Significance
Adult hippocampal neurogenesis is a lifelong process by which new neurons are derived from a pool of resident stem and progenitor cells. Research on the functional role of this process has, to date, focused on the contributions of new, highly plastic neurons to memory function. We show here a previously unidentified functional role of undifferentiated neural stem and progenitor cells in the adult hippocampus as secretory cells that help maintain their own neurogenic niche by secreting large, biologically relevant quantities of the essential growth factor, VEGF. These findings suggest that the function of adult neurogenesis may include the secretome of undifferentiated stem and progenitor cells.
Keywords: adult neurogenesis, vascular endothelial growth factor, stem cell, hippocampus, neural precursor
Abstract
The adult hippocampus hosts a population of neural stem and progenitor cells (NSPCs) that proliferates throughout the mammalian life span. To date, the new neurons derived from NSPCs have been the primary measure of their functional relevance. However, recent studies show that undifferentiated cells may shape their environment through secreted growth factors. Whether endogenous adult NSPCs secrete functionally relevant growth factors remains unclear. We show that adult hippocampal NSPCs secrete surprisingly large quantities of the essential growth factor VEGF in vitro and in vivo. This self-derived VEGF is functionally relevant for maintaining the neurogenic niche as inducible, NSPC-specific loss of VEGF results in impaired stem cell maintenance despite the presence of VEGF produced from other niche cell types. These findings reveal adult hippocampal NSPCs as an unanticipated source of an essential growth factor and imply an exciting functional role for adult brain NSPCs as secretory cells.
In the adult brain, two major neurogenic niches persist throughout the mammalian life span: the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampus. Resident neural stem and progenitor cells (NSPCs) in each of these areas proliferate and give rise to new neurons that migrate and integrate into existing circuitry in the olfactory bulb or dentate gyrus (DG), respectively. Particularly in the DG, where neurogenesis is found in both rodents and humans, newly born neurons play critical roles in facilitating memory function (1, 2). This role of new neurons in memory is currently considered the dominant functional output of adult neurogenesis. However, recent research has revealed that transplanted embryonic stem cells can aid in injury recovery by secreting growth factors while undifferentiated (3, 4). The secretion of functionally relevant growth factors from endogenous adult hippocampal NSPCs has yet to be reported.
We recently showed that cultured neonatal hippocampal progenitors secrete surprisingly large quantities of VEGF compared with astrocytes, microglia, and neurons (5), raising the possibility that NSPCs could be an unexpected source of this essential growth factor in the brain. Within the adult brain, VEGF (also known as VEGF-A) is a potent angiogenic and neurogenic growth factor (6–13). Although several studies have previously noted VEGF expression in cultured adult NSPCs (14, 15), the relative quantity and function of this VEGF are not clear, particularly in vivo, where other cellular sources of VEGF abound. We therefore investigated the contribution of NSPCs to hippocampal VEGF production and the functional role of NSPC-derived VEGF in maintaining the neurogenic niche.
Results
Adult Hippocampal NSPCs Express VEGF in Vivo.
Although several studies have noted detectible levels of VEGF in cultured NSPCs, the in vivo cellular sources of VEGF remain unclear. Using in situ hybridization expression from the Allen Brain Atlas, we found a notable presence of high VEGF-expressing cells in the adult mouse SGZ (Fig. S1A). In contrast to the SGZ, but consistent with previous studies (16), the adult mouse SVZ showed a relative dearth of VEGF expression (Fig. S1A). The choroid plexus (CP), a VEGF-rich tissue adjacent to the SVZ, was heavily populated with high-VEGF–expressing cells.
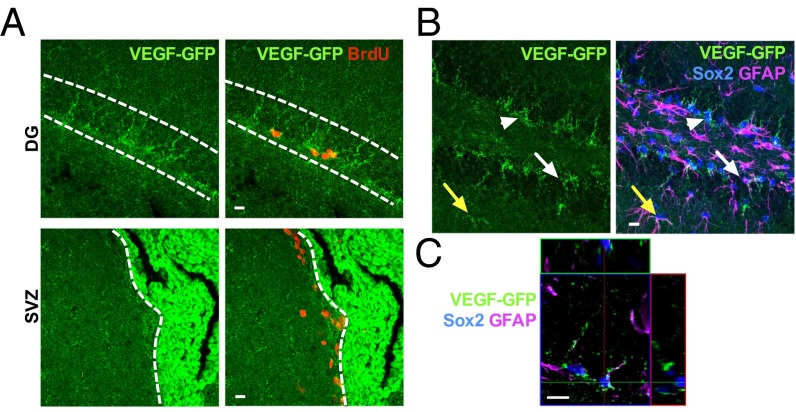
To determine the cellular phenotype of VEGF-expressing cells in the SGZ in vivo, we examined VEGF promoter activity in adult VEGF-GFP reporter mice, which carry a GFP transgene immediately downstream of 2.85 kb of the VEGF promoter and 5′ UTR (17). Similar to the pattern seen in VEGF mRNA, we found strong GFP in the CP and lining the SGZ (Fig. 1 A and B and Fig. S1 B and C) but relatively little expression in the SVZ (Fig. 1A and Fig. S1B).
Fig. 1.
Adult hippocampal NSPCs express VEGF in vivo. (A, Top) Images from adult VEGF-GFP mice showing GFP+ puncta lining the SGZ and surrounding cells labeled with BrdU 2 h before euthanasia. (A, Bottom) SVZ showed weaker GFP expression than the SGZ, whereas the CP showed intense GFP expression. (B) GFP+ puncta were found surrounding Sox2+ TAPs (arrowheads) and filling Sox2+/GFAP+ RGLs (white arrow) in the SGZ. GFAP+ cells with astrocytic morphology also colabeled with GFP (yellow arrow). (C) Orthogonal image of a single 1-μm z-slice showing GFP+ puncta colocalizing with GFAP in a GFAP+/Sox2+ RGL. (Scale bars: 10 μm.)
The NSPC population in the SGZ neurogenic niche can be divided into two broad categories: (i) slowly dividing stem cells, also known as radial glia-like cells (RGLs), and (ii) their more rapidly dividing progeny, transit-amplifying progenitors (TAPs) (2, 18, 19). Immunohistochemical colabeling for the progenitor marker sex determining region Y-box 2 (Sox2) revealed GFP+ puncta surrounding Sox2+ TAPs. Glial fibrillary acidic protein (GFAP) labeling revealed GFP+ puncta filling Sox2+/GFAP+ RGL stem cells (Fig. 1 B and C and Fig. S1D). Consistent with previous studies (9), mature astrocytes also contained GFP+ puncta (Fig. 1B). No GFP was found in hippocampus from a WT mouse (Fig. S1C). These findings suggest that NSPCs express VEGF in the adult hippocampus in vivo and that this high expression may be unique to the SGZ because the SVZ showed minimal VEGF transcriptional activity.
