Significance
Echolocating bats and toothed whales emit a terminal buzz right before capture. The high call rate (≥180 Hz) and short duration should preclude informed reactions, leaving the ubiquitous buzz an enigma. By removing prey from bats right before capture, we showed that the buzz is not inflexible but adaptable and that bats react on a very fast time scale to sudden changes in perceptual feedback. Acoustic and behavioral reactions differed, indicating separate central control of echolocation and capture movements, as well as importance of somatosensory feedback. These results in a naturally behaving animal relying on multimodal integration of actively controlled senses are significant for a central problem in neurobiology: fast decision making.
Keywords: echolocation, bat, buzz, decision, sensory motor
Abstract
Echolocation is an active sense enabling bats and toothed whales to orient in darkness through echo returns from their ultrasonic signals. Immediately before prey capture, both bats and whales emit a buzz with such high emission rates (≥180 Hz) and overall duration so short that its functional significance remains an enigma. To investigate sensory–motor control during the buzz of the insectivorous bat Myotis daubentonii, we removed prey, suspended in air or on water, before expected capture. The bats responded by shortening their echolocation buzz gradually; the earlier prey was removed down to approximately 100 ms (30 cm) before expected capture, after which the full buzz sequence was emitted both in air and over water. Bats trawling over water also performed the full capture behavior, but in-air capture motions were aborted, even at very late prey removals (<20 ms = 6 cm before expected contact). Thus, neither the buzz nor capture movements are stereotypical, but dynamically adapted based on sensory feedback. The results indicate that echolocation is controlled mainly by acoustic feedback, whereas capture movements are adjusted according to both acoustic and somatosensory feedback, suggesting separate (but coordinated) central motor control of the two behaviors based on multimodal input. Bat echolocation, especially the terminal buzz, provides a unique window to extremely fast decision processes in response to sensory feedback and modulation through attention in a naturally behaving animal.
Most sensory systems passively sample the environment by relying on extrinsic energy sources like light or sound to stimulate sensory receptors. Truly active senses, e.g., the electric sense of weakly electric fishes (1) and echolocation (2), where the animal itself produces the energy used to probe the surroundings, are rare (3). The advanced echolocation systems of bats and toothed whales involve dynamic adaptation of the outgoing sound and behavior based on perception of the surroundings through information processing of returning echoes.
The temporal pattern of echolocation signals during prey pursuit changes through three phases: search, approach, and terminal buzz. The buzz, immediately preceding prey capture, is characterized by a dramatic increase in signal repetition rate and is universally present in both bats and whales capturing moving prey (4–8). Repetition rates up to 640 Hz have been reported for porpoises and, contrary to bats, odontocete buzzes usually continue beyond prey contact (6). The buzz of many vespertilionid and molossid bats has two distinct subphases: buzz I with decreasing call durations and intervals, followed by buzz II, with a constant maximum call repetition rate and a characteristic frequency drop of up to an octave (4, 9–14).
The function of the terminal buzz is still not understood (15). It has been hypothesized that odontocete buzzes not only track prey before capture (7), but may also serve to follow escaping prey (6). Bat buzzes have also been hypothesized to help track evasive targets (16). Other suggestions are distance gauging by pitch perception (17), or guidance to a safe landing (18). In contrast, Melcón et al. argue that echo returns from buzz II would reach the bat too late to serve immediate adaptive reactions when buzz II starts around 50 ms before contact, corresponding to the estimated reaction time. Instead, they propose that buzz II provides post hoc information, helping bats assess the cause of unsuccessful capture attempts and eventually react adequately (19).
Here we examined the buzz by provoking very fast acoustic and flight behavior responses in the bat Myotis daubentonii (Vespertilionidae). M. daubentonii catches insects from water surfaces (trawling) or occasionally in air. Its echolocation calls are frequency modulated from 90 down to 40 kHz. In buzz II, call rates increase to 180–200 Hz (12, 20, 21). In two parallel series of experiments, in the field and in a flight room, we suddenly removed the prey in the final phase of pursuit down to a few milliseconds before expected capture. Based on our findings, we discuss the function of the final buzz and rapid dynamic adjustments of motor output and decision making in response to fast sensory feedback in general.
Results
Prey Captures in Air and over Water.
At two field sites, wild M. daubentonii learned to capture mealworms from a string approximately 40 cm above a river surface (Fig. 1A). In control trials without worm removal, bats lowered their hind legs, scooped the worm into the tail membrane, formed a ball, and retrieved the prey with the mouth (Fig. 2A). The whole capture sequence from start (lowering legs) to end (uncurling from ball) lasted 296 (median, 224–367) ms. Lowering the legs and moving the head into the tail membrane lasted only 41 (41–51) and 20 (20–31) ms, respectively, but the ball phase lasted 235 (153–306) ms. Buzz I of the echolocation behavior started approximately 200 ms before prey contact with a median of 11 (10–13) calls. Buzz II started approximately 110 ms before prey contact with 19 (18–21) calls (Figs. 2A and 3).
Fig. 1.

Experimental setups. The two setups used in the field and laboratory. Echolocation and flight behavior of bats attacking the worm were recorded with a T-shaped four-microphone (Mic) array and high-speed video cameras (Cam) illuminated with infrared lights (IR). (A) Field setup with a mealworm tethered to a nylon thread hanging from a fishing rod with the tip bent by a taut fishing line connected to a lifting solenoid. Activation of the solenoid released the tip to flick upwards, instantaneously removing the worm. The two cameras were mounted with perpendicular optical axes (dashes lines) aimed at the mealworm. (B) Lab setup for trawling trials with a mealworm on a prey remover device in a small pond. In removal trials the worm was pulled below the water surface by a small electromotor.
