Abstract
Background
A significant relationship has been reported in which Ki-67/MIB-1 expression is correlated with survival in cervical cancer patients. However, the prognostic value of Ki-67/MIB-1 in cervical cancer is still not well understood.
Material/Method
A meta-analysis was carried out to explore the prognostic value of Ki-67/MIB-1 on overall survival (OS) and/or disease-free survival (DFS) in cervical cancer. The databases of PubMed, ISI Web of Science, EMBASE, Cochrane Central Register of Controlled Trials, ScienceDirect, and Wiley Online Library were used to identify relevant literature.
Results
We included 18 studies covering 1344 patients in the meta-analysis. The effect of Ki-67/MIB-1 on OS for pooled random effects HR estimate was 1.63 (95% confidence intervals (CI) 1.09–2.45; P<0.05). Subgroup analysis by ethnicity suggested that high expression of Ki-67/MIB-1 had association with Asians (1.84, 95% CI 1.04–3.23), but not with Africans (HR=1.53, 95% CI 0.34–6.86) or Europeans (HR=1.29, 95% CI 0.74–2.23). Furthermore, subgroup analysis of diverse treatments revealed no difference in surgery (HR=1.97, 95% CI 0.78–4.99) and radiation therapy (RT) (HR=1.56, 95% CI 0.93–2.63). The pooled HR for DFS was 1.26 (95% CI 0.58–2.73; P>0.05) and the subgroup analysis indicated Ki-67/MIB1 was associated with DFS (HR=3.67, 95% CI 2.65–5.09) in Asians. In the treatment subgroup analysis, no direct value was found among surgery (HR=1.13, 95% CI 0.10–13.53) and RT (HR=1.26, 95% CI 0.71–2.24).
Conclusions
Our meta-analysis concludes that Ki-67/MIB-1 had a prognostic value for OS in cervical cancer patients. To further evaluate the prognostic role of Ki-67/MIB-1 on DFS, studies with larger numbers of patients are needed to validate our findings.
MeSH Keywords: Ki-67 Antigen, Meta-Analysis, Prognosis, Uterine Cervical Neoplasms
Background
Cervical cancer is the fourth most common cancer after breast, colorectal, and lung cancer in women. According to GLOBOCAN 2012 statistics, there were an estimated 266,000 deaths from cervical cancer worldwide in 2012, accounting for 7.5% of all female cancer deaths. In Europe, a total of 58,373 new cases of cervical cancer and 24,385 deaths were reported in 2012. The overall incidence rate of cervical cancer in Europe is 10.6 per 100,000 [1]. Surgery combined with chemotherapy and radiotherapy is the main treatment of cervical cancer. However, the long-term prognosis of cervical cancer is still poor, with expected 5-year survival rate less than 10% [2].
Cervical cancer remains a major health problem in women worldwide. Even with the best currently available therapies, a significant proportion of patients will experience metastasis or recurrence, and eventually death. The prognosis of these patients is unsatisfactory. More effective methods and technology to diagnose and treat cervical cancer are needed to prolong the life of patients and increase the survival rate. In some studies, FIGO stage, lymph node status, and pathological features of primary tumor, such as tumor size, depth of cervical invasion, histological type, lymph-vascular space invasion, diffusion to the uterine body, and parametrical tissue involvement were thought to be relevant to the prognosis of cervical cancer. However, the status of tumor proliferation, such as the expression of Ki-67, has become another factor to identify in the prognosis of cervical cancer.
Ki-67, a protein in the nuclei of growing cells and expresses – in the G1, S, G2 and M phases of the cell cycle, is associated with cellular proliferation. Ki-67 antigen can react with its monoclonal antibodyMIB-1, [3]. Ki-67/MIB-1 is known as predictive factor for tumor development and its expression shows a correlation with poor prognosis in several types of cancer, such as breast cancer [4] and non-small-cell lung carcinoma [5]. In a meta-analysis, Ki-67/MIB-1 holds the potential as a predictor of outcome in early breast cancer [4]. Kritpracha et al. [6] reported that high level of MIB-1 staining above the cut-off point of 7.6% indicated significantly poorer survival in ovarian cancer. However, the role of Ki-67/MIB-1 in the prognosis of cervical cancer is still controversial. Vasilescu et al. [7] confirmed that Ki-67 showed correlation with cancer progression and Ki-67 could be useful to identify those patients who required more aggressive treatment in clinical practice. In contrast, Van de Putte et al. [8] reported that the increased labeling of Ki-67 was not associated with poor outcomes in stage I cervical carcinoma patients.
Because the clinical value of this proliferation marker for prognostication of cervical cancer is still uncertain, we sought to investigate the impact of Ki-67/MIB-1 on overall survival (OS) and/or disease-free survival (DFS) in cervical cancer by performing this meta-analysis study.