Adult Hippocampal NSPCs Synthesize and Secrete VEGF in Vitro.
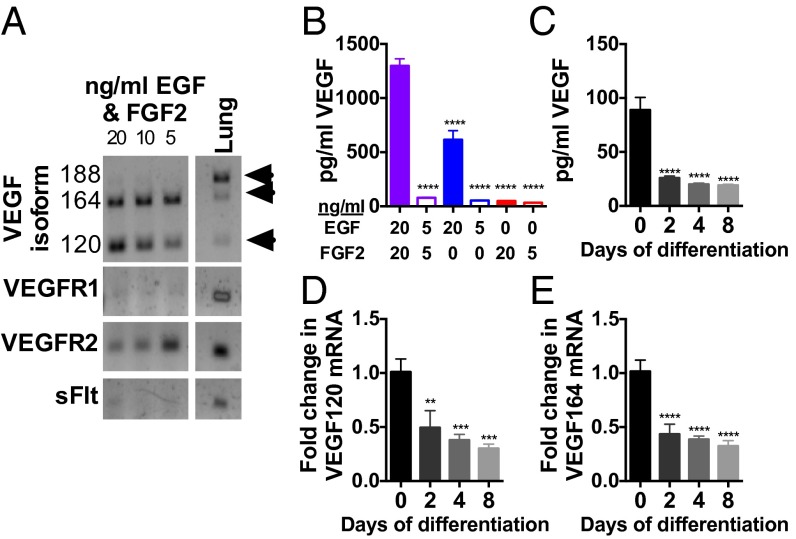
To investigate the quantity and regulation of adult hippocampal NSPC-derived VEGF, we isolated NSPCs from the adult hippocampus and maintained them in standard culture conditions (20). VEGF is synthesized as three major coexpressed splice variants: VEGF120, VEGF164, and VEGF188 (11, 21). Isolated adult hippocampal NSPCs synthesized VEGF120 and VEGF164 mRNA but not VEGF188 (Fig. 2A). NSPCs maintained in optimal growth conditions for 4 d accumulated over 1,298 ± 65.45 pg/mL VEGF protein, whereas culturing in reduced growth factor conditions resulted in up to 10-fold less VEGF secretion (Fig. 2B and Fig. S2A). Reduced growth factor conditions similarly caused rapid decreases in both VEGF120 and VEGF164 mRNA levels (Fig. S2 B and C). Differentiating NSPCs into mature neurons and astrocytes caused a rapid down-regulation of VEGF protein and mRNA (Fig. 2 C–E). These findings demonstrate that adult hippocampal NSPCs synthesize and secrete large quantities of VEGF and that VEGF production is strongly regulated by cell differentiation and the environment.
Fig. 2.
Adult hippocampal NSPCs synthesize and secrete large quantities of VEGF. (A) Isolated adult hippocampal NSPCs treated with different growth factor conditions for 4 d expressed mRNA transcripts for the VEGF120 and VEGF164 but not VEGF188 splice variants. NSPCs also expressed VEGFR2 mRNA but not VEGFR1 or soluble Flt (sFlt), the secreted form of VEGFR1. Adult lung RNA served as a positive control. (B) NSPC secretion of VEGF was measured by ELISA of culture supernatant after 4 d and was greatest in standard proliferative conditions with 20 ng/mL EGF and 20 ng/mL FGF2. Decreasing either EGF or FGF2 reduced secretion of VEGF (ANOVA, P < 0.0001; n = 4–5 wells per group per experiment; two experiments). (C) Adult NSPCs were maintained in 20 ng/mL EGF and 20 ng/mL FGF2 for 2 d (0 d differentiation) or switched to differentiating conditions for 2, 4, or 8 d. VEGF ELISA showed decreased secreted VEGF protein with differentiation (ANOVA, P < 0.0001; n = 3 wells per group per experiment; three experiments). (D and E) VEGF120 and VEGF164 mRNA decreased with differentiation normalized to the MAPK3 housekeeping gene relative to 0 d differentiation (VEGF120: ANOVA, P = 0.0002; VEGF164: ANOVA, P < 0.0001; n = 2–3 wells per group per experiment; three experiments). Data represent mean ± SEM. **P < 0.01; ***P < 0.001; ****P < 0.0001, post hoc Dunnett’s tests.
Adult Hippocampal NSPCs Synthesize a Significant Portion of DG VEGF.
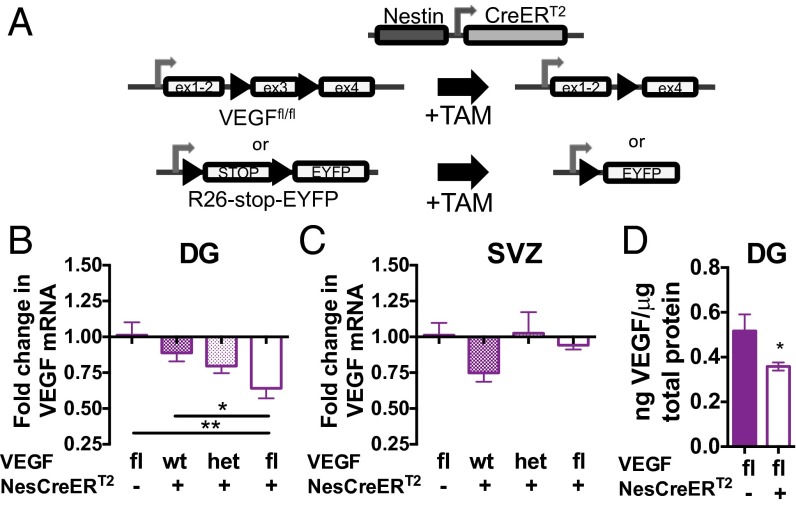
In vivo, multiple sources of VEGF exist, such as mature astrocytes and neurons (9, 11, 14, 22). Although NSPCs represent a small minority of adult DG cells, our findings of enhanced VEGF secretion by NSPCs relative to mature cell types in vitro suggests that NSPCs could contribute disproportionately to VEGF levels in the DG. To determine the relative contribution of NSPCs to VEGF in the neurogenic niche, we created an in vivo, NSPC-specific, inducible VEGF knockdown model by crossing VEGFfl mice (23) with NestinCreERT2 mice (24). When exposed to tamoxifen (TAM), NestinCreERT2 drives high levels of recombination in NSPCs in the adult SGZ, with high specificity and low toxicity (24–26). Crossing the NestinCreERT2 mouse with an R26-enhanced YFP reporter mouse (27) confirmed that TAM treatment selectively induced recombination throughout the SGZ in both Sox2+/GFAP+ RGLs and Sox2+/GFAP− TAPs (Fig. 3A and Fig. S3).