Fig. 2.
Aerial captures in the field. (A–D) Acoustic behavior (spectrograms) and capture behavior (video snapshots) during prey captures in the field experiment. (A) Control trial without worm removal, (B) late removal, (C) intermediate removal, and (D) early removal relative to expected prey contact. Numbers in spectrograms correspond to the numbers in each of the snapshots Below. Letters denote the different phases of the echolocation sequence. (a) Start of buzz I, (b) end of buzz I/start of buzz II, (c) end of buzz II. Black vertical lines indicate time of prey removal. The earlier the prey is removed, the shorter the capture behavior and the buzz II duration. With early removal (D) the bat does not emit a buzz II and no capture behavior is initiated.
Fig. 3.
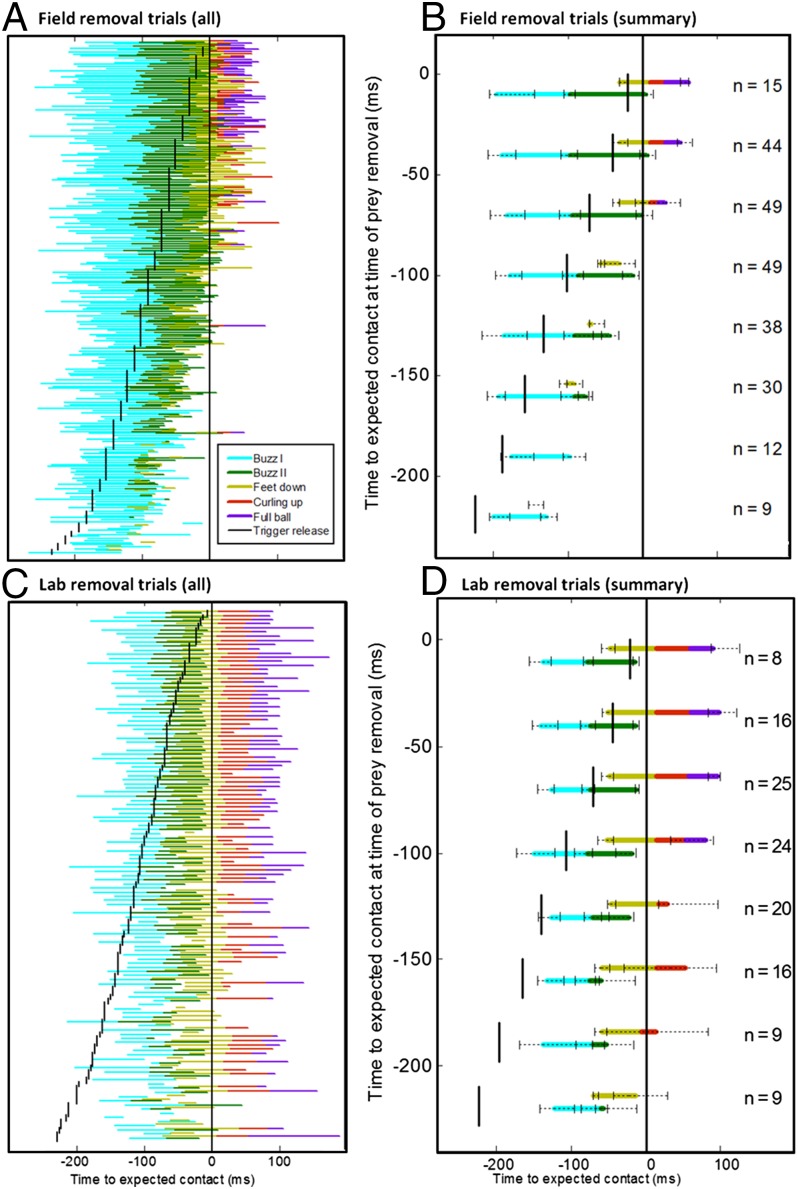
Controls trials showing duration of echolocation buzz (buzz I, cyan and buzz II, green) and of the three behavioral capture phases (feet down, yellow-green; curling up, red-brown; and full ball, violet) relative to bat–prey contact (time = 0 ms). (A) Stacked presentation of all control trials for aerial captures in the field. (B) Stacked presentation of all control trials for trawling captures in the laboratory with the worm attached to the prey remover. (C) Summary figure showing median, first, and third quartiles of durations in control trials in the field (top half) and laboratory (bottom half) (n = number of control trials).
In the flight room, three M. daubentonii were trained to capture mealworms from a water surface, floating freely or pinned to a prey remover device (Fig. 1B). The bats lowered the hind legs, grabbed the prey with the feet, instead of the tail membrane as in aerial captures, and moved the head and feet together to bite into the worm. The capture behavior was shorter than in air: 157 (median, 145–185) ms with the worm floating, and slightly longer, 177 (150–212) ms, with the worm fixed to the removal device. Hence, the latter were used as controls for statistical comparisons. The first two capture phases were longer when trawling than in air: 67 (57–77) ms for lowering the legs and 50 (43–60) ms to move head to feet. The ball phase was much shorter than in air: 52 (37–93) ms. The echolocation behavior was qualitatively similar to aerial field captures, but with shorter buzz I, starting approximately 130 ms before prey contact with 6 (5–6) calls and buzz II, starting approximately 80 ms before prey contact with 11 (10–14) calls (Figs. 2 and 3).
Prey Removal in Aerial Field Captures.