Material and Methods
Publication selection and eligibility criteria
In this meta-analysis, we selected studies that appraised the association between Ki-67/MIB-1 and prognosis in cervical cancer, which were published until 7th, October 2014. The studies were established in PubMed, ISI Web of Science, EMBASE, Cochrane Central Register of Controlled Trials, ScienceDirect, and Wiley Online Library, using the following keywords: cervical cancer, Ki-67, MIB-1, proliferative marker, proliferative index, prognosis, survival. Criteria used to determine study eligibility were as follows: 1) the studies must be published in English and is a full essay, 2) inclusion of patients with primary cervical cancer, 3) investigation of the correlation between Ki-67/MIB-1 expression and the prognosis of cervical cancer, 4) prospective or retrospective cohort design with a clearly defined source population and justifications for all excluded eligible cases, 5) hazard ratios (HR) and 95% CI for OS or DFS were provided or could be calculated from the sufficient data, 6) when the same or similar samples of patients were investigated in several publications, the latest and most complete study was selected to avoid duplication.
The 4 researchers independently read the title and abstract of identified studies, and subsequently excluded the irrelevant ones. Then, the full texts of selected studies were scrutinized. After comprehensive evaluation according to the inclusion criteria, the 4 researchers determined whether the studies were to be included. If disagreements occurred in the eligibility of studies, the researchers would conduct a discussion or resort to the fifth researcher until a consensus was eventually reached.
Data extraction
The information of publications was carefully extracted by the 4 authors. The data collected were described as: year published, first author’s name, number of patients, antibody detecting Ki-67/MIB-1, defined cut-off values, follow-up period, and data provided for us to evaluate the relationship between the expression of Ki-67/MIB-1 and OS and/or DFS. The minimal duration of median follow-up and the minimal number of patients were not defined in the included studies for this meta-analysis. The following exclusion criteria were implemented in all studies: (1) reviews, case reports, conference papers, letters, expertise public opinion, zoopery, and non-English essays were excluded; (2) non-cervical cancer-related studies and studies without information on ki-67/MIB1 impact on survival or studies in which the HR of survival could not calculated from the given information were excluded;(3) articles with duplicated data from the same or similar population or articles with data that could not be extracted were excluded.
Statistical methods
The expression of Ki-67/MIB-1 was divided into a negative and a positive group depending on the cut-off points given in the articles by the authors. To quantitatively aggregate the survival results, HRs was used to valuate the effect of the expression of Ki-67/MIB-1 on survival. The relationship of Ki-67/MIB-1 expression and patient prognosis was estimated by using the HRs and their 95% confidence intervals (CIs). In every study, the HR was evaluated with a method based on the outcome provided in the initial publication. We used 2 of the following parameters to recover the estimated HR value and its variance: the log-rank statistic or its P-value, the HR value estimate, and the O-E statistic or its variance. This method is known to be the most accurate in HR retrieval. If these parameters were not provided in the published articles, we searched for the case of patients exposed to the risk in each group, the log-rank statistic or the P-value, and the whole number of the events, which allowed us to calculate an approximation of the HRs. Furthermore, if the only exploitable survival data were in the form of figures (for example, the Kaplan-Meier curves), we read the curves by using Engauge Digitizer version 4.1 to extracted survival-relative data at stipulated time points and then filled-in the HR calculations spreadsheet with the extracted data to reconstruct the HRs and its standard error (SE) [9]. This spreadsheet was crucial to the estimation of HRs from published summary statistics or data extracted from Kaplan-Meier curves.
Four persons analyzed the survival curves independently to reduce the inaccuracy of the analyzing variations. If the authors described survival of more than 2 groups (for instance, the author utilized several cut-off values to divide the patients into different groups according to the percentage of Ki-67/MIB protein expression in the nucleus), we combined the data of some groups so that we could make a comparison between the 2 groups. For each study, the OS and/or DFS were analyzed. Whenever possible, HRs for subgroups was calculated, such as ethnicity (Africans, Europeans, or Asians) and various treatments (surgery and RT). We verified the results from the original publication at least 3 times to assure the conformance, in particular when the survival rate calculations were based on the survival curves.
All the data analyses were performed with Stata version 11.0 software. We combined the individual HRs estimated into an overall HR with the method reported by Yusuf S et al. [10]. We used Q-tests and P-values to estimate the heterogeneity. A P-value greater than 0.05 indicated that no heterogeneity was found among the studies, implying that a fixed-effects model was used to calculate the HR and its 95% CI according to the method of Mantel and Haenszel [11]. If the Q-tests implied that there was heterogeneity between the studies, we used a random-effects model instead of a fixed-effects model. According to the convention, an observed HR>1 indicated that the positive expression group of Ki-67/MIB-1 has a worse survival compare to the negative group. The result of Ki-67/MIB-1 impact on survival would have statistical significance if the 95% CI of the overall HR did not overlap 1. Possible source of heterogeneity was investigated by subgroup analysis. We carried out a subgroup analysis in accordance with the ethnicity and treatment of patients. Begg’s funnel plot was used to estimate the publication biases. A P value >0.05 indicated that no publication biases was found.
We performed the calculations of all the statistics for our meta-analysis with individual computing.