Fig. 3.
Knockdown of VEGF in adult NSPCs reduces total DG VEGF. (A) NestinCreERT2 mice were crossed over multiple generations with VEGFfl mice to create VEGFfl;NestinCreERT2+ mice, allowing TAM-inducible knockdown of VEGF in NSPCs in adulthood. (B) DG was dissected from VEGFfl;NestinCreERT2+ mice (n = 8) and from VEGFfl;NestinCreERT2− (n = 4), VEGFwt;NestinCreERT2+ (n = 8), and VEGFhet;NestinCreERT2+ (n = 10) littermates after 2 wk of TAM treatment. Total DG VEGF120 mRNA was significantly decreased by VEGF knockdown in VEGFfl;NestinCreERT2+ mice compared with both VEGFfl;NestinCreERT2− and VEGFwt;NestinCreERT2+ controls (ANOVA, P = 0.0083). fl, homozygous floxed; het, heterozygous floxed. *P < 0.05; **P < 0.01, Tukey’s post hoc comparisons. (C) SVZ was dissected from VEGFfl;NestinCreERT2+ mice (n = 4) and from VEGFfl;NestinCreERT2− (n = 4), VEGFwt;NestinCreERT2+ (n = 3), and VEGFhet;NestinCreERT2+ (n = 8) littermates. Total SVZ VEGF120 mRNA did not differ by genotype (ANOVA, P = 0.58). (D) DG was dissected 2 wk after 5 d of TAM treatment and assessed for VEGF protein by ELISA. VEGFfl;NestinCreERT2+ mice (n = 3) had significantly less total DG VEGF than controls (n = 6). *P = 0.048, Mann–Whitney test. Data represent mean ± SEM mRNA normalized to the actin housekeeping gene relative to VEGFfl;NestinCreERT2− control.
Remarkably, TAM-induced knockdown of VEGF in adult NSPCs caused a 27.7–37.1% reduction of total DG VEGF relative to VEGFwt/wt;NestinCreERT2+ or VEGFfl/fl;NestinCreERT2− controls (Con), respectively (Fig. 3 A and B). We replicated this finding in two independent cohorts using shorter courses of TAM treatment, showing 20.9% and 20.8% reductions in VEGF following NSPC-specific VEGF knockdown (Fig. S4A). VEGF mRNA in the SVZ was not altered by NSPC-specific knockdown (Fig. 3C), further confirming the in situ and reporter findings (Fig. 1) that SVZ NSPCs are not major sources of VEGF in vivo. Levels of the VEGF188 mRNA isoform were not altered in the DG or SVZ by NSPC-VEGF knockdown (Fig. S4 B and C), which is consistent with our finding that NSPCs do not synthesize this transcript (Fig. 2A). Total DG VEGF protein was also reduced by 30.7% in induced NSPC-VEGF knockdown mice (0.36 ± 0.02 ng of VEGF per microgram of protein) relative to controls (0.52 ± 0.07 ng of VEGF per microgram of protein) (Fig. 3D). These results demonstrate that NSPCs are a surprisingly large source of VEGF expression in the adult DG, especially given the relatively small size of the NSPC population.
Loss of NSPC-Derived VEGF Alters Stem Cell Dynamics in Vivo.
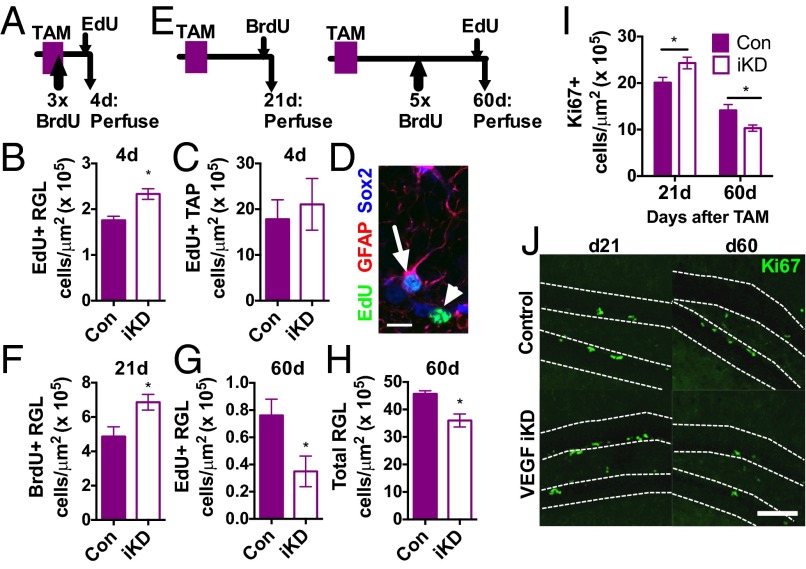
Previous studies show that exogenous infusions of VEGF stimulate NSPC proliferation (10–13), suggesting a role for VEGF in regulation of neurogenesis in the adult hippocampus. To determine the functional role of VEGF derived from NSPCs in regulating their own proliferation, we treated 8- to 9-wk-old VEGFfl/fl;NestinCreERT2+ (VEGF-iKD) and Con mice with TAM and then quantified cell proliferation at multiple time points (Fig. 4 A and E). Surprisingly, 4 d after TAM treatment, VEGF-iKD mice showed an increase in the number of 5-ethynyl-2′-deoxyuridine (EdU)-labeled, proliferating RGL stem cells (2.33 ± 0.12 cells per area) compared with controls (1.76 ± 0.09 cells per area) (Fig. 4 B and D and Fig. S5A). The number of dividing TAPs did not differ (Fig. 4 C and D and Fig. S5A), however, suggesting that loss of self-secreted VEGF caused an increase in proliferation of the stem cell population specifically.
Fig. 4.