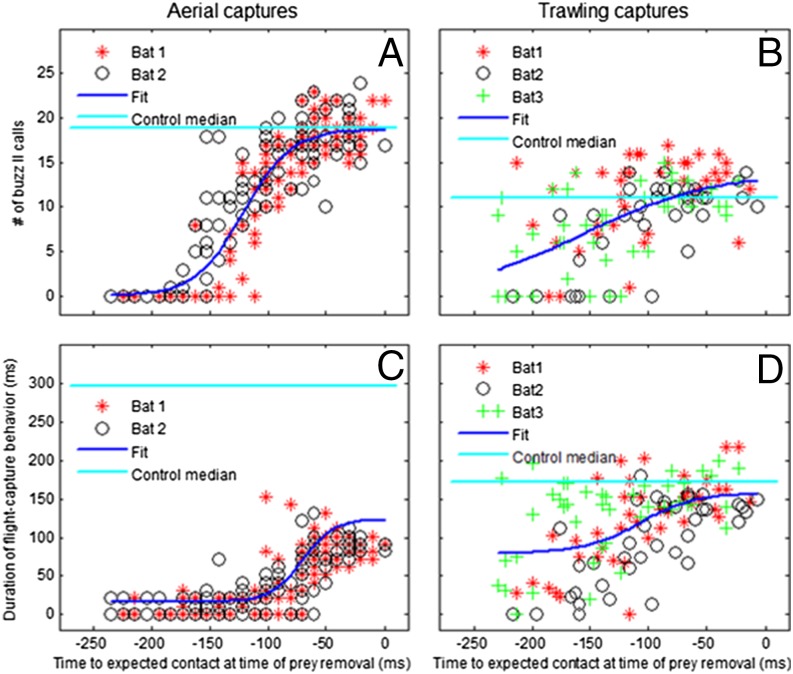
In the field, we reconstructed flight paths in real time from high-speed stereo video recordings. The mealworm was removed instantaneously (Fig. 1A) at bat–prey distances of 2–80 cm, i.e., 6–240 ms before expected contact (Fig. 4). Echolocation behavior was identical to controls without prey removal at late removals <90 ms before expected contact. Earlier removals caused shortened echolocation sequences with significantly fewer buzz II calls (P < 0.01). Notably, the number of buzz II calls decreased gradually depending on how early prey was removed. The latest removal with no buzz II calls was 112 ms before expected contact. Buzz II calls were absent in most trials (71%) with removals earlier than 150 ms and in all trials with removals earlier than 190 ms before expected contact (Figs. 4B and 5A).
Fig. 4.
Prey removal. Effect of removing the prey on echolocation and capture behavior in air in the field (A and B) and over water in the laboratory (C and D). Short black vertical lines indicate the time of removal. (A and C) Timing of buzz I (cyan), buzz II (green), and the three capture behavior phases (feet down, yellow-green; curling up, red-brown; and full ball, violet) relative to expected bat–prey contact for all field removal trials. (B and D) Summary figures showing median, first, and third quartiles of acoustic and capture behavior durations in aerial field removals with trials pooled into 30-ms bins (n = number of trials per bin).
Fig. 5.
Number of buzz II calls and duration of capture behavior. Comparison of the number of buzz II calls (A and B) and the duration of capture behavior (C and D) in removal trials relative to the time of prey removal. Aerial field captures are shown on the Left (A and C) and trawling laboratory captures on the Right (B and D). Data are shown for two bats at two different field sites and three bats in the laboratory as indicated by symbols and colors. A sigmoid function was fitted to each dataset (all bats) for the two parameters: number of buzz II calls and capture behavior duration (blue line). For comparison, the horizontal cyan lines give control values: median number of buzz II calls (Top) and median duration of capture behavior (Bottom) in control trials in air in the field (A and C) and trawling in the laboratory (B and D).
In contrast to vocal response (echolocation), capture behavior in air—prey seizing and handling—was always significantly shorter in removal trials compared with controls (P < 0.01, Fig. 4). At early removals, bats only lowered the hind legs or did not initiate capture behavior (Figs. 2 B–D, 4, and 5C). At removals earlier than 150 ms, 53% of trials showed no capture motions, and capture behavior was absent in some trials even with removal as late as 60 ms before expected contact (Figs. 4A and 5C). The ball phase was shortened or absent even at removals immediately before expected contact.
Prey Removal When Trawling for Prey in the Laboratory.
In the laboratory, the mealworm was removed by pulling it under the water surface (Fig. 1B). As in air, the echolocation sequences were similar to controls at late prey removals (<120 ms before expected capture). Earlier removals (>120 ms) resulted in a significant gradual decrease in buzz II calls correlated to how early prey was removed (P < 0.01, Figs. 4 and 5). Buzz II was completely omitted in 38% of removals >150 ms and absent at some removals down to 97 ms before expected contact.
In open air, capture behavior was always aborted, even at very late prey removals, but over water, late removals (<60 ms before expected contact) resulted in capture behavior identical to controls (Figs. 3–5). At earlier removals (>60 ms), capture behavior was shortened (P < 0.01). Even in removals >150 ms before expected contact, capture behavior was initiated in 88% of all trials. The “feet down” phase was the most persistent behavioral element present at removals >200 ms before expected contact (Fig. 4). When trawling, the reactions were more variable than in air, e.g., complete behaviors were sometimes executed even at early removals (Fig. 5 A and C vs. B and D and Movie S1).
Reaction Times.