Results
Literature search and characteristics of the included studies
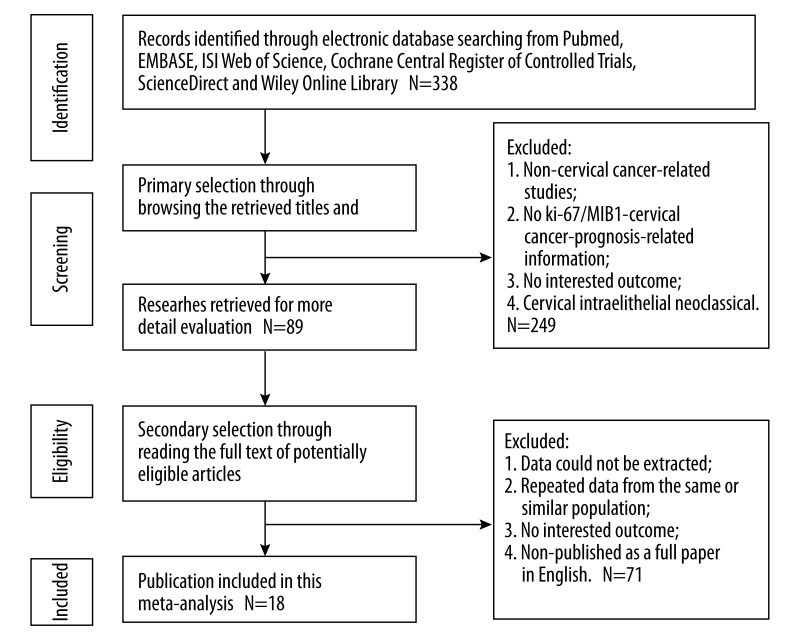
Figure 1 shows the flow diagram of studies included in our meta-analysis. Eighteen studies published between 1993 and 2014, including 1344 patients, were identified in this meta-analysis [12–29]. Tables 1 and 2 present the main information of the studies. The population of patients in each study varied from 30 to 180 cases and the follow-up time ranged from 13 months to 222 months (mean). Anti Ki-67 and MIB-1 were applied to detect the Ki-67 expression. Out of 13 studies for OS, subgroup analyses were possible in all of the 13 studies with ethnicity and treatment. In the analysis of DFS, 7 studies were estimated for aggregation results; the 7 studies were used for ethnic subgroup analysis and another subgroup analysis was in 6 studies with treatment that the patients received.
Figure 1.
Flow diagram of studies included in the meta-analysis.
Table 1.
Main characteristics of all studies included in the meta-analysis for overall survival.
| First author (pub year) | Patients Ki-67or MIB1 ± (total) | Median FU (mos) | Antibody | test method | Threshold (chosen by) | HR (95% CI) |
|---|---|---|---|---|---|---|
| Avall-Lundqvist EH (1997) | 109 | 82 (mean) | MIB-1 | IHC | 50%(PI) | 0.98 (0.84–1.14) |
| Bahnassy AA (2007) | 43 | NR | MIB-1 | IHC | 1%(PI) | 1.53 (1.03–20.7) |
| Dellas A (1997) | 76 | NR | MIB-1 | IHC | 34% (LI) | 1.04 (0.48–2.24) |
| Ho DM (2000) | 97 | 83.4 (mean) | MIB-1 | IHC | 55%(LI) | 3.62 (1.3–10.09) |
| Kuniyuki O (1996) | 186 | 60 | Anti-MIB-1 | IHC | 43% (PI) | 0.86 (0.49–1.52) |
| Klimek M (2011) | 122 | 5y | Anti-Ki-67 | IHC | 52% (PI) | 2.28 (1.27–4.11) |
| Oka K (2000) | 75 | NR | Anti-MIB-1 | IHC | 40% (PI) | 3.4 (0.53–22.15) |
| Shiohara S 1 (2005) | 103 | 65.1 (mean) | Anti-Ki-67 | IHC | 50% (PI) | 1.57 (0.53–4.66) |
| Shiohara S 2 (2005) | 103 | 65.1 (mean) | Anti-Ki-67 | IHC | 50% (PI) | 1.44 (0.32–6.39) |
| Sultana H (2003) | 30 | 5y | Anti-Ki-67 | IHC | 33% (LI) | 0.46 (0.04–5.99) |
| Suzuki M (2000) | 67 | 78 | Anti-MIB-1 | IHC | 26.4% (PI) | 0.49 (0.15–2.53) |
| Yang M (2014) | 180 | 64 (mean) | Anti-Ki-67 | IHC | 10% (PI) | 3.80 (1.80–4.70) |
| Zhang T (2012) | 40 | NR | Anti-Ki-67 | IHC | 34.62% (PI) | 3.44 (0.7–18.34) |
| Zhang T (2012) | 48 | NR | Anti-Ki-67 | IHC | 32.74% (PI) | 3.04 (0.73–13.26) |
FU – follow-up; HR – hazard ratio; CI – confidence interval; N+ – node-positive; NR – not reported + – positive; ‘−’ – negative; y – years; PI – proliferation index; LI – labeling index.
Table 2.