Loss of NSPC-derived VEGF disrupts NSPC self-regulation in vivo. (A) Adult control (n = 3) and VEGF-iKD mice (n = 7) were treated with TAM for 5 d and then perfused 4 d later. (B) VEGF-iKD led to an increase in the number of EdU+ proliferating RGL stem cells. *P = 0.017, Mann–Whitney test. (C) VEGF-iKD did not significantly alter the number of proliferating TAPs. P > 0.1, Mann–Whitney test. (D) Example EdU+/GFAP+/Sox2+ RGLs (arrow) and EdU+/Sox2+ TAPs (arrowhead) in a 1-μm z-slice. (Scale bar: 10 μm.) (E) Adult control and VEGF-iKD mice were treated with TAM for 5 d and then perfused after 21 d (n = 10 control and n = 11 VEGF-iKD mice) or 60 d (n = 4 control and n = 10 VEGF-iKD mice). (F) VEGF-iKD increased proliferation of the RGL stem cell population 21 d after knockdown. *P = 0.0124, t test. (G) Sixty days after knockdown, RGL stem cell proliferation was decreased in VEGF-iKD mice relative to controls. *P = 0.0465, Mann–Whitney test. (H) At 60 d, the number of GFAP+/Sox2+ RGLs was decreased in VEGF-iKD mice relative to controls. *P = 0.024, Mann–Whitney test. (I) VEGF-iKD led to an increase in Ki67+ proliferating cells in the SGZ after 21 d (d21) but a decrease after 60 d (d60) (two-way ANOVA: interaction, P = 0.0042; day, P < 0.0001; genotype, P = 0.94). Post hoc planned comparisons within day (21 d: P = 0.0343, t test; 60 d, P = 0.0140, Mann–Whitney test). (J) Example images of proliferating Ki67 cells in control and VEGF-iKD mice at day 21 and day 60. (Scale bar: 100 μm.) Data represent mean ± SEM.
To determine the longer term impact of loss of self-secreted VEGF on NSPC dynamics, we compared VEGF-iKD mice at 21 and 60 d after TAM treatment (Fig. 4E). Similar to our findings 4 d after TAM-induced knockdown, VEGF-iKD mice showed an increase in RGL proliferation after 21 d (Con: 4.86 ± 0.57 vs. VEGF-iKD: 6.86 ± 0.46 cells per area) (Fig. 4F and Fig. S5 B–D). However, after 60 d, the number of EdU+ proliferating RGL stem cells was decreased in VEGF-iKD mice (Con: 0.76 ± 0.12 vs. VEGF-iKD: 0.34 ± 0.11 cells per area) (Fig. 4G). In addition, at this later time point, the total number of RGLs was decreased by 21.3% in VEGF-iKD mice (Con: 45.69 ± 1.123 vs. VEGF-iKD: 36.00 ± 2.347 cells per area) (Fig. 4H and Fig. S5 B–E). Comparison of the total proliferating population using the endogenous proliferative marker Ki67 revealed a similar pattern as in the RGL stem cells: increased proliferation after 21 d (Con: 20.40 ± 1.07 vs. VEGF-iKD: 24.40 ± 1.36 cells per area) followed by reduced proliferation after 60 d (Con: 14.14 ± 1.26 vs. VEGF-iKD: 10.31 ± 0.68 cells per area) (Fig. 4 I and J).
In previous studies, loss of factors essential for stemness led to a pattern similar to what we observed in VEGF-iKD mice, showing an increase in proliferation 21 d after TAM followed by a decrease after 60 d, relative to controls (28). This pattern typically emerges because loss of stemness causes differentiation of slow-dividing stem cells into rapidly dividing progenitors, followed by exhaustion of proliferation when stem cells cannot replenish the progenitor pool (28–30). Consistent with this pattern, our findings show an increase in stem cell division (at day 4) followed by a surge in the total proliferative population (day 21) and, finally, a collapse in proliferation and a loss of total RGL stem cells (day 60).
To determine whether more extensive knockdown of NSPC-derived VEGF could cause more dramatic impairment of NSPC maintenance, we treated 8- to 9-wk-old VEGF-iKD and control mice with TAM repeatedly over 12 wk (Fig. S6A). This TAM treatment regimen has been used previously to show the necessity of the cell cycle factor p63 for maintenance of stemness (31). In this more extensive knockdown paradigm, VEGF-iKD mice had 54.5% fewer proliferating EdU+ cells in the SGZ than control littermates (Con: 6.59 ± 0.59 vs. VEGF-iKD: 3.02 ± 0.49 cells per area) (Fig. S6B), consisting of 50% fewer proliferating TAPs (Con: 4.87 ± 0.81 vs. VEGF-iKD: 2.46 ± 0.41 cells per area) (Fig. S6 C and F) and 64.3% fewer proliferating RGLs (Con: 0.69 ± 0.18 vs. VEGF-iKD: 0.25 ± 0.09 cells per area) (Fig. S6 D and F). The total number of RGLs was also reduced by 33.3% (Con: 35.67 ± 1.83 vs. VEGF-iKD: 22.96 ± 2.38 cells per area) (Fig. S6 E and F). Combined, our findings suggest that loss of NSPC-derived VEGF disrupts NSPC maintenance in vivo, resulting in a depletion of RGL stem cells. However, the partial maintenance of proliferation and stem cells even with extensive TAM-induced knockdown suggests that the remaining VEGF from other cell types may help partially maintain the population.
Loss of NSPC-VEGF Does Not Alter Cell Fate Choice.
To investigate the effects of NSPC-VEGF knockdown on cell fate choice, we labeled dividing cells with BrdU at three different time points 4–40 d before perfusion. The percentage of BrdU-labeled cells adopting a neuronal fate did not differ at any time point assessed (Fig. S7). These findings suggest that loss of NSPC-derived VEGF does not dramatically alter differentiation of NSPCs once they have exited the cell cycle.
VEGF Acts Directly on NSPCs.
Loss of NSPC-VEGF in vivo could have an impact on NSPCs via multiple pathways, acting either within the NSPC population or via indirect interactions with other niche cell types. Because VEGF is highly angiogenic, we first investigated whether NSPC-VEGF might be influencing NSPC maintenance via changes in vasculature. However, we found no change in the vasculature, as revealed by CD31+ endothelial cell area in the combined DG and hilus or in the SGZ after NSPC-VEGF knockdown (Fig. S6 G–J).
