp.104.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Röhl T, van Wijk KJ. In vitro reconstitution of insertion and processing of cytochrome f in a homologous chloroplast translation system. J Biol Chem. 2001;276(38):35465–35472. doi: 10.1074/jbc.M103005200. [DOI] [PubMed] [Google Scholar]
- 13.van Wijk KJ, Bingsmark S, Aro EM, Andersson B. In vitro synthesis and assembly of photosystem II core proteins. The D1 protein can be incorporated into photosystem II in isolated chloroplasts and thylakoids. J Biol Chem. 1995;270(43):25685–25695. doi: 10.1074/jbc.270.43.25685. [DOI] [PubMed] [Google Scholar]
- 14.Uniacke J, Zerges W. Chloroplast protein targeting involves localized translation in Chlamydomonas. Proc Natl Acad Sci USA. 2009;106(5):1439–1444. doi: 10.1073/pnas.0811268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voelker R, Barkan A. Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO J. 1995;14(16):3905–3914. doi: 10.1002/j.1460-2075.1995.tb00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voelker R, Mendel-Hartvig J, Barkan A. Transposon-disruption of a maize nuclear gene, tha1, encoding a chloroplast SecA homologue: In vivo role of cp-SecA in thylakoid protein targeting. Genetics. 1997;145(2):467–478. doi: 10.1093/genetics/145.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Wijk KJ, Knott TG, Robinson C. Evidence for SecA- and delta pH-independent insertion of D1 into thylakoids. FEBS Lett. 1995;368(2):263–266. doi: 10.1016/0014-5793(95)00668-y. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson R, Brunner J, Hoffman NE, van Wijk KJ. Interactions of ribosome nascent chain complexes of the chloroplast-encoded D1 thylakoid membrane protein with cpSRP54. EMBO J. 1999;18(3):733–742. doi: 10.1093/emboj/18.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Paakkarinen V, Suorsa M, Aro EM. A SecY homologue is involved in chloroplast-encoded D1 protein biogenesis. J Biol Chem. 2001;276(41):37809–37814. doi: 10.1074/jbc.M105522200. [DOI] [PubMed] [Google Scholar]
- 20.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324(5924):218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoschke R, Watkins KP, Barkan A. A rapid ribosome profiling method elucidates chloroplast ribosome behavior in vivo. Plant Cell. 2013;25(6):2265–2275. doi: 10.1105/tpc.113.111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friemann A, Hachtel W. Chloroplast messenger RNAs of free and thylakoid-bound polysomes from Vicia faba L. Planta. 1988;175(1):50–59. doi: 10.1007/BF00402881. [DOI] [PubMed] [Google Scholar]
- 23.Bibi E. Early targeting events during membrane protein biogenesis in Escherichia coli. Biochim Biophys Acta. 2011;1808(3):841–850. doi: 10.1016/j.bbamem.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Denks K, et al. The Sec translocon mediated protein transport in prokaryotes and eukaryotes. Mol Membr Biol. 2014;31(2-3):58–84. doi: 10.3109/09687688.2014.907455. [DOI] [PubMed] [Google Scholar]
- 25.Matlack KE, Walter P. The 70 carboxyl-terminal amino acids of nascent secretory proteins are protected from proteolysis by the ribosome and the protein translocation apparatus of the endoplasmic reticulum membrane. J Biol Chem. 1995;270(11):6170–6180. doi: 10.1074/jbc.270.11.6170. [DOI] [PubMed] [Google Scholar]
- 26.Jan CH, Williams CC, Weissman JS. Principles of ER cotranslational translocation revealed by proximity-specific ribosome profiling. Science. 2014;346(6210):1257521. doi: 10.1126/science.1257521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson CM, Gray J. Cleavage of the precursor of pea chloroplast cytochrome f by leader peptidase from Escherichia coli. FEBS Lett. 1991;280(2):383–386. doi: 10.1016/0014-5793(91)80337-3. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki Y, Sekiguchi K, Nagano Y, Matsuno R. Chloroplast envelope protein encoded by chloroplast genome. FEBS Lett. 1993;316(1):93–98. doi: 10.1016/0014-5793(93)81743-j. [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi S, et al. Uncovering the protein translocon at the chloroplast inner envelope membrane. Science. 2013;339(6119):571–574. doi: 10.1126/science.1229262. [DOI] [PubMed] [Google Scholar]
- 30.Barkan A. Expression of plastid genes: Organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol. 2011;155(4):1520–1532. doi: 10.1104/pp.110.171231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barkan A. Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and polycistronic mRNAs. EMBO J. 1988;7(9):2637–2644. doi: 10.1002/j.1460-2075.1988.tb03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chua NH, Blobel G, Siekevitz P, Palade GE. Periodic variations in the ratio of free to thylakoid-bound chloroplast ribosomes during the cell cycle of Chlamydomonas reinhardtii. J Cell Biol. 1976;71(2):497–514. doi: 10.1083/jcb.71.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurewitz J, Jagendorf AT. Further characterization of ribosome binding to thylakoid membranes. Plant Physiol. 1987;84(1):31–34. doi: 10.1104/pp.84.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibhaya D, Jagendorf AT. Synthesis of subunit III of CF0 by thylakoid-bound polysomes from pea chloroplasts. Plant Mol Biol. 1984;3(5):277–280. doi: 10.1007/BF00017781. [DOI] [PubMed] [Google Scholar]
- 35.Shinohara K, Minami E, Watanabe A. Synthesis and assembly of H+-ATPase complex by isolated “rough” thylakoids. Arch Biochem Biophys. 1988;260(1):452–460. doi: 10.1016/0003-9861(88)90469-9. [DOI] [PubMed] [Google Scholar]