Main characteristics of all studies included in the meta-analysis for disease-free survival.
| First Author (pub year) | Patients Ki-67 or MIB1 ± (total) | Median FU (mos) | Antibody | Test method | Threshold (chosen by) | HR (95% CI) |
|---|---|---|---|---|---|---|
| Bahnassy AA (2006) | 38 | 13 (mean) | NR | IHC | NR | 1.38 (0.66–2.87) |
| Graflund M 1 N+ (2002) | 37 | 222 (mean) | MIB-1 | IHC | 1% (PI) | 0.30 (0.10–0.91) |
| Graflund M 2 N+ (2002) | 37 | 222 (mean) | MIB-1 | IHC | 1% (PI) | 0.28 (0.01–0.85) |
| Klimek M (2011) | 122 | 5y | Anti-Ki-67 | IHC | 52% (PI) | 1.19 (1.05–3.50) |
| Morimura Y (1998) | 34 | NR | Anti-MIB-1 | IHC | 25% (PI) | 1.86 (0.20–17.82) |
| Nakano T (1993) | 45 | 3y (minimum) | Anti-Ki-67 | IHC | 33% (PMI) | 0.8 (0.06–12.05) |
| Oka K (2000) | 75 | NR | Anti-MIB-1 | IHC | 40% (PI) | 4.88 (0.42–55.98) |
| Yang M (2014) | 180 | 64 (mean) | Anti-Ki-67 | IHC | 10% (PI) | 3.80 (2.50–4.90) |
HR – hazard ratio; CI – confidence interval; N+ – node-positive; NR – not reported; + – positive; ‘−’ – negative; y – years; PI – proliferation index; PMI – mitotic index of proliferating cell population.
Meta-analysis
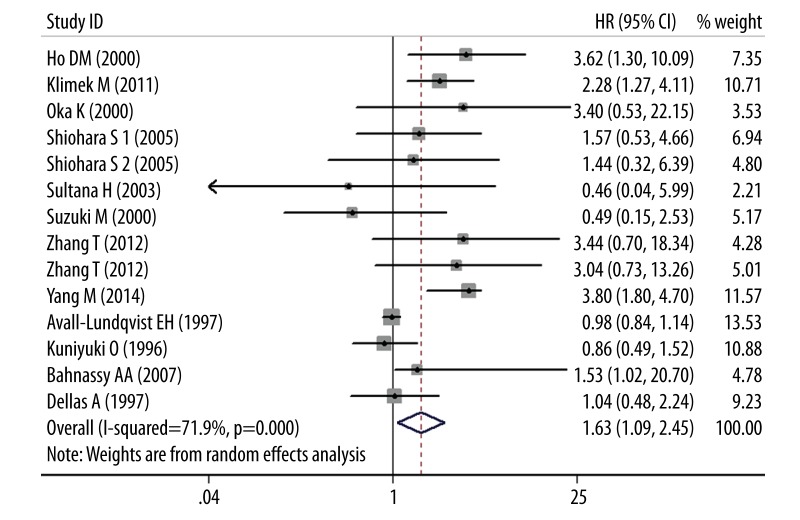
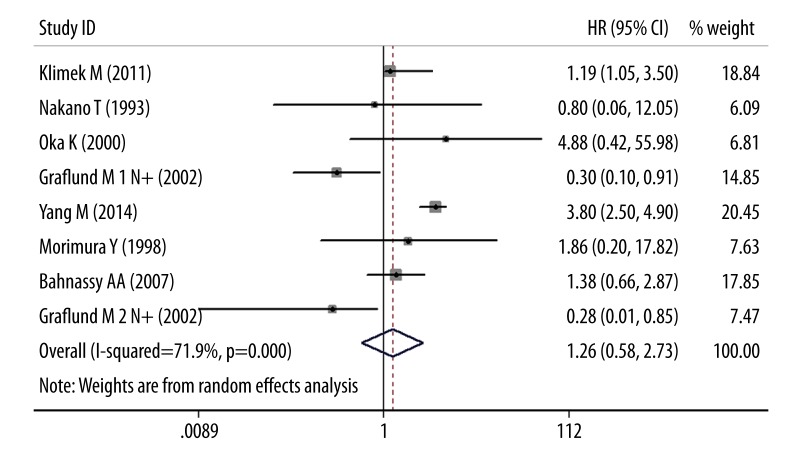
Figure 2 shows the meta-analysis results of OS. Thirteen studies used immunohistochemistry (IHC) for OS, the pooled random HR was 1.63 (95% CI 1.09–2.45; P<0.05), with significant evidence of heterogeneity between studies (I2=71.9%, Q=2.36, P<0.05). Restricting analysis to ethnicity, data failed to show a correlation in Africans (HR=1.53, 95% CI 0.34–6.86) and Europeans (HR=1.29, 95% CI 0.74–2.23), but a statistically difference was observed in Asians (HR=1.84, 95% CI 1.04–3.23). By treatment subgroup analysis, no dramatic differences were discovered in surgery (HR=1.97, 95% CI 0.78–4.99) or RT treatment (HR=1.56, 95% CI 0.93–2.63). Figure 3 shows the meta-analysis results of DFS. Seven studies assessed DFS with the pooled random HR of 1.26 (95% CI 0.58–2.73; P>0.05) and there was evidence of heterogeneity (I2=78.3%, Q=0.59, P>0.05). Altogether, 7 studies assessed ethnicity. The subgroup analysis revealed that Asian ethnicity was associated with DFS (HR=3.67, 95% CI 2.65–5.09), but no association was found in Europeans (HR=0.81, 95% CI 0.39–1.67). Of 5 studies eligible for treatment subgroup, analysis of these data showed that DFS was not positively associated with surgery (HR=1.13, 95% CI 0.10–13.53) and RT (HR=1.26, 95% CI 0.71–2.24).
Figure 2.