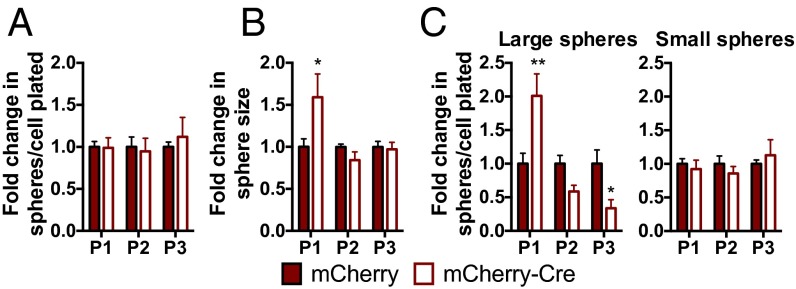
To determine whether NSPC-derived VEGF could act directly on NSPCs independent of interaction with other niche cell types, we isolated NSPCs from adult VEGFfl/fl mice and infected them with an mCherry-Cre–expressing lentiviral vector or an mCherry-only control lentivirus. In VEGFfl/fl NSPCs, mCherry-Cre expression significantly reduced VEGF secretion (25.84 ± 5.68 ng/mL mCherry vs. 7.50 ± 0.38 ng/mL mCherry-Cre; P = 0.0231). When NSPCs were grown as spheres after VEGF knockdown, we observed a transient increase in sphere size without a change in the total number of spheres (Fig. 5 A and B). This increase in sphere size was due to an increase in the formation of large spheres with a diameter over 100 μm in the first passage after knockdown (Fig. 5C). However, after multiple passages, VEGF knockdown NSPCs formed fewer large spheres than controls (Fig. 5C and Fig. S8F). WT NSPCs were unaffected by mCherry-Cre expression relative to mCherry in VEGF secretion, sphere number, and sphere size (Fig. S8 A–E).
Fig. 5.
VEGF knockdown in isolated NSPCs disrupts NSPC self-regulation. (A) Total number of spheres formed over multiple passages after VEGF knockdown by mCherry-Cre expression in VEGFfl/fl NSPCs did not change relative to mCherry control (two-way ANOVA: interaction, P = 0.82; passage, P = 0.082; Cre, P = 0.87). (B) VEGF knockdown led to an increase in sphere size after P1 (two-way ANOVA: interaction, P = 0.0299; passage, P = 0.0299; Cre, P = 0.26). *P < 0.05, post hoc Sidak’s comparisons within passage. (C) VEGF knockdown NSPCs formed more spheres over 100 μm in diameter (large) after P1, but over subsequent passages, they formed fewer spheres than mCherry-only–expressing controls (two-way ANOVA: interaction, P = 0.0002; passage, P = 0.0002; Cre, P = 0.89). Small spheres (diameter ≤100 μm) were not altered. *P < 0.05; **P < 0.01, post hoc Holm–Sidak’s multiple comparisons tests within passage. Data are mean ± SEM normalized to mCherry control within experiment per passage (n = 2–3 wells per group per experiment; three experiments). P1, passage 1; P2, passage 2; P3, passage 3.
This pattern of a surge followed by a decrease in large sphere formation is suggestive of impaired stem cell maintenance (28–30). These data are therefore consistent with our in vivo findings of a reduced RGL population after in vivo NSPC-specific knockdown of VEGF.
Adult Hippocampal NSPCs Self-Regulate via VEGF Receptor 2.
To determine the receptor responsible for the impaired NSPC maintenance with loss of VEGF, we next investigated the expression and regulation of VEGF receptors in NSPCs. VEGF signals through two receptors: VEGF receptor 1 (VEGFR1; also known as Flt1) and VEGFR2 (also known as KDR/Flk1) (9, 22). Isolated adult hippocampal NSPCs expressed VEGFR2 but not VEGFR1 (Fig. 2A). Similar to VEGF, NSPC VEGFR2 expression was down-regulated with decreasing levels of EGF and FGF2 (Fig. S9 A–C) and with differentiation (Fig. S9 D–F). Immunohistochemical staining for VEGFR2 in the adult hippocampus revealed widespread VEGFR2-immunoreactivity (ir) in the SGZ and granular layer of the DG, a finding that is consistent with previous studies (12) (Fig. S10A). VEGFR2-ir was found in all newly born proliferative cells, nestin+/GFAP+ RGL stem cells, and early differentiating doublecortin+ neurons (Fig. S10 A–C). These findings conform to those findings of previous studies (10, 12, 14, 15, 32–34) demonstrating VEGFR2 expression on NSPCs in vitro and in vivo.
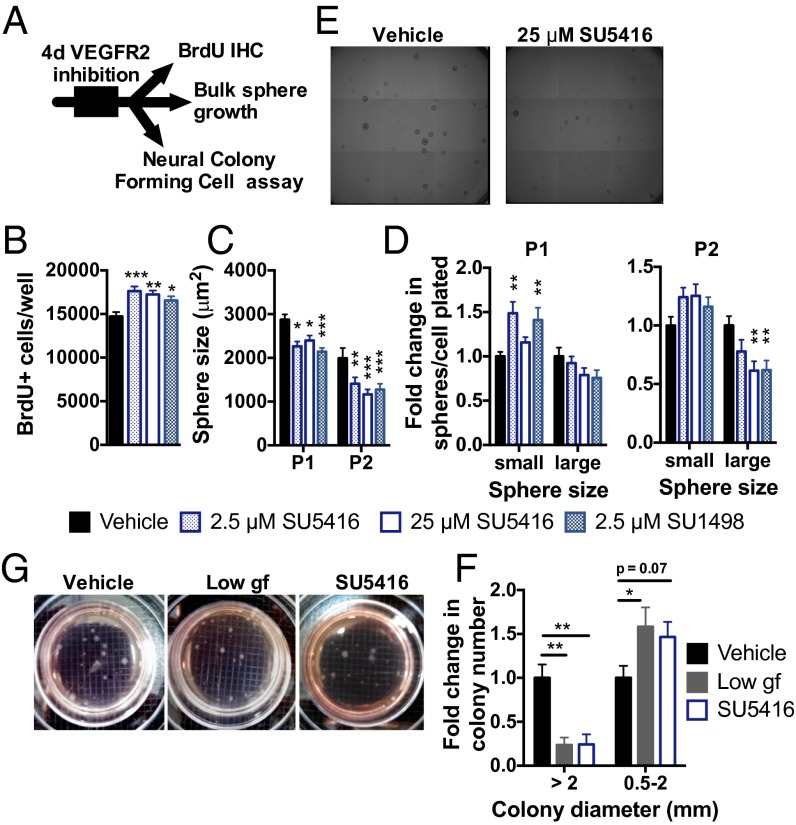
To test the necessity of VEGFR2 signaling for NSPC maintenance in adult hippocampal NSPCs, we used two highly selective pharmacological VEGFR2 inhibitors (10, 32, 35): SU5416 and SU1498 (36, 37). Treating NSPCs with inhibitor for 4 d increased proliferation, as measured by BrdU incorporation (Fig. 6 A and B). However, if we then returned NSPCs to normal growth conditions with no inhibitor present, sphere size was reduced due to a decrease in large spheres and/or an increase in small spheres (Fig. 6 C–E). This increase in proliferation and small sphere formation with a long-term loss in the ability to form large spheres is again consistent with impaired stem cell maintenance.