We estimated reaction times from mealworm removal to the first deviation from controls in acoustic and capture behavior. The median acoustic reaction time (delay from removal to buzz interruption) was 87 (76–101) ms (n = 138) in aerial captures and 123 (100–152) ms (n = 54) in trawling captures. The median behavioral reaction time (removal to abortion of capture behavior) was 82 (71–102) ms (n = 183) in aerial captures and 178 (150–217) ms (n = 98) when trawling. Thus, acoustic and behavioral reaction times were short and nearly identical in aerial captures. Note that even when the prey was removed shortly (20 ms) before capture in air, it took the bats more than 80 ms to react. When trawling, both acoustic and behavioral reaction times were more variable than in aerial captures and behavioral reaction took much longer than acoustic reaction.
We also determined minimum reaction times of 20 (median, 17–27) ms (n = 3, n = 59) for a startle response to a loud click (delay from click onset to beginning of ear movements).
Discussion
Here we present the reactions of sonar-guided bats to sudden prey removals during the terminal phase of prey pursuit. We show how perceptual sensory inputs elicit fast and flexible adaptations of vocal and capture behavior. Vocal behavior was similar in air and over water in controls and late prey removals, but capture behavior differed distinctly between air and water, with respect both to duration of individual phases and reaction time, revealing the importance of multimodal (acoustic and somatosensory) feedback. The results indicate separate control of echolocation behavior and capture maneuvers with flexible synchronization of the two motor programs.
Echolocation.
In the search phase, both bats and odontocetes process each echo before broadcasting the next signal, but the extremely short call intervals (22) in the buzz phase leave no time for that, rendering the function of the buzz enigmatic. Indeed, the buzz has been suggested to function mainly after prey capture, i.e., for porpoises to track escaping prey (6) or for bats to improve future capture success by evaluating failures (19). However, our data show that even in the terminal buzz, echolocation is dynamically adjusted to echo feedback. Specifically, buzz II duration gradually decreased according to prey removal times. The median acoustic reaction time was around 100 ms (air, 87; trawling, 123 ms) corresponding to an approximately 30-cm bat–prey distance (flight speed of 3 m/s) (12) and only a 2-ms echo delay (two-way transit time at 30 cm). Thus, the reaction time is comparable to control duration of buzz II, but shorter than the total buzz duration, particularly in air, where buzz I started 200 ms before expected capture. Over water, buzz I started 130 ms before expected capture in controls. The gradual decrease in buzz II duration from 80 to 110 ms down to 0 ms demonstrates the fast, flexible nature of buzz II and speaks against a role only in postaction sensing. Field data confirm the adaptability: both aerial and trawling captures are part of M. daubentonii’s natural repertoire, with highly variable buzz durations up to 262 ms (buzz II durations of 38–69 ms) (12). Buzz durations of 100–150 ms have been reported for aerial captures in the laboratory (14, 21, 23) and around 140 ms in the field (24). We found longer buzz durations in aerial captures (Figs. 3–5), which may relate to the need for 3D (air) versus 2D (water surface) localization and/or the difference in background clutter (25). The wide range of buzz II durations (40–200 ms) in Pipistrellus kuhlii (26) corroborate the flexibility of buzz II.
Our data reveal fast adaptation of the buzz in both aerial and trawling M. daubentonii. However, the case is not closed. If the prey is removed very late, the bats reach a “point of no return” and emit the full echolocation sequence, including buzz II. Also, in the wild, M. daubentonii occasionally emit full buzz sequences even where prey is missed (12). This is puzzling, because the final part of buzz II leaves no reaction time for acoustic adjustments based on returning information. Our results show that bats adjust buzz duration to the situation and that even buzz II, once initiated, does not have to run its full course. So why do bats continue to emit signals at this late stage? Sound production mechanisms have been suggested to explain postcapture buzzing in odontocetes (6, 27). For bats, biomechanics may also be the key: Continuation of buzz II may allow flexibility to prolong this phase if need be, for example, in case of sudden prey escape maneuvers within the bat’s acoustic field of view (14, 28). Reports of long buzzes are rare, but always related to evasive prey reactions (29). It may be easier to continue an ongoing buzz than to stop and restart.
Capture Behavior.
Interestingly, whereas echolocation was similar, capture behavior differed considerably between air and water: Aerial captures lasted nearly twice as long (296 ms, Fig. 3C) as trawling captures. Kalko and Schnitzler found capture behaviors to last 150–200 ms and noted an occasional prolonged head-down stage, probably due to prey retrieval difficulties (12). Differences in capture duration (and in individual phases) may stem from the situation, i.e., air and field vs. water and laboratory, or from the difference in prey presentation (floating or attached to a device or string that may produce sound when released). Regardless, the differences demonstrate the dynamic adaptability of capture behavior.
The median aerial behavioral reaction time was only 82 ms (close to vocal reaction time) and capture behavior was always shortened, even in late removals, where vocal behavior was similar to controls; so buzz II could occur without simultaneous late phase capture maneuvers (Fig. 4). When trawling, the reaction time was longer, 178 ms, and at late removals, full capture behavior was shown. Sometimes bats trawled with the feet in the water even beyond the position of the removed prey, which may explain the large variation in trawling captures (Figs. 3–5). This strategy resembles raking in Noctilio albiventris (30), and probably reflects that prey may sometimes be available just below the surface although no longer detectable by echolocation, indicating that prolonged trawling (feet down) is part of M. daubentonii’s natural repertoire. The movie showed water surface ripples after removal, changing from trial to trial probably due to variations in worm placement. This might also sometimes deceive the bats (Movie S1). Thus, our results contradict phase locking between echolocation and capture behavior (12), instead implying independent control of vocalization and capture maneuvers. Accordingly, adaptive coordination serves to synchronize echolocation and capture motions.