Results of the meta-analysis with all evaluable studies for OS. A HR >1 implies a worse OS for the group with increased Ki-67/MIB-1. The center of the lozenge gives the combined HR for the meta-analysis, and its extremities give the 95% CI.
Figure 3.
Results of the meta-analysis with all evaluable studies for DFS. No association was found between Ki-67/MIB-1 and DFS, and the 95% CI for the overall HR did overlap 1. The center of the lozenge gives the combined HR for the meta-analysis, and its extremities give the 95% CI.
Sensitivity analyses
Sensitivity analyses were performed to evaluate the meta-results stability. For OS and DFS, the results indicated that random-effects estimate before and after the deletion studies were similar, suggesting stability of the meta-analysis.
Publication bias
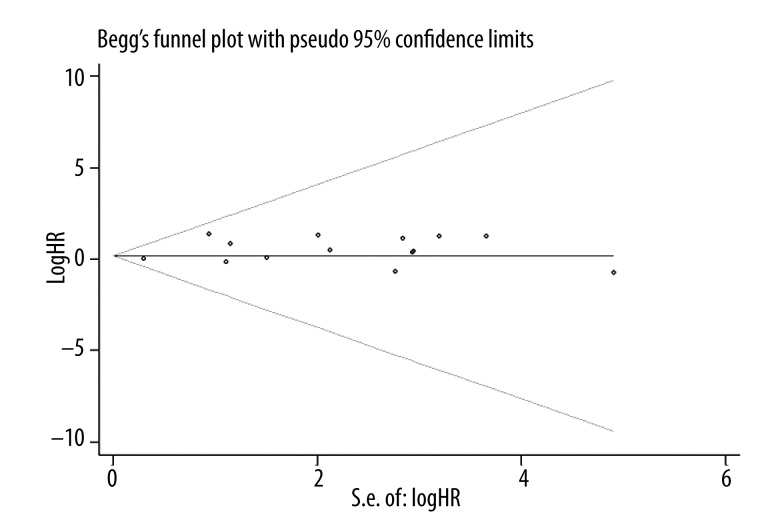
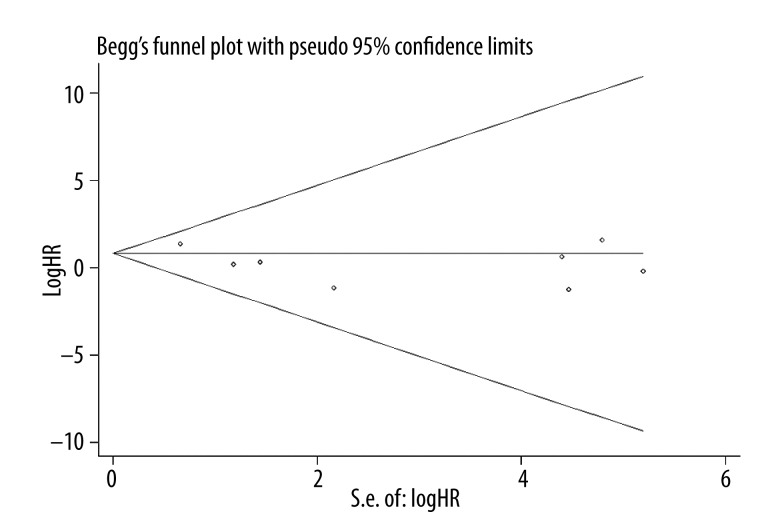
Publication bias statistics were determined by using Begg’s test. No publication biases were found in 13 OS studies (P=0.907) (Figure 4), but significant difference was found in 7 studies on DFS (P=0.014) (Figure 5).
Figure 4.
Begg test was constructed to detect the publication bias risk of OS (P=0.907).
Figure 5.
Begg test was constructed to detect the publication bias risk of DFS (P=0.014).
Discussion
Ki-67/MIB-1 plays an important role as a tumor marker in cancers due to its close correlation with cell proliferation. It was reported that overexpression of Ki-67/MIB-1 had significant association with poor prognosis in cervical cancer [14,18–20,23, 25–30]. In this meta-analysis, our results confirm that an increase of Ki-67/MIB-1 expression was closely correlated with the prognosis of cervical cancer, demonstrating that it might be used as a potential predictor of prognosis in cervical cancer.
It is well known that the development of cervical cancer is a multistep process, based directly or indirectly on cell proliferation. Ki-67/MIB-1 protein is a cellular marker for proliferation, which can be detected within the cell nucleus with monoclonal antibody MIB-1 and can act as a predictive factor for tumor development. Ki-67 is present during all active phases of the cell cycle (G1, S, G2, and mitosis), but is absent from resting cells (G0). Assessment of Ki-67 protein expression by IHC is a reliable method of evaluating the proliferation of tumor cells. However, the mechanism governing Ki-67 gene expression remains unknown. Tian et al. [31] reported that Sp1 plays a role in transcriptional regulation of the Ki-67 gene. The Ki-67 transcriptional activity was inhibited through down-regulating the Sp1 expression using siRNA-Sp1 and mithramycin, which may be useful for gene therapy. Studies have shown a correlation between Ki67/MIB-1 expression and poor prognosis in several types of cancer, such as ovarian cancer [6], breast cancer [4], esophageal squamous cell carcinoma [32], lung cancer [33], and carcinoma of the bile duct and gallbladder [34], which indicated that Ki-67/MIB-1 might be used as a potential predictor for the survival of patients with some cancers. Similarly to the aforementioned cancers, the finding from the available research demonstrates that high expression of Ki-67 can predict poor prognosis in cervical cancer [35].