Fig. 6.
Inhibition of VEGFR2 signaling disrupts NSPC self-regulation. (A) NSPCs were treated in a monolayer for 4 d with VEGFR2 kinase inhibitors: SU5416 or SU1498. (B) Number of BrdU+ proliferating NSPCs was increased by exposure to either SU5416 or SU1498 (ANOVA: P = 0.0010; n = 3–5 wells per group per experiment; three experiments). (C) Four days of VEGFR2 inhibition led to a smaller sphere size over subsequent passages (two-way ANOVA: interaction, P = 0.46; passage, P < 0.0001; VEGFR2 inhibition, P < 0.0001; n = 6 wells per group per experiment; three experiments). (D) Formation of small spheres (diameter ≤100 μm) was enhanced in P1 after VEGFR2 inhibition. In P2, formation of large spheres (diameter >100 μm) was inhibited (P1, two-way ANOVA: interaction, P = 0.0029; size, P < 0.0001; VEGFR2 inhibition, P = 0.24; P2, two-way ANOVA: interaction, P = 0.0012; size, P < 0.0001; VEGFR2 inhibition, P = 0.31). (E) Example images of spheres after P2. (Grid: 1.5 μm.) (F) Neural colony-forming cell (NCFC) assay revealed that treatment with 5 ng/mL EGF/FGF2 [low growth factor (gf)] or 25 μM SU5416 decreased formation of stem cell-derived colonies with diameter >2 mm and caused a trend toward an increase in smaller, progenitor-derived colonies (two-way ANOVA: interaction, P = 0.0019; size, P < 0.0001; treatment, P = 0.90; n = 2–3 wells per experiment; three experiments). All post hoc comparisons were Dunnett’s comparisons to vehicle: *P < 0.05; **P < 0.01; ***P < 0.001. Data are mean ± SEM normalized to vehicle within experiment. (G) Example NCFC spheres after 3 wk in culture. (Grid: 2 mm.)
To confirm further that the changes in sphere formation after VEGFR2 inhibition resulted from a loss of stem cells as our in vivo data suggested, we used the NeuroCult neural colony-forming cell assay (StemCell Technologies). In this assay, NSPCs are plated in a semisolid gel that allows colonies to grow from a single cell (38). After 3 wk, colonies that grow to over 2 mm in diameter derive from a stem cell with self-renewal and multipotent potential (38) (Fig. S11). We found that both VEGFR2 inhibition with SU5416 and low growth factor treatment (5 ng/mL EGF/FGF2, a positive control for impairing stem cell maintenance) inhibited the formation of stem cell-derived large colonies (Fig. 6 F and G).
Together, these findings suggest that NSPCs maintain their stem cell capacity via self-secreted VEGF interacting with VEGFR2.
Discussion
VEGF is an essential molecule for brain plasticity. In the adult brain, overexpressing or infusing VEGF stimulates neovascularization, improved cognition, and hippocampal neurogenesis (7, 10, 33). Blocking endogenous VEGF, in contrast, impairs the cognitive and neurogenic responses to stimuli like antidepressants and environmental enrichment (33, 34). However, although neural precursors in the developing brain are well-recognized sources of VEGF (39), in the adult brain, secretion of VEGF has largely been attributed to mature astrocytes (7, 9, 21, 22).
We show here that endogenous adult NSPCs are a previously unidentified, significant source of VEGF in the adult hippocampus. NSPCs represent a small minority of the cells in the neurogenic niche. Estimates of the nestin+ NSPC population have indicated around 20,000 NSPCs reside in a healthy adult mouse DG (40). In contrast, there are over 1 million DG granule cell neurons (41) and ∼70,000 astrocytes (42) in the niche. Our results therefore demonstrate that this small population may be a surprisingly potent contributor to secreted growth factors in the adult neurogenic niche, and they are the first demonstration, to our knowledge, that adult hippocampal NSPCs can shape their own niche via secreted proteins. It should be noted, however, that up to two-thirds of DG VEGF remained after NSPC-specific knockdown, suggesting that other cell types (astrocytes, neurons, oligodendrocytes, microglia, or others) are also potent sources of VEGF in vivo. This remaining VEGF may explain why, even after repeated TAM-induced knockdown of NSPC-VEGF, not all stem cells were eliminated from the neurogenic niche. Whether the functional role of VEGF in the neurogenic niche differs depending on its cellular source remains an open question.
In support of our findings of high levels of VEGF expression in NSPCs, the adult SGZ has previously been shown to display high levels of constitutively active hypoxia-inducible factor 1α (HIF1α), the transcriptional driver of VEGF expression (43), suggesting conspicuous activation of VEGF in NSPCs relative to other granule layer cells. In addition, previous work shows VEGF expression in adult NSPCs in vitro (14, 15) and VEGF antibody immunoreactivity in some SGZ populations in vivo (12, 44). However, the relative abundance and potential functional role of this VEGF remain unclear in these studies.
The present findings may be cause for reinterpretation of the functional relevance of adult neurogenesis. Numerous studies have explored the effects of loss of adult hippocampal neurogenesis by inhibiting NSPC proliferation, via antimitotic agents, irradiation, or genetic knockdown (45). The memory impairments resulting from the loss of the neurogenic population in these studies are typically attributed to a loss of new neurons. However, given that VEGF levels also influence hippocampal cognition (9, 33), the present findings suggest that the long-term effects of loss of NSPCs could rely partially on reduced growth factor availability. This secretory role of NSPCs might be particularly relevant to the adaptive significance of rapid NSPC proliferative changes seen after interventions such as exercise and stress (46, 47).
Determining the independent role of NSPC-VEGF in hippocampal function remains a difficult challenge due to the dependence of NSPCs on self-secreted VEGF to maintain proliferation and new neuron production. Although studies that manipulate new neuron survival without affecting NSPCs confirm an independent role of new neurons in supporting memory function (48), a system for depleting NSPC-VEGF without having an impact on NSPC proliferation and new neuron production would be required to determine the independent role of NSPC-derived VEGF in the hippocampal niche.