The results point to the importance of multimodal sensing through echolocation and somatosensory feedback: Capture behavior was always aborted in aerial prey removal trials where the bats got no feedback from tail membrane mechanoreceptors, but over water, full capture was executed at late removals, probably because in these trials trawling bats lower their feet into the water and get mechanoreceptor input even without prey. We repeatedly observed trawling bats partly uncurling, to curl up again as if rechecking for the prey they thought they had grasped (Movie S1). Somatosensory feedback has been little studied in bats, but is required along with echo feedback for flight control (31). The full vocal repertoire after late removals both in air and over water indicates that vocal behavior is adjusted only according to echo feedback. However, the aborted capture in air even at late removals indicates that capture behavior requires both acoustic (to initiate capture before prey contact) and somatosensory feedback, to be completed after prey contact. Undoubtedly, bats’ natural behavior also relies on other senses (vision, olfaction, etc.) as well as memory and experience.
Fast Decision Making Based on Perceptual Feedback.
Bat echolocation is a superior model for studying active perception, because ongoing dynamic adaptation of echolocation signals provides a direct window to scene analysis in a naturally behaving animal (32–34), allowing general inferences for active motor response to perceptual sensory feedback. The acoustic and behavioral median reaction times of around 80–90 ms (Figs. 4 and 5) were much longer than startle responses (approximately 20 ms), but startle response may only be relevant for reflex-like behaviors, whereas reacting to prey removal involves attention, central processing, decision, and complex multistage behaviors. Drosophila use 200–300 ms to escape from a looming stimulus (35), and humans need around 400 ms to reach an arm toward one of two buttons in response to visual sensory input (36). Human visual reaction time determined by oculomotor latency is faster, around 200 ms, when adapting secondary saccades to correct for movement of a visual target during the first saccade (37–39), but this only involves moving the eye. The bats’ reactions require complex processing and motor control, but only took 80–90 ms, i.e., much faster than other nonreflex systems requiring higher order processing of sensory input (35, 36). However, the reaction times here are within the range suggested by other echolocation experiments (19, 40–42). Conceivably, echolocation allows for very short reaction times because the temporal precision of the auditory system far exceeds that of other sensory modalities (43). However, not only vocal, but also behavioral reaction times were very fast suggesting a strong evolutionary constraint for fast reaction. The relatively short detection ranges of echolocation in air (44–46) probably created a need for speed as bats must perform difficult sensory evaluations under strong time pressure, and perceptual urgency to react on a very fast time scale drove evolution of extremely fast capture behaviors.
The bat provides an attractive animal model for studying decision making, allowing not only for recording the response but, by observing adjustments of the echolocation output, also the processing involved. The acoustic gaze—the distance and direction where the bat focuses its sonar beam—directly measures attention (47). Recording echolocation sounds does not disturb bats performing natural behavior, whereas e.g., quantifying eye movements involves wearing eye-tracking devices. Our data show that the motor programs underlying the vocalization rates and body movements of bats in the last phase of prey capture are not fixed but dynamically adapted on an exceptionally fast time scale in response to sensory feedback. Thus, echolocation and especially the terminal buzz provides a unique window to observe and understand very fast decision processes at the neural and behavioral level and their modulation through attention.
Materials and Methods
Aerial Field Setup.
M. daubentonii were recorded catching mealworms (Tenebrio molitor larvae) tethered to a nylon thread 40 cm above the river Würm, Germany. To ensure including more than one bat, we recorded at two field sites several kilometers apart, Pasing (48°8′1.57′′ N/11°26′53.94′′ E) and Planegg (48°6′18.16′′ N/11°25′23.35′′ E), April–October 2010 and 2011. The thread hung from the tip of a fishing rod pulled down by a taut, thin (0.25-mm diameter) fishing line and was released by a lifting solenoid (Fig. 1A), which instantaneously flung the worm upwards and away.
The bats’ vocal behavior was recorded in control and prey removal trials in random order with an array of four ultrasonic microphones (Sanken CO100K), three spaced horizontally 1.5 m apart and one 1.5 m above the center. Two high-speed video cameras (Basler A600f, with infrared light: IR-Strahler MEGA-LED XL; ABUS) were mounted 2.9 m apart on the microphone array, with optical axes perpendicular to enable reconstruction of 3D flight paths. The worm hung about 1.5 m from the array close to the interception point of the cameras' optical axes (Fig. 1A).
A laptop running MATLAB 7.5 (Mathworks) with the audio tool SoundMexPro (HörTech) controlled data acquisition. Synchronized video acquisition (frame rate 98 Hz) was ensured with a trigger signal sent to the cameras every 10.2 ms via an audio interface (Motu Traveler). A second laptop running a customized version of EyeSeeCam (37) with the eye-tracking module replaced by a bat-tracking module used camera information to position the bat and calculate the distance from bat to worm for each frame (every 10.2 ms). At a predefined distance (2–80 cm from the worm), the program activated the solenoid, instantaneously (before next video frame) removing the worm. When the experimenter stopped the trial, the last 5 s (490 frames) from each camera and acoustic recording (sampling rate 192 kHz per channel) were stored along with camera trigger signals and the electrical signal activating the solenoid. Twenty-eight controls and 126 removal trials were recorded at Pasing and 75 controls and 124 removal trials at Planegg.
Trawling Laboratory Setup.