According to our analysis results, no publication biases were found in OS studies, but publication biases were found in DFS studies (Figures 1 and 2). The source of the bias can come from many factors. Here, we discuss the source of bias and some limitations of our meta-analysis. Our meta-analysis is a literature-based study. Therefore, study selection might generate some limitations. We restricted our analysis to studies published as full papers in English; there were several non-English studies that met the eligibility criteria but were excluded due to language. Because of limitations, some of the articles were not included in the current meta-analysis and only the fully published studies were considered. There are still some unpublished personal data or meeting abstracts that are unavailable. We did not extend the search to unpublished studies and abstracts because the data were often only obtained from the full publication studies, as the methodology we used required, which would lead to comprehensive information collection and might influence our results.
In the HR estimates, variation can come from the method we used for extrapolating HR. For certain studies, we obtained HR directly from the original data provided in the study. When no HR was available in a study, we extrapolated survival-relative data from Kaplan-Meier curves and calculated the HRs. Four authors independently analyzed the curves and extrapolated the HR to reduce variability. The estimated HR and its statistical significance were compared with the original results presented in the study. Among our results and the results presented in the study, we are not confirming whether there was any contradiction.
A potential source of bias may come from the techniques we used to detect the expression of Ki-67 /MIB-1. The convenience and high sensitivity of MIB-1 make it the most common and frequently used antibody for use in staining Ki-67 rather than other antibodies in recent studies. In our meta-analysis, the antibody used in IHC to identify Ki-67 protein was not always performed with the same antibody: some studies used anti-MIB-1, some used anti-Ki-67 or MIB-1, and some studies provided no information of antibodies. Two recent studies compared different available Ki-67/MIB-1 equivalent antibodies, showing that the 4 tested Ki-67 equivalent antibodies appeared to make differences in quantitative and qualitative staining feature [36,37] According to these 2 studies, MIB-1 antibody is the most suitable to detect Ki-67 antigen because it has the highest sensitivity and gives the clearest staining when compared to other available antibodies and the use of varied concentrations of the antibody also has an effect on the staining result. With positive nuclear staining for a common standard, only when the tumor cells stained to a certain extent, Ki-67/MIB-1 expression can be considered to be positive. Therefore, the use of different antibodies and a protocol to count the number of cells stained by the antibody without a received standard may produce alterations among the studies.
The use of different cut-off points for IHC is of great significance. In our meta-analysis, arbitrary and varied cut-off points were used to define cervical cancer with a Ki-67/MIB-1 positive expression by different investigators. Some research took the median values as the cut-off points, while other studies selected the most suitable cut-off values or arbitrary values, which might make it difficult to determine a standard critical value in clinical practice. Altman et al. [38] stated that selection of the cut-off value depending on the minimum P-value approach would lead to seriously biased conclusions. If a preferred cut-off is usually selected arbitrarily, choosing the median value to define the expression levels appears to be a more standardized approach to analysis of prognostic factors, although it may cause some loss of information [38]. In a recent research of breast cancer, Spyratos et al. [39] used 5 different Ki-67/MIB-1 cut-offs to describe the optimal one for classifying tumors with high and low proliferation indexes in therapeutic trials. They found that with a Ki-67/MIB-1 cut-off of 10%, few tumors with low proliferation rate were under the misclassification. In contrast, a Ki-67/MIB-1 cut-off of 25% was more acceptable to identify a highly proliferative tumor. In accordance with the study of Walts et al. [40], an operating characteristic cure analysis has shown that a Ki-67 index cutoff value of 5% provides the best fit for specificity and sensitivity in predicting overall survival. Therefore, using different Ki-67/MIB1 cut-offs might have an influence on the classification of low and high proliferative tumors. In summary, an appropriate threshold still needs to be determined for Ki-67/MIB-1 to validate for cervical cancer.
Furthermore, we only assessed the univariate prognostic value of Ki-67/MIB-1, which might be another limitation of this meta-analysis. Besides Ki-67/MIB-1 positive expression, other features can also have an effect on the prognosis, including tumor clinical grading, lymph node metastasis, and auxiliary treatment. Hence, we are unable to conclude that Ki-67/MIB-1 can serve as an independent factor in the prognosis of cervical cancer. A prospective study with a larger number of patients is needed to answer this question.
In spite of these limitations, we can still characterize the role of Ki-67/MIB1 as a prognostic marker in cervical cancer, according to the finding that patients with high Ki-67/MIB1 expression had significantly shorter OS than patients with low expression of Ki-67/MIB1 (P<0.001). If we had the necessary data completely provided in all chosen studies, or if these studies had consulted the recommended REMARK, our literature-based meta-analysis would better assess the role of Ki-67/MIB1 in prognosis in cervical cancer. Furthermore, it is necessary that better designed studies need to be enrolled into future meta-analyses to provide a stronger conclusion about the relationship between Ki-67/MIB1 expression and prognosis of patients with cervical cancer. In addition, a simpler, quantitative, and reproducible assay needs to be developed and validated for the detection of Ki-67/MIB1. The value of Ki-67/MIB1 for molecular staging of cervical cancer also needs to be confirmed in controlled trials involving larger numbers of patients with longer follow-up.