Hippocampal NSPCs are a heterogeneous population (2, 18, 19, 49, 50). How each of these subtypes of NSPCs is regulated has not been fully characterized, although some important players have been identified. For example, loss of RBPJκ, a mediator of Notch signaling, leads to a similar surge and collapse of proliferation as we observed after conditional deletion of VEGF from hippocampal NSPCs (28). Loss of either the stem cell factor p21 or the Notch binding partner Dll1 similarly causes a proliferation surge and collapse in SVZ NSPCs (29, 30). We demonstrate here a functional role for self-secreted VEGF in regulating the hippocampal NSPC population that resembles these other known regulators of stem cell dynamics, causing a biphasic change in proliferation and a long-term loss of stem cells.
Self-secreted VEGF has been implicated in self-regulation of stem cells outside the brain, such as hematopoietic stem cells and tumor cells (39, 51, 52). This study is the first published report to our knowledge showing that VEGF (from any source) plays a role in adult hippocampal stem cell maintenance, a finding that likely went unnoticed in previous studies of VEGF and adult neurogenesis because (i) NSPCs were not considered to produce their own VEGF, and therefore were not selectively targeted, particularly in vivo, and (ii) the time course needed to detect population-level changes in proliferation due to a change in stem cell dynamics is longer than many previous studies of VEGF inhibition allowed (7, 10, 34).
We found evidence consistent with previous studies (16, 53) that SVZ NSPCs may not synthesize significant amounts of VEGF, and therefore may differ in their dependence on self-sustained VEGF signaling for maintaining the NSPC pool. The conspicuously close proximity of the CP, an intense source of secreted VEGF, to the SVZ could explain this divergence between SGZ and SVZ dependence on self-derived VEGF. SVZ stem and progenitor cells are also known to differ from SGZ NSPCs cell-intrinsically in multiple ways (54, 55). Dependence on VEGF could similarly be a cell-intrinsic difference between these two populations. Potentially in contrast to these and previous findings (16), however, a recent study suggests that SVZ progenitors may require HIF1α, a transcriptional regulator of VEGF and several other hypoxia-related genes, to maintain their vascular niche (56). Further studies will therefore be necessary to determine fully the quantity and contribution of NSPC-derived VEGF in the SVZ neurogenic niche.
VEGF, also known as VEGF-A, is the most abundant form of VEGF in the CNS and the most heavily investigated member of a broader VEGF family (22). Other VEGF family members include VEGF-B, VEGF-C, VEGF-D, and VEGF-E, some of which also regulate neurogenesis in either the SGZ or SVZ (57, 58). These different family members share some structural similarities, but they are separable molecules from VEGF-A, with unique receptor binding capabilities (VEGF-B, for example, binds only VEGFR1 and not VEGFR2 or VEGFR3, whereas VEGF-A binds both VEGFR1 and VEGFR2) (22). Whether adult NSPCs are significant sources of any of these other members of the VEGF family remains to be determined.
Given the multifaceted role of VEGF in the hippocampus, our characterization of its expression in adult hippocampal NSPCs provides an important potential role for undifferentiated cells in regulating hippocampal function. We show a functional role for NSPC-derived VEGF in self-regulating the NSPC pool in the adult SGZ, but the practical implications could extend throughout the neurogenic niche and DG, having an impact on numerous other cell types. VEGF is tied to the hippocampal response not only to positive hippocampal stimuli but also to injury response and neurodegeneration (59). These findings therefore open a previously unexplored avenue for investigation of how VEGF from endogenous adult NSPCs may shape hippocampal function in both physiological and pathological conditions.
Methods
Animals.
Male and female mice were used at the age of 8–9 wk. Genotyping primers are detailed in Table S1. All animal use was in accordance with institutional guidelines approved by the Veterans Administration Palo Alto Committee on Animal Research. More details are available in SI Methods.
Lentivirus Infection of NSPCs.
VEGFfl/fl or WT NSPCs were plated in an adherent monolayer on 96-well plates and then infected with mCherry or mCherry-Cre lentivirus (SI Methods). After 48 h, infected cells were passaged and replated in sphere culture conditions (on uncoated plates) at 3,000 cells per well. Passaging was performed with Cell Dissociation Buffer (Gibco).
Qualitative and Quantitative Assessment of RNA.
RNA was quantified using standard real-time PCR techniques (SI Methods). Qualitative assessment of RNA was determined by gel electrophoresis of PCR products using isoform-specific primers (SI Methods).
VEGFR2 Inhibition in Vitro.
SU5416 and SU1498 (Sigma) were dissolved in DMSO (10 mM) and added to adherent monolayer cultures of isolated NSPCs (5,000 cells per well). Immunostaining was done using standard procedures (SI Methods).
Immunohistochemical Staining.
Antibody staining was performed using standard procedures (46) (SI Methods and Table S2).
Quantification of in Vivo Immunohistochemical Stains.
All in vivo staining was quantified by one of two blinded observers. EdU, BrdU, and Ki67+ cells were counted throughout the DG, and the area sampled was measured using LSM700 Zen software (Zeiss). Total GFAP/Sox2+ type I stem cells and colabeling of BrdU or EdU were assessed in 1-μm z-stacks on an LSM700 confocal microscope with a 40× oil objective.
Quantification of Protein.
VEGFR2 protein from isolated NSPCs was quantified using Western blot analysis (SI Methods and Table S2). A VEGF ELISA (R&D Systems) was performed as per the manufacturer’s instructions on RIPA Lysis and Extraction Buffer (Thermo Scientific)-extracted DG lysates and tissue culture supernatant.
Supplementary Material
Acknowledgments
This study was funded by NIH Grant AG045034 (to T.W.-C.) and the Department of Veterans Affairs. E.D.K. was supported by a postdoctoral National Research Service Award from the National Institute of Neurological Disorders and Stroke. A.A.K. was supported by a California Institute for Regenerative Medicine Bridges to Stem Cell Research Award (Grant TB1-01190). R.L.M. was supported by the Stanford Summer Research Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422448112/-/DCSupplemental.