Three M. daubentonii (two males, one female, caught at Odense River, Denmark (55°22′17.5′′ N/10°22′54.9′′ E) were trained to catch mealworms from the surface of an indoor pond (2.5 × 1 m, water level 15 cm) in a flight room (7 × 4.8 × 2.5 m) at University of Southern Denmark. Bats were only fed during experiments, but had unlimited access to water.
We recorded audio data with a four-microphone array (G.R.A.S. 1/4 inch, 40 BF; G.R.A.S. Sound and Vibration), three spaced 40 cm horizontally and one 35 cm above the center. Signals were preamplified (G.R.A.S. 26AC), amplified (G.R.A.S. 12AA, +40 dB, 15 kHz high-pass filters), sampled at 300 kHz per channel (Avisoft UltraSoundGate A/D converter 1216H; Avisoft Bioacoustics) and stored on a laptop. High-speed video data (Mikrotron EoSens MC1362 camera and Inspecta-5 PCI-X frame grabber card, three infrared lights, λ 850 nm, model 995JH; Kinapriser) were digitized at 300 frames per second and stored as 2.5-s files with 1.5-s (450 frames) pre- and 1-s (300 frames) posttrigger time. The custom-built trigger unit removed the prey and gave a 5-V synchronization signal that was recorded on the audio and turned on a green diode within view of the high-speed video camera. The delay from triggering until the worm disappeared was measured for each trial from the high-speed video.
The prey remover was anchored at the bottom of the pond and had a metal tip to which the mealworm was attached. It was manually triggered to pull the worm below the water surface via a small electromotor (controlled by an Arduino board; Dangi Internet Electronics) connected by a carbon fishing line to a spring at the bottom of the device (Fig. 1B and Movie S1). We carried out three types of trials in randomized order. In removal trials, the mealworm was removed between 3 and 240 ms before expected time of contact. In surface control trials, the tip of the prey remover was anchored below the water surface and the mealworm was floating freely on the water above the remover device. In worm-on-device controls, the mealworm was attached to the metal tip of the remover as in removal trials, but the electromotor was unplugged, so the worm did not move when the setup was triggered.
Reaction Times.
We estimated minimum reaction times by recording startle responses to intense clicker sounds for the three M. daubentonii from the laboratory experiments. Each bat was wrapped gently in cloth and kept immobilized with the head free. We used the high-speed video camera and two channels on the audio A/D converter (one channel recording the clicker sound, another the trigger pulse, synchronized to illumination of the green diode within the video frame) to time the delay from onset of the clicker sound until the bats’ pinnae flicked and cross checked the synchronization by counting the number of video frames from clicker activation to flicking of the pinnae.
Data Analysis.
From video and audio recordings we measured temporal features of the echolocation (call timing, number of calls in buzz I and buzz II, respectively) and behavioral capture phases (lowering of the hind legs, moving the head toward the tail membrane, and ball stage until uncurling). As controls, we analyzed the behavior in aerial hawking and trawling captures without removals. In all analyses, the reference time, measured from the video, was “time of contact” or, in prey removals, expected time of contact, when the bat reached the position of the worm before removal.
Sound recordings were analyzed in MATLAB v. 7.7 and BatSound v. 4.0 (Pettersson Elektronik). Call rates were extracted manually from spectrograms of field recordings [Hamming window, FFT (Fast Fourier Transform) size: 256, overlap: 98%] and by a custom call detection MATLAB script for flight room recordings (cross-checked manually in Batsound). The first buzz I call was defined as the call following the last pulse interval (i.e., time between start of two consecutive calls) >15 ms. Buzz II was characterized by intervals of <6.5 ms.
Video sequences from the field were converted into multiimage TIFF files for analyses, whereas flight room sequences were analyzed using MotionBlitz software (MotionBlitz Director v. 3.04.0003 and MotionBlitz Cube v. 1.11.28; Mikrotron). We determined the timing and duration of each behavioral stage by counting the number of frames where the bat performed the corresponding behavior.
We determined medians (and interquartile range) in MATLAB v. 7.5 (Statistics Toolbox). The bats’ echolocation and capture reactions at prey removals were tested (Kruskal–Wallis for multiple comparison followed by Tukey's honestly significant difference criterion post hoc tests; significance level of P < 0.05) against controls by subdividing trials with prey removal times between 0 and 240 ms before contact into eight bins of 30 ms.
All experiments were according to European law. Laboratory experiments in Denmark complied with the Danish law on animal experimentation (lov om dyreforsøg, LBK nr 474 af 15/05/2014) and the European directive (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes), and permit to A.S. to catch and keep bats, J.nr. NST-3446-00001 from the Danish Ministery of Environment and University of Southern Denmark.
Supplementary Material
Acknowledgments
We thank Thomas Dera (Faculty of Medicine, Ludwig Maximilians University) for developing the 3D bat tracking system used in the field and John Hallam (Maersk-McKinney Moeller Institute, University of Southern Denmark) for discussions and for designing the prey remover device used in the lab. This study was funded by the Danish Council for Natural Sciences, European Union 7th Framework Program: ChiRoPing project (ICT-2007-1 STREP Project 215370), the Deutsche Forschungsgemeinschaft (DFG Wi 1518/11), and Human Frontiers (RGHP0062/2009).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424457112/-/DCSupplemental.