Conclusions
The aim of our meta-analysis was to explore the relationship between the expression of Ki-67/MIB-1 and prognosis of cervical cancer. Although there were several limitations, we concluded that Ki-67/MIB-1 had a prognostic value for OS in patients with cervical cancer. To further evaluate the prognostic role of Ki-67/MIB-1 on DFS, studies with larger numbers of patients are needed to validate our findings.
Footnotes
Conflict of interests
The authors report no conflict of interests. The authors alone are responsible for the content and writing of the paper.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Source of support: The study was supported by the fund of “Future Star in Science” in Guangxi, China (No. WLXSZX1414)
References
- 1.Kesic V, Poljak M, Rogovskaya S. Cervical cancer burden and prevention activities in Europe. Cancer Epidemiol Biomarker. 2012;21(9):1423–33. doi: 10.1158/1055-9965.EPI-12-0181. [DOI] [PubMed] [Google Scholar]
- 2.Eskander RN, Tewari KS. Beyond angiogenesis blockade: targeted therapy for advanced cervical cancer. J Gynecol Oncol. 2014;25(3):249–59. doi: 10.3802/jgo.2014.25.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Key G, Becker MH, Baron B, et al. New Ki-67-equivalent murine monoclonal antibodies (MIB 1–3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Inves. 1993;68(6):629–36. [PubMed] [Google Scholar]
- 4.de Azambuja E, Cardoso F, de Castro G, Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96(10):1504–13. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin B, Paesmans M, Mascaux C, et al. Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer. 2004;91(12):2018–25. doi: 10.1038/sj.bjc.6602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kritpracha K, Hanprasertpong J, Chandeying V, et al. Survival analysis in advanced epithelial ovarian carcinoma in relation to proliferative index of MIB-1 immunostaining. J Obstet Gynaecol Res. 2005;31(3):268–76. doi: 10.1111/j.1447-0756.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 7.Vasilescu F, Ceausu M, Tanase C, et al. P53, p63 and Ki-67 assessment in HPV-induced cervical neoplasia. Rom J Morphol Embryol. 2009;50(3):357–61. [PubMed] [Google Scholar]
- 8.Van de Putte G, Kristensen GB, Lie AK, et al. Cyclins and proliferation markers in early squamous cervical carcinoma. Gynecol Oncol. 2004;92(1):40–46. doi: 10.1016/j.ygyno.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335–71. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 11.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. [PubMed] [Google Scholar]
- 12.Avall-Lundqvist EH, Silfversward C, Aspenblad U, et al. The impact of tumour angiogenesis, p53 overexpression and proliferative activity (MIB-1) on survival in squamous cervical carcinoma. Eur J Cancer. 1997;33(11):1799–804. doi: 10.1016/s0959-8049(97)00161-5. [DOI] [PubMed] [Google Scholar]
- 13.Bahnassy AA, Zekri AR, Alam El-Din HM, et al. The role of cyclins and cyclins inhibitors in the multistep process of HPV-associated cervical carcinoma. J Egypt Natl Canc Inst. 2006;18(4):292–302. [PubMed] [Google Scholar]
- 14.Bahnassy AA, Zekri AR, Saleh M, et al. The possible role of cell cycle regulators in multistep process of HPV-associated cervical carcinoma. BMC Clin Pathol. 2007;7:4. doi: 10.1186/1472-6890-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cattoretti G, Becker MH, Key G, et al. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168(4):357–63. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 16.Dellas A, Schultheiss E, Almendral AC, et al. Altered expression of mdm-2 and its association with p53 protein status, tumor-cell-proliferation rate and prognosis in cervical neoplasia. Int J Cancer. 1997;74(4):421–25. doi: 10.1002/(sici)1097-0215(19970822)74:4<421::aid-ijc10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 17.Graflund M, Sorbe B, Karlsson M. MIB-1, p53, bcl-2, and WAF-1 expression in pelvic lymph nodes and primary tumors in early stage cervical carcinomas: correlation with clinical outcome. Int J Oncol. 2002;20(5):1041–47. [PubMed] [Google Scholar]
- 18.Ho DM, Hsu CY, Chiang H. MIB-1 labeling index as a prognostic indicator for survival in patients with FIGO stage IB squamous cell carcinoma of the cervix. Gynecol Oncol. 2000;76(1):97–102. doi: 10.1006/gyno.1999.5663. [DOI] [PubMed] [Google Scholar]
- 19.Klimek M, Kruczak A, Rys J, et al. Clinico-morphological parameters affecting survival of patients with advanced cervical cancer. Pol J Pathol. 2011;62(4):250–56. [PubMed] [Google Scholar]
- 20.Morimura Y, Yanagida K, Hashimoto T, et al. Evaluation of immunostaining for MIB1 and nm23 products in uterine cervical adenocarcinoma. Tohoku J Exp Med. 1998;185(3):185–97. doi: 10.1620/tjem.185.185. [DOI] [PubMed] [Google Scholar]