- 36.Tzvetkova-Chevolleau T, et al. Canonical signal recognition particle components can be bypassed for posttranslational protein targeting in chloroplasts. Plant Cell. 2007;19(5):1635–1648. doi: 10.1105/tpc.106.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatzi KE, Sardis MF, Economou A, Karamanou S. SecA-mediated targeting and translocation of secretory proteins. Biochim Biophys Acta. 2014;1843(8):1466–1474. doi: 10.1016/j.bbamcr.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Huber D, et al. SecA interacts with ribosomes in order to facilitate posttranslational translocation in bacteria. Mol Cell. 2011;41(3):343–353. doi: 10.1016/j.molcel.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 39.Fluman N, Navon S, Bibi E, Pilpel Y. mRNA-programmed translation pauses in the targeting of E. coli membrane proteins. eLife. 2014;3:e03440. doi: 10.7554/eLife.03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pechmann S, Chartron JW, Frydman J. Local slowdown of translation by nonoptimal codons promotes nascent-chain recognition by SRP in vivo. Nat Struct Mol Biol. 2014;21(12):1100–1105. doi: 10.1038/nsmb.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J, Klein PG, Mullet JE. Ribosomes pause at specific sites during synthesis of membrane-bound chloroplast reaction center protein D1. J Biol Chem. 1991;266(23):14931–14938. [PubMed] [Google Scholar]
- 42.Lareau LF, Hite DH, Hogan GJ, Brown PO. Distinct stages of the translation elongation cycle revealed by sequencing ribosome-protected mRNA fragments. eLife. 2014;3:e01257. doi: 10.7554/eLife.01257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldridge C, Cain P, Robinson C. Protein transport in organelles: Protein transport into and across the thylakoid membrane. FEBS J. 2009;276(5):1177–1186. doi: 10.1111/j.1742-4658.2009.06875.x. [DOI] [PubMed] [Google Scholar]
- 44.van der Laan M, Bechtluft P, Kol S, Nouwen N, Driessen AJ. F1F0 ATP synthase subunit c is a substrate of the novel YidC pathway for membrane protein biogenesis. J Cell Biol. 2004;165(2):213–222. doi: 10.1083/jcb.200402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson PJ, Woolhead CA. Post-translational membrane insertion of an endogenous YidC substrate. Biochim Biophys Acta. 2013;1833(12):2781–2788. doi: 10.1016/j.bbamcr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Li M, Schnell DJ. Reconstitution of protein targeting to the inner envelope membrane of chloroplasts. J Cell Biol. 2006;175(2):249–259. doi: 10.1083/jcb.200605162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tripp J, Inoue K, Keegstra K, Froehlich JE. A novel serine/proline-rich domain in combination with a transmembrane domain is required for the insertion of AtTic40 into the inner envelope membrane of chloroplasts. Plant J. 2007;52(5):824–838. doi: 10.1111/j.1365-313X.2007.03279.x. [DOI] [PubMed] [Google Scholar]
- 48.Singh ND, Li M, Lee SB, Schnell D, Daniell H. Arabidopsis Tic40 expression in tobacco chloroplasts results in massive proliferation of the inner envelope membrane and upregulation of associated proteins. Plant Cell. 2008;20(12):3405–3417. doi: 10.1105/tpc.108.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skalitzky CA, et al. Plastids contain a second sec translocase system with essential functions. Plant Physiol. 2011;155(1):354–369. doi: 10.1104/pp.110.166546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schottkowski M, et al. Biogenic membranes of the chloroplast in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2012;109(47):19286–19291. doi: 10.1073/pnas.1209860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maier RM, Neckermann K, Igloi GL, Kössel H. Complete sequence of the maize chloroplast genome: Gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. J Mol Biol. 1995;251(5):614–628. doi: 10.1006/jmbi.1995.0460. [DOI] [PubMed] [Google Scholar]
- 52.Lohse M, Drechsel O, Kahlau S, Bock R. OrganellarGenomeDRAW—A suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013;41(Web Server issue):W575–W581. doi: 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berends T, Gamble PE, Mullet JE. Characterization of the barley chloroplast transcription units containing psaA-psaB and psbD-psbC. Nucleic Acids Res. 1987;15(13):5217–5240. doi: 10.1093/nar/15.13.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhelyazkova P, et al. Protein-mediated protection as the predominant mechanism for defining processed mRNA termini in land plant chloroplasts. Nucleic Acids Res. 2012;40(7):3092–3105. doi: 10.1093/nar/gkr1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lezhneva L, Meurer J. The nuclear factor HCF145 affects chloroplast psaA-psaB-rps14 transcript abundance in Arabidopsis thaliana. Plant J. 2004;38(5):740–753. doi: 10.1111/j.1365-313X.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 56.Pfalz J, Bayraktar OA, Prikryl J, Barkan A. Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J. 2009;28(14):2042–2052. doi: 10.1038/emboj.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berends Sexton T, Jones JT, Mullet JE. Sequence and transcriptional analysis of the barley ctDNA region upstream of psbD-psbC encoding trnK(UUU), rps16, trnQ(UUG), psbK, psbI, and trnS(GCU) Curr Genet. 1990;17(5):445–454. doi: 10.1007/BF00334526. [DOI] [PubMed] [Google Scholar]
- 58.Stahl DJ, Rodermel SR, Bogorad L, Subramanian AR. Co-transcription pattern of an introgressed operon in the maize chloroplast genome comprising four ATP synthase subunit genes and the ribosomal rps2. Plant Mol Biol. 1993;21(6):1069–1076. doi: 10.1007/BF00023603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.