References
- 1.Lissmann HW, Machin KE. The mechanism of object location in Gymnarchus niloticus and similar fish. J Exp Biol. 1958;35(2):451–486. [Google Scholar]
- 2.Griffin DR. Echolocation in blind men, bats, and radar. Science. 1944;100(2609):589–590. doi: 10.1126/science.100.2609.589. [DOI] [PubMed] [Google Scholar]
- 3.Nelson ME, MacIver MA. Sensory acquisition in active sensing systems. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192(6):573–586. doi: 10.1007/s00359-006-0099-4. [DOI] [PubMed] [Google Scholar]
- 4.Simmons JA, Fenton MB, O’Farrell MJ. Echolocation and pursuit of prey by bats. Science. 1979;203(4375):16–21. doi: 10.1126/science.758674. [DOI] [PubMed] [Google Scholar]
- 5.Griffin DR. 1958. Listening in the Dark (Yale Univ Press, New Haven, CT)
- 6.Deruiter SL, et al. Acoustic behaviour of echolocating porpoises during prey capture. J Exp Biol. 2009;212(19):3100–3107. doi: 10.1242/jeb.030825. [DOI] [PubMed] [Google Scholar]
- 7.Johnson M, Madsen PT, Zimmer WMX, de Soto NA, Tyack PL. Foraging Blainville’s beaked whales (Mesoplodon densirostris) produce distinct click types matched to different phases of echolocation. J Exp Biol. 2006;209(Pt 24):5038–5050. doi: 10.1242/jeb.02596. [DOI] [PubMed] [Google Scholar]
- 8.Miller PJO, Johnson MP, Tyack PL. Sperm whale behaviour indicates the use of echolocation click buzzes “creaks” in prey capture. Proc Biol Sci. 2004;271(1554):2239–2247. doi: 10.1098/rspb.2004.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora EC, Macías S, Vater M, Coro F, Kössl M. Specializations for aerial hawking in the echolocation system of Molossus molossus (Molossidae, Chiroptera) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190(7):561–574. doi: 10.1007/s00359-004-0519-2. [DOI] [PubMed] [Google Scholar]
- 10.Guillén-Servent A, Ibáñez C. Unusual echolocation behavior in a small molossid bat, Molossops temminckii, that forages near background clutter. Behav Ecol Sociobiol. 2007;61:1599–1613. [Google Scholar]
- 11.Griffin DR, Webster FA, Michael CR. The echolocation of flying insects by bats. Anim Behav. 1960;8:141–154. [Google Scholar]
- 12.Kalko EKV, Schnitzler HU. The echolocation and hunting behavior of Daubenton's bat, Myotis daubentoni. Behav Ecol Sociobiol. 1989;24:225–238. [Google Scholar]
- 13.Neuweiler G. Foraging ecology and audition in echolocating bats. Trends Ecol Evol. 1989;4(6):160–166. doi: 10.1016/0169-5347(89)90120-1. [DOI] [PubMed] [Google Scholar]
- 14.Jakobsen L, Surlykke A. Vespertilionid bats control the width of their biosonar sound beam dynamically during prey pursuit. Proc Natl Acad Sci USA. 2010;107(31):13930–13935. doi: 10.1073/pnas.1006630107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratcliffe JM, Elemans CPH, Jakobsen L, Surlykke A. How the bat got its buzz. Biol Lett. 2013;9(2):20121031. doi: 10.1098/rsbl.2012.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnitzler HU, Kalko EKV. Echolocation by insect-eating bats. Bioscience. 2001;51(7):557–569. [Google Scholar]
- 17.Nordmark J. Perception of distance in animal echo-location. Nature. 1960;188(4755):1009–1010. doi: 10.1038/1881009a0. [DOI] [PubMed] [Google Scholar]
- 18.Russo D, Jones G, Arlettaz R. Echolocation and passive listening by foraging mouse-eared bats Myotis myotis and M. blythii. J Exp Biol. 2007;210(Pt 1):166–176. doi: 10.1242/jeb.02644. [DOI] [PubMed] [Google Scholar]
- 19.Melcón ML, Denzinger A, Schnitzler HU. Aerial hawking and landing: Approach behaviour in Natterer’s bats, Myotis nattereri (Kuhl 1818) J Exp Biol. 2007;210(Pt 24):4457–4464. doi: 10.1242/jeb.007435. [DOI] [PubMed] [Google Scholar]
- 20.Zsebok S, et al. Trawling bats exploit an echo-acoustic ground effect. Front Physiol. 2013;4:65. doi: 10.3389/fphys.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elemans CPH, Mead AF, Jakobsen L, Ratcliffe JM. Superfast muscles set maximum call rate in echolocating bats. Science. 2011;333(6051):1885–1888. doi: 10.1126/science.1207309. [DOI] [PubMed] [Google Scholar]
- 22.Madsen PT, Surlykke A. Functional convergence in bat and toothed whale biosonars. Physiology (Bethesda) 2013;28(5):276–283. doi: 10.1152/physiol.00008.2013. [DOI] [PubMed] [Google Scholar]
- 23.Boonman A, Jones G. Intensity control during target approach in echolocating bats; stereotypical sensori-motor behaviour in Daubenton’s bats, Myotis daubentonii. J Exp Biol. 2002;205(Pt 18):2865–2874. doi: 10.1242/jeb.205.18.2865. [DOI] [PubMed] [Google Scholar]
- 24.Britton ARC, Jones G. Echolocation behaviour and prey-capture success in foraging bats: Laboratory and field experiments on Myotis daubentonii. J Exp Biol. 1999;202(Pt 13):1793–1801. doi: 10.1242/jeb.202.13.1793. [DOI] [PubMed] [Google Scholar]