- 21.Nakano T, Oka K. Differential values of Ki-67 index and mitotic index of proliferating cell population. An assessment of cell cycle and prognosis in radiation therapy for cervical cancer. Cancer. 1993;72(8):2401–8. doi: 10.1002/1097-0142(19931015)72:8<2401::aid-cncr2820720818>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.Oka K, Arai T. MIB1 growth fraction is not related to prognosis in cervical squamous cell carcinoma treated with radiotherapy. Int J Gynecol Pathol. 1996;15(1):23–27. doi: 10.1097/00004347-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Oka K, Suzuki Y, Nakano T. High growth fraction at 9 grays of radiotherapy is associated with a good prognosis for patients with cervical squamous cell carcinoma. Cancer. 2000;89(7):1526–31. [PubMed] [Google Scholar]
- 24.Shiohara S, Shiozawa T, Miyamoto T, et al. Expression of cyclins, p53, and Ki-67 in cervical squamous cell carcinomas: overexpression of cyclin A is a poor prognostic factor in stage Ib and II disease. Virchows Arch. 2005;446(6):626–33. doi: 10.1007/s00428-005-1252-0. [DOI] [PubMed] [Google Scholar]
- 25.Sultana H, Kigawa J, Kanamori Y, et al. Chemosensitivity and p53-Bax pathway-mediated apoptosis in patients with uterine cervical cancer. Ann Oncol. 2003;14(2):214–19. doi: 10.1093/annonc/mdg071. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M, Tsukagoshi S, Saga Y, et al. Assessment of proliferation index with MIB-1 as a prognostic factor in radiation therapy for cervical cancer. Gynecol Oncol. 2000;79(2):300–4. doi: 10.1006/gyno.2000.5950. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Liu YD, Wang YY, et al. Ubiquitin-specific protease 22: a novel molecular biomarker in cervical cancer prognosis and therapeutics. Tumour Biol. 2014;35(2):929–34. doi: 10.1007/s13277-013-1121-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhang T, Zhao C, Chen D, Zhou Z. Overexpression of AQP5 in cervical cancer: correlation with clinicopathological features and prognosis. Med Oncol. 2012;29(3):1998–2004. doi: 10.1007/s12032-011-0095-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Zhao C, Luo L, et al. The expression of Mcl-1 in human cervical cancer and its clinical significance. Med Oncol. 2012;29(3):1985–91. doi: 10.1007/s12032-011-0005-y. [DOI] [PubMed] [Google Scholar]
- 30.Costa S, Terzano P, Santini D, et al. Neoadjuvant chemotherapy in cervical carcinoma: regulators of cell cycle, apoptosis, and proliferation as determinants of response to therapy and disease outcome. Am J Clin Pathol. 2001;116(5):729–37. doi: 10.1309/8B4E-57PR-T50F-VRQT. [DOI] [PubMed] [Google Scholar]
- 31.Tian H, Qian GW, Li W, et al. A critical role of Sp1 transcription factor in regulating the human Ki-67 gene expression. Tumour Biol. 2011;32(2):273–83. doi: 10.1007/s13277-010-0119-4. [DOI] [PubMed] [Google Scholar]
- 32.Chen M, Huang J, Zhu Z, et al. Systematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancer. BMC Cancer. 2013;13:539. doi: 10.1186/1471-2407-13-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scagliotti GV, Micela M, Gubetta L, et al. Prognostic significance of Ki67 labelling in resected non small cell lung cancer. Eur J Cancer. 1993;29A(3):363–65. doi: 10.1016/0959-8049(93)90387-u. [DOI] [PubMed] [Google Scholar]
- 34.Shrestha ML, Miyake H, Kikutsuji T, Tashiro S. Prognostic significance of Ki-67 and p53 antigen expression in carcinomas of bile duct and gallbladder. J Med Invest. 1998;45(1–4):95–102. [PubMed] [Google Scholar]
- 35.Cole DJ, Brown DC, Crossley E, et al. Carcinoma of the cervix uteri: an assessment of the relationship of tumour proliferation to prognosis. Br J Cancer. 1992;65(5):783–85. doi: 10.1038/bjc.1992.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindboe CF, Torp SH. Comparison of Ki-67 equivalent antibodies. J Clin Pathol. 2002;55(6):467–71. doi: 10.1136/jcp.55.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindboe CF, von der Ohe G, Torp SH. Determination of proliferation index in neoplasms using different Ki-67 equivalent antibodies. APMIS. 2003;111(5):567–70. doi: 10.1034/j.1600-0463.2003.1110505.x. [DOI] [PubMed] [Google Scholar]
- 38.Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86(11):829–35. doi: 10.1093/jnci/86.11.829. [DOI] [PubMed] [Google Scholar]
- 39.Spyratos F, Ferrero-Pous M, Trassard M, et al. Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer. 2002;94(8):2151–59. doi: 10.1002/cncr.10458. [DOI] [PubMed] [Google Scholar]
- 40.Walts AE, Ines D, Marchevsky AM. Limited role of Ki-67 proliferative index in predicting overall short-term survival in patients with typical and atypical pulmonary carcinoid tumors. Mod Pathol. 2012;25(9):1258–64. doi: 10.1038/modpathol.2012.81. [DOI] [PubMed] [Google Scholar]