References
- 1.Aimone JB, et al. Regulation and function of adult neurogenesis: From genes to cognition. Physiol Rev. 2014;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun SMG, Jessberger S. Adult neurogenesis: Mechanisms and functional significance. Development. 2014;141(10):1983–1986. doi: 10.1242/dev.104596. [DOI] [PubMed] [Google Scholar]
- 3.Anthony DF, Shiels PG. Exploiting paracrine mechanisms of tissue regeneration to repair damaged organs. Transp Res. 2013;2(1):10. doi: 10.1186/2047-1440-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horie N, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29(2):274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosher KI, et al. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci. 2012;15(11):1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunewald M, et al. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Licht T, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci USA. 2011;108(12):5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenstein JM, Mani N, Silverman WF, Krum JM. Patterns of brain angiogenesis after vascular endothelial growth factor administration in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95(12):7086–7091. doi: 10.1073/pnas.95.12.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Licht T, Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell Mol Life Sci. 2013;70(10):1727–1737. doi: 10.1007/s00018-013-1280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier NM, Lee B, Banasr M, Elsayed M, Duman RS. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology. 2012;63(4):642–652. doi: 10.1016/j.neuropharm.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6(2):107–114. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci USA. 2008;105(32):11352–11357. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wittko-Schneider IM, Schneider FT, Plate KH. Brain homeostasis: VEGF receptor 1 and 2-two unequal brothers in mind. Cell Mol Life Sci. 2013;70(10):1705–1725. doi: 10.1007/s00018-013-1279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 15.Maurer MH, Tripps WKC, Feldmann RE, Jr, Kuschinsky W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci Lett. 2003;344(3):165–168. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- 16.Licht T, et al. VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development. 2010;137(2):261–271. doi: 10.1242/dev.039636. [DOI] [PubMed] [Google Scholar]
- 17.Fukumura D, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94(6):715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 18.Bonaguidi MA, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145(7):1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonaguidi MA, Song J, Ming G-L, Song H. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr Opin Neurobiol. 2012;22(5):754–761. doi: 10.1016/j.conb.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babu H, et al. A protocol for isolation and enriched monolayer cultivation of neural precursor cells from mouse dentate gyrus. Front Neurosci. 2011;5:89. doi: 10.3389/fnins.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmeliet P, Ruiz de Almodovar C. VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cell Mol Life Sci. 2013;70(10):1763–1778. doi: 10.1007/s00018-013-1283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89(2):607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- 23.Gerber HP, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 24.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27(46):12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Y-W, et al. Inducible and conditional deletion of extracellular signal-regulated kinase 5 disrupts adult hippocampal neurogenesis. J Biol Chem. 2012;287(28):23306–23317. doi: 10.1074/jbc.M112.344762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun M-Y, Yetman MJ, Lee T-C, Chen Y, Jankowsky JL. Specificity and efficiency of reporter expression in adult neural progenitors vary substantially among nestin-CreER(T2) lines. J Comp Neurol. 2014;522(5):1191–1208. doi: 10.1002/cne.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehm O, et al. RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30(41):13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaguchi D, Furutachi S, Kawai H, Hozumi K, Gotoh Y. Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nat Commun. 2013;4:1880. doi: 10.1038/ncomms2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marqués-Torrejón MÁ, et al. Cyclin-dependent kinase inhibitor p21 controls adult neural stem cell expansion by regulating Sox2 gene expression. Cell Stem Cell. 2013;12(1):88–100. doi: 10.1016/j.stem.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cancino GI, et al. p63 Regulates adult neural precursor and newly born neuron survival to control hippocampal-dependent Behavior. J Neurosci. 2013;33(31):12569–12585. doi: 10.1523/JNEUROSCI.1251-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fournier NM, Duman RS. Role of vascular endothelial growth factor in adult hippocampal neurogenesis: Implications for the pathophysiology and treatment of depression. Behav Brain Res. 2012;227(2):440–449. doi: 10.1016/j.bbr.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao L, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 34.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA. 2007;104(11):4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schänzer A, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14(3):237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fong TA, et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59(1):99–106. [PubMed] [Google Scholar]
- 37.Boguslawski G, McGlynn PW, Harvey KA, Kovala AT. SU1498, an inhibitor of vascular endothelial growth factor receptor 2, causes accumulation of phosphorylated ERK kinases and inhibits their activity in vivo and in vitro. J Biol Chem. 2004;279(7):5716–5724. doi: 10.1074/jbc.M308625200. [DOI] [PubMed] [Google Scholar]
- 38.Azari H, Louis SA, Sharififar S, Vedam-Mai V, Reynolds BA. Neural-colony forming cell assay: An assay to discriminate bona fide neural stem cells from neural progenitor cells. J Vis Exp. 2011;(49):2639. doi: 10.3791/2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilley JA, Yang C-P, Kernie SG. Developmental profiling of postnatal dentate gyrus progenitors provides evidence for dynamic cell-autonomous regulation. Hippocampus. 2011;21(1):33–47. doi: 10.1002/hipo.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seress L. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans. Prog Brain Res. 2007;163:23–41. doi: 10.1016/S0079-6123(07)63002-7. [DOI] [PubMed] [Google Scholar]
- 42.Long JM, et al. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19(5):497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 43.Roitbak T, Surviladze Z, Cunningham LA. Continuous expression of HIF-1α in neural stem/progenitor cells. Cell Mol Neurobiol. 2011;31(1):119–133. doi: 10.1007/s10571-010-9561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernal GM, Peterson DA. Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging Cell. 2011;10(3):466–482. doi: 10.1111/j.1474-9726.2011.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirby ED, et al. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. eLife. 2013;2:e00362. doi: 10.7554/eLife.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer TJ, Walker TL, Overall RW, Brandt MD, Kempermann G. Acute effects of wheel running on adult hippocampal precursor cells in mice are not caused by changes in cell cycle length or S phase length. Front Neurosci. 2014;8:314. doi: 10.3389/fnins.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gage FH, Temple S. Neural stem cells: Generating and regenerating the brain. Neuron. 2013;80(3):588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 50.DeCarolis NA, et al. In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus. 2013;23(8):708–719. doi: 10.1002/hipo.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med. 2013;17(1):30–54. doi: 10.1111/jcmm.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerber H-P, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417(6892):954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 53.Bozoyan L, Khlghatyan J, Saghatelyan A. Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling. J Neurosci. 2012;32(5):1687–1704. doi: 10.1523/JNEUROSCI.5531-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317(5836):381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 55.Obernier K, Tong CK, Alvarez-Buylla A. Restricted nature of adult neural stem cells: Re-evaluation of their potential for brain repair. Front Neurosci. 2014;8:162. doi: 10.3389/fnins.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, et al. Hypoxia inducible factor-1α (HIF-1α) is required for neural stem cell maintenance and vascular stability in the adult mouse SVZ. J Neurosci. 2014;34(50):16713–16719. doi: 10.1523/JNEUROSCI.4590-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calvo C-F, et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011;25(8):831–844. doi: 10.1101/gad.615311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y, et al. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: Evidence from knockout mice and growth factor administration. Dev Biol. 2006;289(2):329–335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9(3):169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.