- 25.Moss CF, Bohn K, Gilkenson H, Surlykke A. Active listening for spatial orientation in a complex auditory scene. PLoS Biol. 2006;4(4):e79. doi: 10.1371/journal.pbio.0040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnitzler HU, Kalko E, Miller L, Surlykke A. The echolocation and hunting behavior of the bat, Pipistrellus kuhli. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1987;161(2):267–274. doi: 10.1007/BF00615246. [DOI] [PubMed] [Google Scholar]
- 27.Madsen PT, Wisniewska D, Beedholm K. Single source sound production and dynamic beam formation in echolocating harbour porpoises (Phocoena phocoena) J Exp Biol. 2010;213(Pt 18):3105–3110. doi: 10.1242/jeb.044420. [DOI] [PubMed] [Google Scholar]
- 28.Matsuta N, et al. Adaptive beam-width control of echolocation sounds by CF-FM bats, Rhinolophus ferrumequinum nippon, during prey-capture flight. J Exp Biol. 2013;216(Pt 7):1210–1218. doi: 10.1242/jeb.081398. [DOI] [PubMed] [Google Scholar]
- 29.Acharya L, Fenton MB. Echolocation behaviour of vespertilionid bats (Lasiurus cinereus and Lasiurus borealis) attacking airborne targets including arctiid moths. Can J Zool. 1992;70:1292–1298. [Google Scholar]
- 30.Kalko EKV, Schnitzler HU, Kaipf I, Grinnell AD. Echolocation and foraging behavior of the lesser bulldog bat, Notilio albiventris: Preadaptation for piscivory? Behav Ecol Sociobiol. 1998;42:305–319. [Google Scholar]
- 31.Sterbing-D’Angelo S, et al. Bat wing sensors support flight control. Proc Natl Acad Sci USA. 2011;108(27):11291–11296. doi: 10.1073/pnas.1018740108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genzel D, Geberl C, Dera T, Wiegrebe L. Coordination of bat sonar activity and flight for the exploration of three-dimensional objects. J Exp Biol. 2012;215(Pt 13):2226–2235. doi: 10.1242/jeb.064535. [DOI] [PubMed] [Google Scholar]
- 33.Moss CF, Chiu C, Surlykke A. Adaptive vocal behavior drives perception by echolocation in bats. Curr Opin Neurobiol. 2011;21(4):645–652. doi: 10.1016/j.conb.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewicki MS, Olshausen BA, Surlykke A, Moss CF. Scene analysis in the natural environment. Front Psychol. 2014;5:199. doi: 10.3389/fpsyg.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vries SEJ, Clandinin TR. Loom-sensitive neurons link computation to action in the Drosophila visual system. Curr Biol. 2012;22(5):353–362. doi: 10.1016/j.cub.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman J, Brown S, Finkbeiner M. Linking cognitive and reaching trajectories via intermittent movement control. J Math Psychol. 2013;57(3–4):140–151. [Google Scholar]
- 37.Schneider E, et al. EyeSeeCam: An eye movement-driven head camera for the examination of natural visual exploration. Ann N Y Acad Sci. 2009;1164(1):461–467. doi: 10.1111/j.1749-6632.2009.03858.x. [DOI] [PubMed] [Google Scholar]
- 38.Panouillères M, Urquizar C, Salemme R, Pélisson D. Sensory processing of motor inaccuracy depends on previously performed movement and on subsequent motor corrections: A study of the saccadic system. PLoS ONE. 2011;6(2):e17329. doi: 10.1371/journal.pone.0017329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madelain L, Champrenaut L, Chauvin A. Control of sensorimotor variability by consequences. J Neurophysiol. 2007;98(4):2255–2265. doi: 10.1152/jn.01286.2006. [DOI] [PubMed] [Google Scholar]
- 40.Ghose K, Horiuchi TK, Krishnaprasad PS, Moss CF. Echolocating bats use a nearly time-optimal strategy to intercept prey. PLoS Biol. 2006;4(5):e108. doi: 10.1371/journal.pbio.0040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corcoran AJ, Conner WE. Sonar jamming in the field: Effectiveness and behavior of a unique prey defense. J Exp Biol. 2012;215(Pt 24):4278–4287. doi: 10.1242/jeb.076943. [DOI] [PubMed] [Google Scholar]
- 42.Wensveen PJ, Huijser LAE, Hoek L, Kastelein RA. Equal latency contours and auditory weighting functions for the harbour porpoise (Phocoena phocoena) J Exp Biol. 2014;217(Pt 3):359–369. doi: 10.1242/jeb.091983. [DOI] [PubMed] [Google Scholar]
- 43.Rabinowitz NC, King AJ. Auditory perception: Hearing the texture of sounds. Curr Biol. 2011;21(23):R967–R968. doi: 10.1016/j.cub.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brinkløv S, Kalko EKV, Surlykke A. Dynamic adjustment of biosonar intensity to habitat clutter in the bat Macrophyllum macrophyllum (Phyllostomidae) Behav Ecol Sociobiol. 2010;64:1867–1874. [Google Scholar]
- 45.Surlykke A, Kalko EKV. Echolocating bats cry out loud to detect their prey. PLoS ONE. 2008;3(4):e2036. doi: 10.1371/journal.pone.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung K, Kalko EKV, von Helversen O. Echolocation calls in Central American emballonurid bats: Signal design and call frequency alternation. J Zool (Lond) 2007;272:125–137. [Google Scholar]
- 47.Ulanovsky N, Moss CF. What the bat’s voice tells the bat’s brain. Proc Natl Acad Sci USA. 2008;105(25):8491–8498. doi: 10.1073/pnas.0703550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.